Abstract
Purpose
Short life expectancy influences decision-making when treating very old patients with acute ischemic stroke (AIS). We investigated mortality and survival duration in very old AIS patients (≥ 80 years) who received hospital care.
Patients and Methods
Mortality data were obtained from medical records, structured telephone inquiries, death certificates from the Korean National Statistical Office, and social security data 5 ± 1.9 years after stroke onset. Age, gender, vascular risk factors, and functional outcomes from modified Rankin scales (MRS) at discharge were analyzed as predictors of mortality.
Results
Among 134 patients, 92 (68.7%) died. On Kaplan-Meier analysis, duration of survival of patients aged 80 - 84 years was longer than those aged 85 - 89 or 90 - 94 (24 ± 6.4, 8 ± 7.3, 7 ± 2.0 months, respectively, p = 0.002). Duration of survival of patients discharged in a state of MRS 0 - 1 was longer than the remaining groups at 47 ± 4.8 months (p < 0.001). In Cox proportional hazard analysis, age and MRS at discharge were independent predictors of mortality.
Conclusion
Long-term outcomes of very old patients with AIS are not uniformly grave, therefore predictors of mortality and estimated duration of survival should be considered during decision- making for treatment.
Keywords: Aging, ischemic stroke, prognosis, mortality
INTRODUCTION
With prolonged life expectancy, the number of very old patients in acute stroke units has been increasing. Early or in-hospital mortality of very old patients is higher and their active medical care is poorer than younger patients.1-4 Short life expectancy may influence active medical care in very old patients.
Despite the lack of evidence-based studies on patients beyond the age of 80 years, carotid endarterectomy or thrombolysis has been performed in a selected numbers of very old patients.5-9 Recent case studies recommended that the very old should not be denied treatment on the basis of age alone, therefore other prognostic factors would be useful in selecting candidates for active intervention among very old patients with acute ischemic stroke (AIS).7,8
Most previous studies compared the prognosis of very old stroke patients with that of younger patients or assessed the prognosis at discharge or 3 months later.2-4,9-11 Very few studies have focused on the long-term outcomes or survival duration of very old stroke patients. The Copenhagen stroke study analyzed long-term prognosis in very old stroke patients but prognostic factors among very old stroke patients were not separately analyzed.11 Imbalance in baseline variables between very old and younger groups may contribute to the poorer prognosis in very old stroke patients after acute treatment.9 Long-term prognosis for very old stroke patients was not uniformly poor, therefore analysis of long-term prognostic factors among very old stroke patients may be important in deciding on the treatment of very old AIS patients.
This study investigated long-term mortality, its predictors, and duration of survival among very old patients with AIS.
PATIENTS AND METHODS
This study was based on data from Hallym Stroke Registry (HSR) of the Hallym University Medical Center, a prospective, multicenter, hospital-based stroke data bank of acute stroke patients who were admitted within 7 days of onset of stroke.12 The diagnosis of ischemic stroke was based on clinical findings and neuroimaging studies simultaneously reviewed by 2 or more experienced neurologists. Data on ischemic stroke patients aged ≥ 80 years were obtained from the HSR of Hangang Sacred Heart Hospital from February 1996 to August 2003 and the HSR of Hallym University Sacred Heart Hospital from February 1999 to August 2003. Seventy of 463 (15.1%) patients in the HSR of Hangang Sacred Heart Hospital and 64 of 781 (8.2%) patients in the HSR of Hallym University Sacred Heart Hospital served as subjects. Hallym University Sacred Heart Hospital was founded by former employees of Hangang Sacred Heart Hospital in 1999. A total of 134 patients were selected from the 2 hospitals.
Conventional risk factors for stroke such as previous history of stroke, hypertension, diabetes mellitus, hyperlipidemia, smoking habits, atrial fibrillation, and NIH stroke scale score upon admission were obtained from HSR data on each patient.12 Hypertension was defined as systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure of ≥ 90 mm Hg based on repeated measurements or a history of taking anti- hypertensive medication. Diabetes mellitus was diagnosed if a patient had a history of diabetes mellitus with current treatment, or fasting blood glucose level ≥ 126 mg/dL. A patient was considered to have hyperlipidemia if there was a history of hyperlipidemia with current treatment, if fasting serum cholesterol level was ≥ 240 mg/dL, or if fasting triglyceride level was ≥ 200 mg/dL. Current infarcts were classified according to TOAST criteria,13 and functional outcomes were measured by means of modified Rankin scales (MRS) at discharge as follows: no symptom (MRS 0), symptom but no disability (MRS 1), mild disability (MRS 2), moderate disability with independent walking (MRS 3), severe disability (MRS 4), bedridden state (MRS 5), and death (MRS 6).14
Information on mortality was obtained from January to July 2005, through medical records, structured telephone phone inquiries, death certificates from the Korean National Statistical Office from 1996 to 2002, and social security data. Probable causes of death were categorized based on patients' medical charts, information from families through phone interviews, and death certificates from the Korean National Statistical Office. The categories were index stroke, recurrent stroke, heart disease, infection, cancer, another known cause, and unknown causes.
Cox proportional hazard analysis was used to calculate the hazard ratio (HR) and 95% confidence interval (CI) for mortality. Demographic characteristics, conventional risk factors for stroke, and functional status at discharge were analyzed for predictors of mortality. A p value < 0.05 was chosen for statistical significance. Kaplan-Meier survival analysis was done with significant predictors of mortality. Statistical analyses were undertaken using SPSS software.
RESULTS
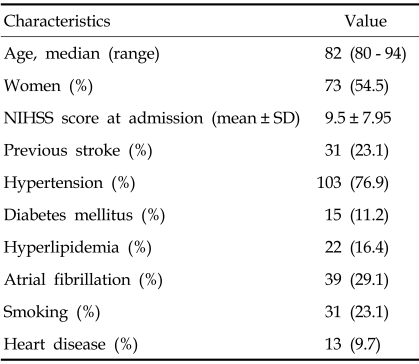
A total of 134 patients were included in this study: 61 men and 73 women (median age, 82 years; range, 80 - 94). Demographic characteristics and vascular risk factor are shown in Table 1. After admission, all patients were treated with antiplatelet agents, 6 patients with an intravenous recombinant tissue plasminogen activator (rtPA), and 22 patients with intravenous conventional heparin, based on physician's decisions.
Table 1.
Baseline Characteristics, Hospital Stay, and Functional Status at Discharge (n = 134)
NIHSS, National Institutes of Health Stroke Scale; SD, standard deviation.
According to TOAST classification, 44 patients were classified as having large vessel disease, 37 as cardioembolism, 33 as small vessel disease, 19 as undetermined causes, and 1 as another determined cause.
Median length of hospital stay was 10 days. Within 90 days, 132 patients (98.5%) were discharged. Functional status at discharge was MRS 0 - 1 in 22 patients (16.4%), MRS 2 - 3 in 47 (35.1%), MRS 4 - 5 in 45 (33.6%), and MRS 6 (death during hospital stay) in 20 (14.9%). A total of 101 patients were discharged to their homes and 13 were transferred to other hospitals. Secondary preventive medications at hospital discharge were antiplatelet agents for 91 patients and oral anticoagulants for 16. Seven patients did not receive any antiplatelet agent or oral anticoagulant at discharge because of bleeding complications during hospital stay.
Mortality data were obtained at mean 5 ± 1.9 years (range, 2 - 9 years) after stroke onset: 58 patients from medical records, 45 from structured telephone phone inquiries, 21 from the Korean National Statistical Office from 1996 to 2002, and 10 from social security data. Ninety-two patients (68.7%) died of the following causes: index stroke (44 patients), recurrent stroke (3), heart disease (7), infection (5), cancer (8), or another known or unknown cause (25). Among 92 patients who died during follow up, 54 (58.7%) died of vascular causes and 38 (41.3%) of non vascular or unknown causes.
On Kaplan-Meier analysis, median duration of estimated survival was 24 ± 6.4 months for 91 patients aged 80 - 84 years, 8 ± 7.3 months for 34 patients aged 85 - 89 years, and 7 ± 2.0 months for 9 patients aged 90 - 94 years (Fig. 1, p = 0.002). Median survival duration according to functional status at discharge was 47 ± 4.8 months for MRS 0 - 1, 39 ± 9.0 months for MRS 2 - 3, 5 ± 1.1 months for MRS 4 - 5, and 1 month for MRS 6 (p < 0.001).
Fig. 1.
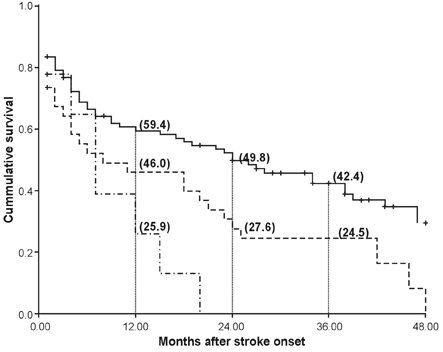
Survival plot by Kaplan-Meier method according to 3 age groups. Solid line represents 91 patients aged 80 - 84 years, dotted line represents 34 patients aged 85 - 89 years, and broken line represents 9 patients aged 90 - 94 years. Numbers in brackets show percentage of survival at the time. P value of log rank test is 0.002.
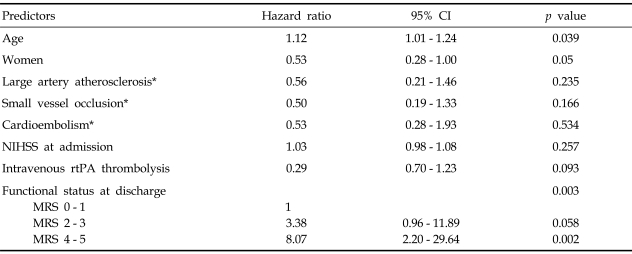
After excluding patients who died in hospital, demographic characteristics, vascular risk factors, NIH stroke scale at admission, stroke subtype, treatment with intravenous rtPA, and functional status at discharge were analyzed for predictors of mortality in a Cox proportional hazard model. As shown in Table 2, age (HR 1.12, 95% CI 1.01 - 1.24, p = 0.039) and MRS 4 - 5 status at discharge (HR 8.1, 95% CI 2.20 - 29.64, p = 0.002) were significant predictors of mortality. When the analysis was performed in all 134 patients, independent predictors of mortality did not change.
Table 2.
Cox Proportional Hazard Analysis of Death After Excluding Patients Who Died in Hospital
CI, confidence interval; NIHSS, National Institutes of Health Stroke Scale; rtPA, recombinant tissue plasminogen activator; MRS, modified Rankin scale.
*Large artery disease, small vessel occlusion, and cardioembolism were classified according to TOAST.
DISCUSSION
According to in this hospital-based retrospective study, about two-thirds of AIS patients aged ≥ 80 years died at a mean of 5 ± 1.9 years (range, 2 - 9 years) after stroke onset. To obtain more information about follow-up status, national statistical data based on the personal identification number can be used to study long-term prognoses.1,11 Age 80 was selected as the cutoff, similar to recent studies focused on the treatment of the very old.7-8,15
This study had some limitations. First, data from 2 hospitals were studied because the number of patients aged 80 years and older per hospital was not sufficient for analysis of predictors of mortality. The initial clinical data of the two hospitals were similar. Second, although collection of patient outcomes at a fixed interval was reasonable, functional status was assessed only upon discharge.16 Median duration of hospital stay was 10 days and length of hospital stay was not an independent predictor of mortality in a separate analysis. Third, pre-stroke disability, comorbidity other than vascular risk factors, and recurrence of stroke may influence mortality after a stroke,1,11,17 but these variables were not assessed due to limitations because of the nature of retrospective study. Finally, we did not compare the mortality of older and younger stroke patients. The comparison may be able to identify specific predictors of mortality in older patients compared to younger patients.
Age and functional status at discharge were significant predictors of mortality. We classified patients into 3 age groups and median duration of survival was analyzed separately. Since the number of patients aged 90 - 94 years was small, median survival durations of the 2 octogenarian groups could be clinically useful as a reference value.
Functional status at discharge was a significant predictor of mortality. NIH stroke scale scores were not significant because of the correlation between the outcomes at discharge and NIH stroke scale scores upon admission (r = 0.736, p < 0.001). Functional status at discharge is just one short-tem outcome parameter with more influence on long-term outcome.
Atrial fibrillation was not an independent predictor of mortality in this study. The significance of atrial fibrillation was inconsistent with previous studies.1,4,11 In a study that separately analyzed the prognostic factors of AIS patients aged ≥ 80 years, atrial fibrillation was not a significant predictor for older patients,1 and previous histories of stroke did not influence mortality. A possible explanation is that only patients with mild disability from previous strokes could survive until their 80s and most very old patients with first-ever stroke in the study had a silent infarct on MRI.18 Subtypes of stroke were not an independent predictor of mortality. De Jong et al. reported stroke subtypes were one of the independent predictors of 30-day case fatality but not a predictor of later mortality.19
In the present study, 6 patients received thrombolysis with rtPA and the treatment did not give significantly influence long-term mortality. However, the number of patients was very small and the functional outcomes at final follow up were not analyzed, indicating that the efficacy of intravenous thrombolysis in stroke patients aged ≥ 80 years was not assessed in this study. Recent systemic review also suggested that it is reasonable to include older patients in randomized placebo-controlled trials to analyze efficacy and safety of treatment.9
In recent studies, the proportion of patients aged ≥ 80 years was 30.2 - 53.7% among patients with AIS.1,4 However, long-term outcomes or survival duration after stroke in those patients have not been frequently studied. Miller et al. reported that median survival of patients with carotid stenosis after carotid endarterectomy was 6.6 years in patients aged ≥ 80 years, but median patient survival with ischemic stroke was not separately analyzed.7 Residual life expectancy of less than 5 years was one of the exclusion criteria for carotid endarterectomy in symptomatic patients,5 however, the longest median duration of survival of any subgroup in the present study was less than 4 years in this study, and about 10% of the patients aged ≥ 85 years were independently alive 5 years after stroke onset in the Copenhagen stroke study.11 Further study with a larger population seems to be needed to evaluate survival after stroke and establish more practical criteria of active intervention for very old AIS patients.
In conclusion, age and functional outcomes at discharge were significant predictors of long-term mortality. These predictors and estimated duration of survival should be considered during the decision-making process on the treatment of very old AIS patients.
References
- 1.Di Carlo A, Lamassa M, Pracucci G, Basile AM, Trefoloni G, Vanni P, et al. Stroke in the very old: clinical presentation and determinants of 3-month functional outcome: A European perspective. European BIOMED Study of Stroke Care Group. Stroke. 1999;30:2313–2319. doi: 10.1161/01.str.30.11.2313. [DOI] [PubMed] [Google Scholar]
- 2.Sharma JC, Fletcher S, Vassallo M. Strokes in the elderly-higher acute and 3-month mortality-an explanation. Cerebrovasc Dis. 1999;9:2–9. doi: 10.1159/000015889. [DOI] [PubMed] [Google Scholar]
- 3.Olindo S, Cabre P, Deschamps R, Chatot-Henry C, René-Corail P, Fournerie P, et al. Acute stroke in the very elderly: epidemiological features, stroke subtypes, management, and outcome in Martinique, French West Indies. Stroke. 2003;34:1593–1597. doi: 10.1161/01.STR.0000077924.71088.02. [DOI] [PubMed] [Google Scholar]
- 4.Altieri M. SPASE-1: a multicenter observational study on pharmacological treatment of acute stroke in the elderly. The Italian Study of Pharmacological Treatment of Acute Stroke in the Elderly group. Neurol Sci. 2002;23:23–28. doi: 10.1007/s100720200019. [DOI] [PubMed] [Google Scholar]
- 5.North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 6.Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, et al. Randomised double-blinded placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998;352:1245–1251. doi: 10.1016/s0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 7.Miller MT, Comerota AJ, Tzilinis A, Daoud Y, Hammerling J. Carotid endarterectomy in octogenarians: does increased age indicate "high risk?". J Vasc Surg. 2005;41:231–237. doi: 10.1016/j.jvs.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 8.Chen CI, Iguchi Y, Grotta JC, Garami Z, Uchino K, Shaltoni H, et al. Intravenous TPA for very old stroke patients. Eur Neurol. 2005;54:140–144. doi: 10.1159/000089086. [DOI] [PubMed] [Google Scholar]
- 9.Engelter ST, Bonati LH, Lyrer PA. Intravenous thrombolysis in stroke patients of > or = 80 versus < 80 years of age-a systematic review across cohort studies. Age Ageing. 2006;35:572–580. doi: 10.1093/ageing/afl104. [DOI] [PubMed] [Google Scholar]
- 10.Arboix A, García-Eroles L, Massons J, Oliveres M, Targa C. Acute stroke in very old people: clinical features and predictors of in-hospital mortality. J Am Geriatr Soc. 2000;48:36–41. doi: 10.1111/j.1532-5415.2000.tb03026.x. [DOI] [PubMed] [Google Scholar]
- 11.Kammersgaard LP, Jørgensen HS, Reith J, Nakayama H, Pedersen PM, Olsen TS. Short- and long-term prognosis for very old stroke patients. The Copenhagen Stroke Study. Age Ageing. 2004;33:149–154. doi: 10.1093/ageing/afh052. [DOI] [PubMed] [Google Scholar]
- 12.Lee BC, Hwang SH, Jung S, Yu KH, Lee JH, Cho SJ, et al. The Hallym Stroke Registry: a web-based stroke data bank with an analysis of 1,654 consecutive patients with acute stroke. Eur Neurol. 2005;54:81–87. doi: 10.1159/000088097. [DOI] [PubMed] [Google Scholar]
- 13.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 14.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1998;19:604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 15.Ovbiagele B, Hills NK, Saver JL, Johnston SC. Secondary-prevention drug prescription in the very elderly after ischemic stroke or TIA. Neurology. 2006;66:313–318. doi: 10.1212/01.wnl.0000196476.10103.52. [DOI] [PubMed] [Google Scholar]
- 16.Dennis MS. Stroke unit versus and neurology ward-a short commentary. J Neurol. 2003;250:1370–1371. doi: 10.1007/s00415-003-0217-y. [DOI] [PubMed] [Google Scholar]
- 17.Hénon H, Durieu I, Lebert F, Pasquier F, Leys D. Influence of prestroke dementia on early and delayed mortality in stroke patients. J Neurol. 2003;250:10–16. doi: 10.1007/s00415-003-0917-3. [DOI] [PubMed] [Google Scholar]
- 18.Minn YK, Cho SJ, Lee JH, Kim SY, Kim CH, Kwon KH, et al. Significance of silent infarcts in acute ischemic stroke patients aged 80 years and older. Cerebrovasc Dis. 2005;20:92–95. doi: 10.1159/000086512. [DOI] [PubMed] [Google Scholar]
- 19.de Jong G, van Raak L, Kessels F, Lodder J. Stroke subtype and mortality: a follow-up study in 998 patients with a first cerebral infarct. J Clin Epidemiol. 2003;56:262–268. doi: 10.1016/s0895-4356(02)00572-3. [DOI] [PubMed] [Google Scholar]