Abstract
Purpose
This study was designed as a multicenter, randomized, open-label study to evaluate the efficacy and tolerability of Clotinab™. We expected to obtain same results as with ReoPro® in improving ischemic cardiac complications in high-risk patients who were about to undergo percutaneous coronary intervention (PCI).
Patients and Methods
Patients of 19 - 80 years of age with acute coronary syndrome (ACS) who were about to undergo PCI were enrolled. After screening and confirmation of eligibility, patients were randomly assigned to different groups. Clotinab™ was given to 84 patients (58.7 ± 10.6 years, M : F = 68 : 16) and ReoPro® (59.0 ± 10.5 years, M : F = 30 : 10) was given to 40 patients before PCI. The primary efficacy endpoint was the onset of major adverse cardiac event (MACE) within 30 days from day 1. The tolerability endpoints were assessed based on bleeding, thrombocytopenia, change in Hb/Hct, human antichimetric antibody development, and adverse events.
Results
The number of Clotinab™ patients experiencing MACE was 0 out of 76 per protocol (PP) patients. The MACE rate was 0%, and its 95% exact CI was [0.00 - 4.74%]. A major bleeding event developed in 3 patients in the ReoPro® group. The probability of MACE onset in Clotinab™ was estimated to be less than 5%. There was no clinically significant result in tolerability variables.
Conclusion
Clotinab™ is an effective and safe medicine in preventing ischemic cardiac complications for high-risk patients who will receive PCI.
Keywords: Clotinab™ ReoPro®, acute coronary syndrome, angioplasty, platelet
INTRODUCTION
Platelet-mediated thrombosis accounts for the pathophysiology of acute coronary syndrome (ACS).1,2 In the treatment of ACS, intravenous platelet glycoprotein (GP) IIb/IIIa receptor antagonists for platelet aggregation may reduce the risk of ischemic complications.3-7 Therefore, in the management of ACS, platelet GP IIb/IIIa receptor inhibitors have been developed as a promising therapy for the reduction of coronary events and the improvement of clinical outcomes.
Abciximab, a platelet GP IIb/IIIa receptor blocker, was developed by Coller in 1985 and named 7E3.8 Abciximab is a chimeric human monoclonal antibody and binds to platelet surface GP IIb/IIIa receptors competitively with adhesive molecules, such as fibrinogen and von Willebrand factor, and blocks the final stage of platelet aggregation.9
Clotinab™ a product manufactured by ISU ABXIS Co., Ltd, was produced by inserting anti-platelet GP IIb/IIIa DNA into a Chinese hamster's ovary cell. Since it contains the same active ingredient as ReoPro®, Clotinab™ is expected to have the same efficacy as ReoPro® as a platelet GP IIb/IIIa receptor inhibitor. Therefore, the aim of this trial was to evaluate the efficacy and tolerability of Clotinab™ as an investigational drug and prevent ischemic cardiac events in high-risk patients scheduled to undergo PCI.
MATERIALS AND METHODS
Patients
Korean men and women between 19 - 80 years of age with ACS meeting 1 of the following conditions and who were scheduled to undergo PCI were enrolled: 1) Patients with ischemic ST change in the electrocardiogram as well as refractory to drug treatment in resting phase or with recurrent angina pectoris; 2) Post-infarction angina patients within 7 days from acute myocardial infarction (AMI) attack refractory to drug treatment with ischemic ST change in the electrocardiogram; 3) Acute Q-Wave myocardial infarction (MI) within 12 hours after the attack that required direct intervention procedures; 4) Acute Q-wave myocardial infarction within 12 hours after the attack that required another rescue intervention after failure to thrombolytic therapy; and 5) Classification of vascular lesions according to the American College of Cardiology/American Heart Association (ACC/AHA) criteria defined by angiography.10
Two type B lesion characteristics
One type C lesion characteristic
Females aged over 65 with one type B lesion characteristic
Diabetes with one type B lesion characteristic
Exclusion criteria
Patients with any of the following were excluded from the study: those in which PCI could not be performed within 24 hours from receiving the investigational product or occlusion of the left main coronary artery was ≥ 50% and bypass surgery was necessary. In addition, subjects with a culprit lesion in the bypass graft; history of major surgery, trauma, or retinal hemorrhage; significant gastrointestinal or genitourinary bleeding in the previous 6 weeks; cerebrovascular attack in the past 2 years or a cerebrovascular attack with a significant residual neurological deficit were excluded. Patients who required oral anticoagulants during the trial; those who had been administrated oral anticoagulants in the past 7 days; severe uncontrollable hypertension (systolic blood pressure > 180 mmHg or diastolic blood pressure > 100 mmHg); thrombocytopenia (< 100,000 cells/mL); hypersensitivity to the test drug or the murine protein; history of hypersensitivity; intracranial neoplasm, arteriovenous malformation; aneurysm; history or diagnosis of vasculitis; renal insufficiency (the level of serum creatinine was 2 times higher than the upper limit of normal of each center); or hepatic disorder (AST, ALT was three times higher than the upper limit of normal) were excluded. Pregnant women, alcoholics, drug abusers, patients who could not take anti-platelet drugs, and those who might have died of causes other than cardiac disease during the trial were also removed from the study.
Study design
This trial was conducted prospectively at 3 medical centers in Korea from May 2005-December 2005 in 2 stages. The first stage was a single-arm, multicenter, open-label study of Clotinab™. Thirty eligible patients were enrolled in the first stage. The original design was to evaluate efficacy and safety of Clotinab™ in the first stage. If the MACE rate was statistically significantly below 20%, the trial was to enter the second stage for comparison to ReoPro®. The second stage was a randomized multicenter open-label clinical trial with 40 patients to be assigned to the Clotinab™ group and 30 to the ReoPro® group. Although patients in the Clotinab™ group were recruited over two stages, the patients who were receiving Clotinab™ were considered as one group because there was no time break between the first and second stage and no protocol change between stages.
During the initial screening, patients underwent complete physical examinations, baseline electrocardiograms, and laboratory assessments. They were randomly assigned into 2 groups according to a computer-generated allocation schedule. A randomization list was made using a block randomization method. To create a 4 : 3 allocation ratio between the treatment and control groups, a block of size 7 was used for each center. The size of the block was not specified in the protocol or known to investigators.
After the screening and confirmation of the eligibility of patients, those who signed written consent forms were randomly assigned to one of the groups prior to the procedure, administration of the investigational product, and treatment by PCI on day 1. One group received 0.25 mg/kg of Clotinab™ IV bolus 10 minutes to 6 hours prior to PCI, followed by an infusion of 0.125 mg/kg/min (Max 10 µg/min) dissolved in sterile saline of 0.9% or 5% dextrose followed for 12 hours. The other group received 0.25 mg/kg of ReoPro® IV bolus 10 minutes to 6 hours prior to PCI, followed by an infusion of 0.125 mg/kg/min (Max 10 µg/min) dissolved in sterile saline of 0.9% or 5% dextrose followed for 12 hours. After the procedure, subjects remained in the intensive care units for observation. At least 2 hours before PCI in both groups, 200 mg of aspirin was administered orally, followed by oral administration of 100 mg/day. In addition to aspirin, a loading dose of 300 mg and maintenance dose of 75 mg/day of clopidogrel were given.
On day 30 after discharge, patients visited the hospital for safety and efficacy assessments. During the clinical visit, subjects were assessed by all clinical and laboratory measures, to unravel, the likelihood of a causal relationship between the study and the study drug ("definitely related", "probably related", "possibly related", "probably not related", "definitely not related", and "unknown"), and they were categorized by the intensity of clinical adverse experiences (CAEs). The institutional review board and ethics committee at each participating center approved the protocol, and written consent forms were obtained from all patients.
Efficacy assessments
The primary efficacy endpoint was the onset of MACE within 30 days from the study drug administration following PCI. MACE included death related to heart disease, recurrence of MI, and emergency revascularization. We classified the cause of death to either "cardiovascular causes" or "non-cardiovascular causes" (If non-cardiovascular causes could not clearly be established, the cause of death was classified to "cardiovascular causes"). The recurrence of MI was categorized as ST-elevation MI (STEMI) or non-ST-elevation MI (NSTEMI) [NSTEMI; CK-MB > 3 times the upper limit of normal value and increase of > 50% in the second sample over the first sample when two samples were collected at two different times]. A return to the catheterization laboratory for emergency revascularization to treat recurrent ischemia was considered as a primary event. A scheduled PCI (staged procedures) nor elective surgery to treat pre-existing multivessel disease was considered primary events. The secondary efficacy endpoint was a change in the electrocardiogram [New change in electrocardiogram was decided by investigator's decision about the ECG result], indicating myocardial ischemia.
Tolerability assessments
Tolerability endpoints were assessed by bleeding, thrombocytopenia, changes in Hb/Hct, HACA (human antichimetric antibody) development, and adverse events. (1) Bleeding: According to TIMI criteria,11 bleeding was categorized into "major bleeding". "minor bleeding", or "insignificant bleeding". (2) Thrombocytopenia: The details were described for patients whose platelet count was < 100.000 and platelet count decreased by ≥ 25% compared to baseline count. (3) Change of Hb/Hct: (4) HACA development: Frequency and proportion were presented to patients showing a positive response to HACA development by group. (5) Adverse events: All adverse events were coded. Frequency and proportion by these codes were generated in 2 groups. The frequency was calculated by group for adverse events that were related to the study drug ("definite", "probable", "possible") and not ("improbable", "none", "unknown"). Frequency and proportion (95% exact CI) were presented for the number of adverse event and the number of subjects in which adverse events occurred at least once.
Manufacturing process of Clotinab™
The active substance abciximab is produced in recombinant Chinese Hamster Ovary (CHO) cells using a serum-free media. The master cell bank (MCB), working cell bank (WCB), and end of production cells were sufficiently characterized. The MCB and WCB were adopted to grow in serum-free media. The manufacturing process of the active ingredient starts with the thawing and expansion of cells from the MCB or WCB derived from the MCB. Cells were expanded using a flask and fermenters. After harvesting, different chromatographic steps were used for purification. With affinity chromatography, unwanted protein and potential endotoxin contaminants were removed. Cation exchange chromatography removes antibody aggregates and fragment and CHO impurities. Anion exchange chromatography is intended to separate DNA, endotoxins, and retroviruses if present. With hydrophobic interaction, the chromatography removes antibody aggregates and fragments and CHO proteins.
Statistical analysis
A 1-sided significance test was applied to the primary efficacy endpoint at α = 0.05. All other significance tests were 2-sided at α = 0.05, and the efficacy analysis was the PP analysis. All statistical analyses were carried out by SAS® (Version 9.1, SAS institute, Cary, NC, USA). Exact confidence intervals were computed by StatXact (version 4.0, Cytel Software Corp). Continuous variables were compared using t-test or Wilcoxon rank sum test by treatment group. Categorical variables were compared using chi-square test or Fisher's exact test. For the primary endpoint, a non-inferiority analysis of the difference in MACE from baseline to day 30 between both study groups was assessed. Chi-square tests or Fisher's exact tests were performed to compare Clotinab™ and ReoPro®. Fisher's exact tests were performed to find the differences between the two groups. McNemar's tests were used to determine if the changes in electrocardiogram from pre- to post-administration were significant.
Any statistical significance tests would be probably underpowered if we make a direct comparison between two drugs. Therefore, we planned a non-inferiority trial, and unplanned posthoc statistical significance tests were performed on the difference in various parameters between Clotinab™ group and ReoPro® group.
Sample size estimation
Clotinab™ has same active ingredient as ReoPro® and is supposed to have a similar effect. The clinical trial was designed to prove that event rate of the primary efficacy endpoint of Clotinab™ was below 20%. This value of 20% was deduced from previous clinical trials.3,9,12-14 The following are the statistical null hypothesis and alternative hypothesis in this trial on Clotinab™.
H0: p ≥ 0.2 versus HA: p < 0.2
It is assumed that the event rate is more than 0.2. If the null hypothesis was rejected by this trial, it was concluded that the event rate of Clotinab™ was less than 0.2. The previous clinical trial set the event rate of 9%.15 Data distributed binomially, sample size, and critical value were obtained to satisfy significance level and power exactly at 0.05 and 0.8, respectively. The efficacy interpretation followed the decision rule. In the analysis of PP population, if 9 or fewer patients among 76 subjects who were treated with Clotinab™ experienced MACE, the MACE rate was estimated to be below 20% and Clotinab™ was considered to be effective. In FAS (Full analysis set, n = 83) and ITT (Intention-to-Treated, n = 84) population, if 10 or fewer patients experienced MACE, the MACE onset rate was estimated to be below 20% and Clotinab™ was considered to be effective. If the number of MACE patients was more than the defined number in each population, the efficacy of Clotinab™ was evaluated after adjusting the critical value based on the MACE rate in ReoPro®.
RESULTS
Patient characteristics
A total of 124 patients were screened for this trial at 3 medical centers. First, 31 patients were given Clotinab™ treatment without randomization. Afterward, 93 patients were admitted and randomized into the Clotinab™ (53) and ReoPro® (40) groups. Out of the 124 subjects screened, one subject was excluded before the treatment started, because the patient was found to be ineligible. After the study drugs were administered and PCI was performed on the 123 patients, one Clotinab™ subject withdrew consent and left the trial. The disposition status of 124 patients is shown in Fig. 1.
Fig. 1.
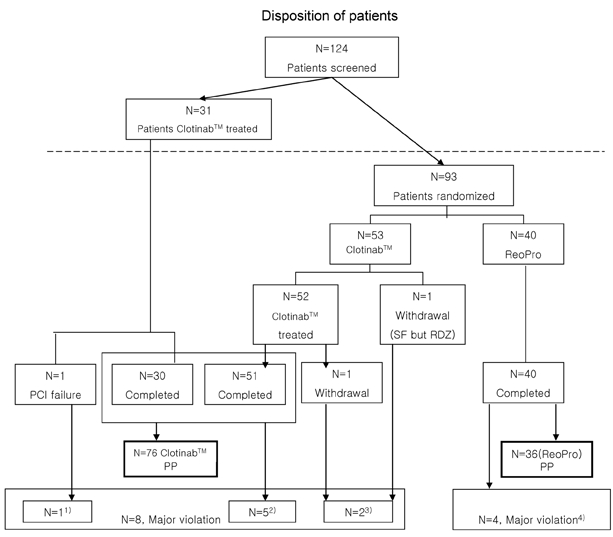
Disposition of patients There were 12 subjects with major protocol deviation. One1 had CABG treatment after PCI failure; 5 Clotinab™ patients2 who completed the trial were considered to be major protocol deviations; of 5 Clotinab™ patients, 4 did not clear exclusion criteria; and 1 patient came for the follow-up visit on day 10 instead of day 30. Two3 patients dropped out of the study, 1 subject withdrew consent on day 2, and 1 randomized subject (RDZ) was dropped because of a screening failure (SF). Four ReoPro® patients4 were considered major protocol violations, 3 subjects did not clear exclusion criteria, and 1 subject occurred an accidental disconnection of the IV line during IV administration of study drug.
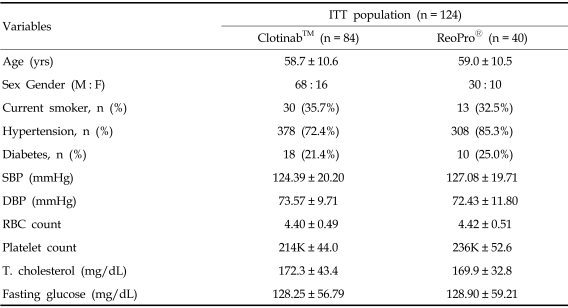
Out of the 124 subjects, including 31 Clotinab™ patients from stage 1, 84 subjects received Clotinab™ and 40 received ReoPro® The ITT set consisted of all 124 patients and the FAS consisted of 123 (Clotinab™: 83 patients, ReoPro®: 40). The PP set consisted of 112 patients (Clotinab™: 76 patients, ReoPro®: 36), PP analysis was the main method used to evaluate the efficacy of the drug and FAS analysis was used to determine the tolerability of the study drug. Table 1 gives the general characteristics of patients included in the study. The distribution of subjects by disease entity is shown in Table 2. There was no significant difference between the two study groups.
Table 1.
Subject Demographics
ITT, intention-to-treated; SBP, systolic blood pressure; DBP, diastolic blood pressure; T. cholesterol, total cholesterol.
Table 2.
Distribution of Subjects by Disease (can select more then one item)
ITT, intention-to-treated.
*The coronary artery stenosis ACC/AHA classification defined by angiography.10
†p value from chi-square test.
‡p value from Fisher's exact test.
Efficacy results
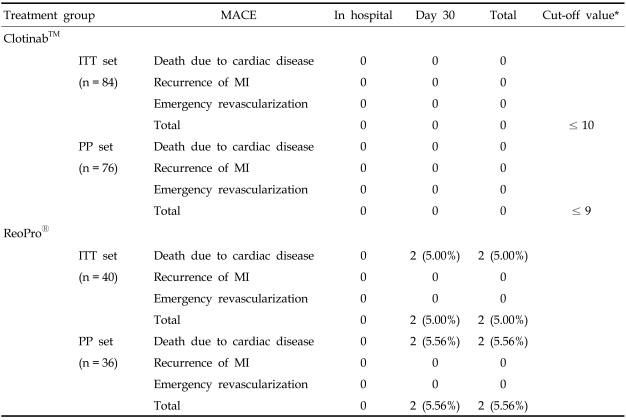
The primary efficacy endpoint was the onset of MACE within 30 days from the study drug administration following PCI. Table 3 shows the primary results of this trial. The number of Clotinab™ patients experiencing MACE was 0 out of 76 PP patients. The MACE rate was 0.00%, and its 95% exact CI was (0.00 - 4.74%). The upper confidence bound was less than 5%. The number of ReoPro® patients experiencing MACE was 2 out of 36 PP patients. The observed MACE rate was 5.56%, and its 95% exact CI was (0.68 - 18.66%). The upper confidence bound was below 20%. The ITT population analysis showed little variation from the PP analysis. None of 84 Clotinab™ subjects experienced MACE. The observed MACE rate was 0.0% and its 95% exact CI was (0.00 - 4.30%). Two out of 40 ReoPro® subjects experienced MACE. The observed MACE rate was 5.00% and its 95% exact CI was (0.61 - 16.92%). Upon analyses of PP, ITT, and FAS populations, it was concluded that Clotinab™ was effective in preventing MACE. The upper bound of 95% CI was all less than 5% regardless of the analysis population, indicating that the MACE rate among patients undergoing PCI will be less than 5% if they are also treated with Clotinab™. In the ReoPro® group, the upper bound of 95% CI was about 16.9 - 18.7% depending on the analysis population (ITT analysis: 16.9%, PP analysis: 18.7%). This indicates that less than 20% of patients undergoing PCI will experience MACE if treated with ReoPro®.
Table 3.
Primary Efficacy Endpoint: Major Adverse Cardiac Event Between Clotinab™ and ReoPro® Groups
MACE, major adverse cardiac event; ITT, intention-to-treated; PP, per protocol.
*The cut-off value comes from decision rule (decision rule is explained in the section of sample size estimation).
The secondary efficacy endpoint was a change of electrocardiogram that indicated myocardial ischemia. One Clotinab™ patient showed changes at 24 h and on day 3. None of the other Clotinab™ subjects showed new changes. One ReoPro® patient showed changes at 12 h, 24 h, and on day 3. None of the other ReoPro® patients showed any changes.
The drop of the status of ischemia rate in the Clotinab™ group from 37.3% at baseline to 20.0% on day 30 was statistically significant by the PP analysis (p < 0.001). The drop in the ReoPro® group from 48.6% to 21.2% was also statistically significant by the PP analysis (p = 0.013). The electrocardiogram data in the ITT and the FAS populations revealed results similar to that of the PP population. Unplanned posthoc analyses showed that there was no significant difference in the electrocardiogram changes (data not shown) between the Clotinab™ and the ReoPro® groups.
Tolerability results
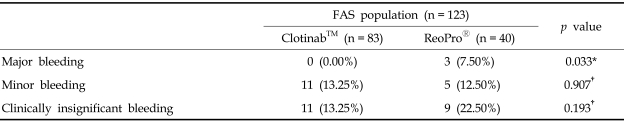
Based on TIMI criteria, no major bleeding occurred in Clotinab™ patients, but 3 subjects (7.50%) experienced major bleeding on ReoPro®. The number of subjects with minor bleeding was 11 (13.25%) for Clotinab™ and 5 (12.50%) for ReoPro®. The number of subjects with clinically insignificant bleeding was 11 (13.25%) for Clotinab™ and 9 (22.50%) for ReoPro®.
Based on TIMI criteria, the number of patients with overall bleeding showed no significant difference between Clotinab™ and ReoPro® at each time point (Table 4). However, a number of patients with major bleeding showed significant differences (p = 0.033). No significant results were noted in thrombocytopenia, change in Hb/ Hct, HACA.
Table 4.
Number of Patients with Bleeding Events
FAS, full analysis set.
Number of patients with at least 1 bleeding event during the trial.
*p value from Fisher's exact test.
†p value from chi-square test.
Serious adverse events occurred in 7 subjects [3 in Clotinab™ (3.61%), 95% exact CI (0.75 - 10.20%)], [4 in ReoPro® (10.00%), 95% exact CI (2.79 - 23.66%)]. Prolongation of existing hospitalization: 2 patients, life-threatening: 1 subject in Clotinab™. Death: 2 subjects, life-threatening: 2 subjects in ReoPro®. But we concluded that these 7 cases were not related to the study drug and there was no significant difference in SAE occurrence rate between Clotinab™ and ReoPro® (p = 0.152).
Sudden cardiac death occurred in two patients. One patient who had taken medication for diabetes mellitus and hypertension was treated with ReoPro®, followed by PCI. The subject died one week after discharge. The other patient was treated with ReoPro® and PCI for STEMI. He was admitted to the emergency room due to chest pain 4 days after discharge. Cardiac arrest occurred, and cardiopulmonary resuscitation was performed but the subject died. The investigator concluded that these two events were definitely not related to the study drug. Of the life-threatening adverse events, there was a case of cardiac tamponade in the ReoPro® group during PCI, one case of cardiac tamponade in the ReoPro® group on day 21 post-PCI, and one case of transient bradycardia with hypotension due to "no-reflow phenomenon" after stenting in the Clotinab™ group. Of the adverse events of prolongation of hospitalization, there was one case of dyspnea in the Clotinab™ group on day 2, and one case of urinary difficulty in the Clotinab™ group on day 23. All subjects, however, recovered and completed the study. We concluded that this was definitely not related to the study drug. The most frequent adverse events in the Clotinab™ and ReoPro® groups were related to general disorders and conditions of administration site. The most frequent adverse events related to PCI were wound secretion, catheter site hematoma, or catheter site hemorrhage in both treatment groups.
Out of 83 subjects in the Clotinab™ and 40 subjects in the ReoPro® groups, the number of subjects whose results were higher than the cut-off values or more than twice at 30 days after the administration, compared to the pre-administration in IgG screening, was 13 and 4 in the Clotinab™ and ReoPro® groups, respectively. After neutralization of the subjects who were selected by IgG screening, 6 subjects [7.2%, 95% exact C.I.(2.70 - 15.07%)] in the Clotinab™ group (83 subjects) were confirmed as HACA seropositive, and 4 among them were V-region and 2 were C-region. One patient [2.5%, 95% exact CI (0.06 - 13.16%)] in the ReoPro® group (40 subjects) was confirmed as HACA seropositive and C-region. Unplanned posthoc analyses showed that there was no significant difference in HACA seropositive response rate between the Clotinab™ and ReoPro® groups(p = 0.426) (Table 5).
Table 5.
HACA Incidence Between Clotinab™ and ReoPro® Groups
HACA, human antichimetric antibody; FAS, full analysis set; CI, confidence interval.
*p value from Fisher's exact test.
DISCUSSION
In this study, we demonstrated the efficacy and tolerability of Clotinab™ in improving ischemic cardiac complications in high-risk patients who were about to undergo PCI.
None of 84 patients in the Clotinab™ group experienced the primary efficacy endpoints of MACE. The same conclusion was also reached in the PP, ITT, and FAS populations. Clotinab™ is effective in preventing MACE. The upper bound of 95% CI was all below 5% regardless of the analysis population, indicating that the MACE rate among patients undergoing PCI will be less than 5% if treated with Clotinab™. Only one Clotinab™ patient experienced a change in electrocardiogram. The rate of ischemia, indicated by electrocardiogram, in the Clotinab™ patients at day 30 dropped significantly by about 17% from the baseline depending on the PP analysis. None of the Clotinab™ patients experienced major bleeding event. Therefore, we can conclude that Clotinab™ is safe and effective in preventing MACE in high-risk patients undergoing PCI.
In contrast, 2 out of 40 patients in the ReoPro® group experienced MACE during the study. The upper bound of 95% CI in the ReoPro® patients was about 16.9 - 18.7%, depending on the analysis population, indicating that less than 20% of the patients undergoing PCI will experience MACE if they are treated with ReoPro.® Only one patient experienced a change of electrocardiogram. The rate of ischemia, indicated by electrocardiogram, in the ReoPro® group at day 30 dropped significantly by about 27% from the baseline, depending on the PP analysis. There was no clinically significant result noted in tolerability variables without a major bleeding event. Three ReoPro® patients experienced a major bleeding event. The major bleeding rate was significantly higher in the ReoPro® group than in the Clotinab™ group. Because this study was not designed to compare the efficacy or tolerability of Clotinab™ with ReoPro® in patients undergoing PCI, we cannot conclude which treatment is safer or more effective. Nevertheless, we can conclude that Clotinab™ is safe and effective in preventing MACE in patients undergoing PCI.
The effect of the platelet GP IIb/IIIa receptor blocker has been proven in numerous clinical trials, such as the EPIC trial,9 EPILOG trial,16 PRAGON trial,17 PRISM trial,18 PRISM-PLUS trial,5 and PURSUIT trial.6 Therefore, the use of GP IIb/IIIa inhibitor has become standard therapy for PCI in high-risk patients. The ACC/AHA recommend the use of platelet GP IIb/IIIa inhibitors in patients undergoing PCI.19 The representative platelet GP IIb/IIIa receptor blockers currently used in clinical practice include abciximab (ReoPro®), which is a chimeric human-murine monoclonal antibody fragment; eptifibatide (Integrilin), which is a peptide; and Tirofiban (Aggrastat®) and Lamifiban, which are peptidomimetics.5,9,16,18,20-22
The most potent antithrombotic effect has been documented with ReoPro®, because it has a prolonged half-life and binds to the vitronectin receptor (found on endothelial and smooth-muscle cells).23,24 Therefore, ReoPro® has the potential to influence the adhesion of platelets with endothelial cells and platelets with white cells. However, the selection of platelet GP IIb/IIIa receptor blockers is often based on the clinician's preference and cost consideration. Because of its relatively high cost, the use of ReoPro® is not an option for some patients. There are reports on the economics of GP IIb/IIIa inhibitor therapy. The PRICE (Prairie ReoPro® Versus Integrilin Cost Evaluation) trial evaluated the differences between groups and concluded that eptifibatide was associated with lower in-hospital and 30-day costs compared to ReoPro®.25 By comparing abciximab and eptifibatide, the PRICE study noted significant differences in hospitalization and acquisition costs despite comparable tolerability and effectiveness.26
There are unequivocal clinical benefits in using GP IIb/IIIa inhibitors to reduce the ischemic complications of PCI. TARGET27 demonstrated the benefit of abciximab, in ischemic outcomes ofpatients treated by PCI compared to tirofiban. Moreover, the meta-analysis on the different GP IIb/IIIa receptor blockers suggests clinical differences among these drugs.28
Because the economics of the GP IIb/IIIa inhibitor therapy are closely linked to drug efficacy and tolerability, the development of GP IIb/IIIa receptor blocker, which is not only biocompatible to ReoPro® in efficacy and tolerability but also low in cost, may be an affordable option. Clotinab™, shown to be effective and safe in this study, may be used, in clinical practice, as a good substitute for ReoPro® because of its low cost. In conclusion, the probability of MACE onset in patients who use Clotinab™ is estimated to be less than 5%. There was no significant outcome in tolerability variables. Therefore, Clotinab™ is an effective medicine in preventing ischemic heart complications for high-risk patients who undergo PCI.
ACKNOWLEDGEMENT
We would like to thank Jeong Youn Bae, Jung Sook Oh, and Chung Kim, the study coordinators, for their assistance in the collection of the data.
Footnotes
This study was supported by ISU Abxis Co., Ltd. in Seoul, Korea.
References
- 1.Coller BS. Platelets and thrombolytic therapy. N Engl J Med. 1990;322:33–42. doi: 10.1056/NEJM199001043220107. [DOI] [PubMed] [Google Scholar]
- 2.Schömig A, Neumann FJ, Kastrati A, Schühlen H, Blasini R, Hadamitzky M, et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med. 1996;334:1084–1089. doi: 10.1056/NEJM199604253341702. [DOI] [PubMed] [Google Scholar]
- 3.Montalescot G, Barragan P, Wittenberg O, Ecollan P, Elhadad S, Villain P, et al. Platelet glycoprotein IIb/IIIa inhibition with coronary stenting for acute myocardial infarction. N Engl J Med. 2001;344:1895–1903. doi: 10.1056/NEJM200106213442503. [DOI] [PubMed] [Google Scholar]
- 4.Randomised placebo-controlled trial of abciximab before and during coronary intervention in refractory unstable angina: the CAPTURE Study. Lancet. 1997;349:1429–1435. [PubMed] [Google Scholar]
- 5.Inhibition of the platelet glycoprotein IIb/IIIa receptor with tirofiban in unstable angina and non-Q-wave myocardial infarction. Platelet Receptor Inhibition in Ischemic Syndrome Management in Patients Limited by Unstable Signs and Symptoms (PRISM-PLUS) Study Investigators. N Engl J Med. 1998;338:1488–1497. doi: 10.1056/NEJM199805213382102. [DOI] [PubMed] [Google Scholar]
- 6.Inhibition of platelet glycoprotein IIb/IIIa with eptifibatide in patients with acute coronary syndromes. The PURSUIT Trial Investigators. Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy. N Engl J Med. 1998;339:436–443. doi: 10.1056/NEJM199808133390704. [DOI] [PubMed] [Google Scholar]
- 7.Kong DF, Califf RM, Miller DP, Moliterno DJ, White HD, Harrington RA, et al. Clinical outcomes of therapeutic agents that block the platelet glycoprotein IIb/IIIa integrin in ischemic heart disease. Circulation. 1998;98:2829–2835. doi: 10.1161/01.cir.98.25.2829. [DOI] [PubMed] [Google Scholar]
- 8.Coller BS. A new murine monoclonal antibody reports an activation-dependent change in the conformation and/or microenvironment of the platelet glycoprotein IIb/IIIa complex. J Clin Invest. 1985;76:101–108. doi: 10.1172/JCI111931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The EPIC Investigation. Use of a monoclonal antibody directed against the platelet glycoprotein IIb/IIIa receptor in high-risk coronary angioplasty. N Engl J Med. 1994;330:956–961. doi: 10.1056/NEJM199404073301402. [DOI] [PubMed] [Google Scholar]
- 10.Ryan TJ, Faxon DP, Gunnar RM, Kennedy JW, King SB, 3rd, Loop FD, et al. Guidelines for percutaneous transluminal coronary angioplasty. A report of the American College of Cardiology/American Heart Association Task Force on Assessment of Diagnostic and Therapeutic Cardiovascular Procedures (Subcommittee on Percutaneous Transluminal Coronary Angioplasty) Circulation. 1988;78:486–502. doi: 10.1161/01.cir.78.2.486. [DOI] [PubMed] [Google Scholar]
- 11.Cannon CP, Battler A, Brindis RG, Cox JL, Ellis SG, Every NR, et al. American College of Cardiology key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes. A report of the American College of Cardiology Task Force on Clinical Data Standards (Acute Coronary Syndromes Writing Committee) J Am Coll Cardiol. 2001;38:2114–2130. doi: 10.1016/s0735-1097(01)01702-8. [DOI] [PubMed] [Google Scholar]
- 12.Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, et al. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA. 2000;284:835–842. doi: 10.1001/jama.284.7.835. [DOI] [PubMed] [Google Scholar]
- 13.Hamm CW, Heeschen C, Goldmann B, Vahanian A, Adgey J, Miguel CM, et al. Benefit of abciximab in patients with refractory unstable angina in relation to serum troponin T levels. c7E3 Fab Antiplatelet Therapy in Unstable Refractory Angina (CAPTURE) Study Investigators. N Engl J Med. 1999;340:1623–1629. doi: 10.1056/NEJM199905273402103. [DOI] [PubMed] [Google Scholar]
- 14.Simoons ML, de Boer MJ, van den Brand MJ, van Miltenburg AJ, Hoorntje JC, Heyndrickx GR, et al. Randomized trial of a GPIIb/IIIa platelet receptor blocker in refractory unstable angina. European Cooperative Study Group. Circulation. 1994;89:596–603. doi: 10.1161/01.cir.89.2.596. [DOI] [PubMed] [Google Scholar]
- 15.Topol EJ, Califf RM, Weisman HF, Ellis SG, Tcheng JE, Worley S, et al. Randomised trial of coronary intervention with antibody against platelet IIb/IIIa integrin for reduction of clinical restenosis: results at six months. The EPIC Investigators. Lancet. 1994;343:881–886. doi: 10.1016/s0140-6736(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 16.Platelet glycoprotein IIb/IIIa receptor blockade and low-dose heparin during percutaneous coronary revascularization. The EPILOG Investigators. N Engl J Med. 1997;336:1689–1696. doi: 10.1056/NEJM199706123362401. [DOI] [PubMed] [Google Scholar]
- 17.International, randomized, controlled trial of lamifiban (a platelet glycoprotein IIb/IIIa inhibitor), heparin, or both in unstable angina. The PARAGON Investigators. Platelet IIb/IIIa Antagonism for the Reduction of Acute coronary syndrome events in a Global Organization Network. Circulation. 1998;97:2386–2395. doi: 10.1161/01.cir.97.24.2386. [DOI] [PubMed] [Google Scholar]
- 18.A comparison of aspirin plus tirofiban with aspirin plus heparin for unstable angina. Platelet Receptor Inhibition in Ischemic Syndrome Management (PRISM) Study Investigators. N Engl J Med. 1998;338:1498–1505. doi: 10.1056/NEJM199805213382103. [DOI] [PubMed] [Google Scholar]
- 19.Braunwald E, Antman EM, Beasley JW, Califf RM, Cheitlin MD, Hochman JS, et al. ACC/AHA 2002 guideline update for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction--summary article: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee on the Management of Patients With Unstable Angina) J Am Coll Cardiol. 2002;40:1366–1374. doi: 10.1016/s0735-1097(02)02336-7. [DOI] [PubMed] [Google Scholar]
- 20.Randomised placebo-controlled trial of effect of eptifibatide on complications of percutaneous coronary intervention: IMPACT-II. Integrilin to Minimise Platelet Aggregation and Coronary Thrombosis-II. Lancet. 1997;349:1422–1428. [PubMed] [Google Scholar]
- 21.Effects of platelet glycoprotein IIb/IIIa blockade with tirofiban on adverse cardiac events in patients with unstable angina or acute myocardial infarction undergoing coronary angioplasty. The RESTORE Investigators. Randomized Efficacy Study of Tirofiban for Outcomes and REstenosis. Circulation. 1997;96:1445–1453. doi: 10.1161/01.cir.96.5.1445. [DOI] [PubMed] [Google Scholar]
- 22.Alexander JH, Harrington RA. Recent antiplatelet drug trials in the acute coronary syndromes. Clinical interpretation of PRISM, PRISM-PLUS, PARAGON A and PURSUIT. Drugs. 1998;56:965–976. doi: 10.2165/00003495-199856060-00002. [DOI] [PubMed] [Google Scholar]
- 23.Kaul DK, Tsai HM, Liu XD, Nakada MT, Nagel RL, Coller BS. Monoclonal antibodies to alphaVbeta3 (7E3 and LM609) inhibit sickle red blood cell-endothelium interactions induced by platelet-activating factor. Blood. 2000;95:368–374. [PubMed] [Google Scholar]
- 24.Thompson RD, Wakelin MW, Larbi KY, Dewar A, Asimakopoulos G, Horton MA, et al. Divergent effects of platelet-endothelial cell adhesion molecule-1 and beta 3 integrin blockade on leukocyte transmigration in vivo. J Immunol. 2000;165:426–434. doi: 10.4049/jimmunol.165.1.426. [DOI] [PubMed] [Google Scholar]
- 25.PRICE Investigators. Comparative 30-day economic and clinical outcomes of platelet glycoprotein IIb/IIIa inhibitor use during elective percutaneous coronary intervention: Prairie ReoPro versus Integrilin Cost Evaluation (PRICE) Trial. Am Heart J. 2001;141:402–409. doi: 10.1067/mhj.2001.113391. [DOI] [PubMed] [Google Scholar]
- 26.Coons JC, Seybert AL, Saul MI, Kirisci L, Kane-Gill SL. Outcomes and costs of abciximab versus eptifibatide for percutaneous coronary intervention. Ann Pharmacother. 2005;39:1621–1626. doi: 10.1345/aph.1G129. [DOI] [PubMed] [Google Scholar]
- 27.Topol EJ, Moliterno DJ, Herrmann HC, Powers ER, Grines CL, Cohen DJ, et al. Comparison of two platelet glycoprotein IIb/IIIa inhibitors, tirofiban and abciximab, for the prevention of ischemic events with percutaneous coronary revascularization. N Engl J Med. 2001;344:1888–1894. doi: 10.1056/NEJM200106213442502. [DOI] [PubMed] [Google Scholar]
- 28.Brown DL, Fann CS, Chang CJ. Meta-analysis of effectiveness and safety of abciximab versus eptifibatide or tirofiban in percutaneous coronary intervention. Am J Cardiol. 2001;87:537–541. doi: 10.1016/s0002-9149(00)01427-2. [DOI] [PubMed] [Google Scholar]