Abstract
Purpose
To assess the incidence of thyroid malignancy in an adult population screened by high-resolution ultrasonography at a medical screening center and to compare the clinical and pathological features of screen-detected thyroid carcinomas to symptomatic overt thyroid carcinomas.
Materials and Methods
We calculated the prevalence of screen-detected thyroid cancer at a medical screening center using high-resolution ultrasonography and fine needle aspiration. We then compared the clinical and pathological features of screen-detected thyroid cancers (n = 46) to clinical symptomatic thyroid cancers (n = 157). We evaluated age, gender, size, perithyroidal extension, lymphovascular extension, stage, histological lymph node metastasis, and the type of cancer. We also compared the above findings of micropapillary carcinomas to papillary thyroid carcinomas that were larger than 1 cm in diameter.
Results
Screen-detected thyroid nodule patients were 2,747 (37%) of 7,491 patients. Nodules selected for fine needle aspiration were 658 and cytology confirmed malignancy were 79 (12%) nodules. When screen-detected thyroid cancers (n = 46) were compared to symptomatic overt thyroid cancers (n = 157), only statistically significant factor was size (p = 0.002). Papillary thyroid carcinomas that were larger than 1 cm had more frequent capsular invasion (p = 0.000) and a higher stage (p = 0.027), and a higher prevalence of lymph node metastases (p = 0.002).
Conclusion
Screen-detected thyroid cancers should be managed as same as symptomatic thyroid cancers in respect to size, and an assessment should strictly be based on the ultrasound features and fine needle aspiration biopsy findings.
Keywords: Ultrasound, thyroid cancer, prevalence
INTRODUCTION
The widespread use of high resolution sonography (HRS)1 as a screening tool for thyroid cancer in medical health care in Korea is increasing,2 and its use has generated a number of issues. There is very limited evidence of the use of the modality to be cost effective.3 Furthermore, the management of thyroid nodules detected during screening with HRS remains controversial. Performing screening HRS for thyroid cancer can lead to the identification of a large number of small benign-appearing nodules and can lead to unnecessary studies thus increasing the cost of health care.3,4 However, the demand for screening HRS by the self-referred patient is on the increase, and the medical faculty does not currently intervene in the decision of patients to undergo screening. The rate of thyroid cancer for screen-detected thyroid nodules at medical centers in Korea is increasing due to better performance of fine-needle aspiration biopsy (FNAB) and ultrasound (US).5-8 The incidence of thyroid cancer for screening thyroid US has been reported in limited studies,9 and previous reports were mainly based on the finding of autopsy cases with a wide range of malignancy rates of 5 - 35%.10-12 We investigated the actual prevalence of thyroid cancer in a voluntary cancer-screening program in healthy subjects without a previous history of malignancy to evaluate the value of ultrasound for screening thyroid cancer.
As previously reported, many autopsy studies have suggested that most occult thyroid carcinomas follow a relatively benign or indolent course,12,13 and there has been a recognition of different biological behavior between the really "occult" and clinically overt cancers.1,14 We, therefore, compared the clinical and pathological features of screen-detected thyroid cancers to symptomatic cancers to evaluate if a different approach of management is necessary.
MATERIALS AND METHODS
Patient selection
From March 2006 to February 2008, 7,491 consecutive subjects underwent screening thyroid HRS at a medical health care center, Kangbuk Samsung Hospital, in the Republic of Korea. Informed consent was waived for all patients who underwent thyroid US and FNAB. Approximately 37% of patients (n = 2,747) had a thyroid nodule or nodules. Among these subjects, 658 patients (24%) underwent fine needle aspiration for having a solid nodule larger than 1 cm or a nodule less than 1 cm, however, showed at least one malignant US feature. Seventy-nine patients (12%) had a cytology result of being positive for a malignancy and 46 patients underwent a surgical procedure at our hospital (Fig. 1).
Fig. 1.
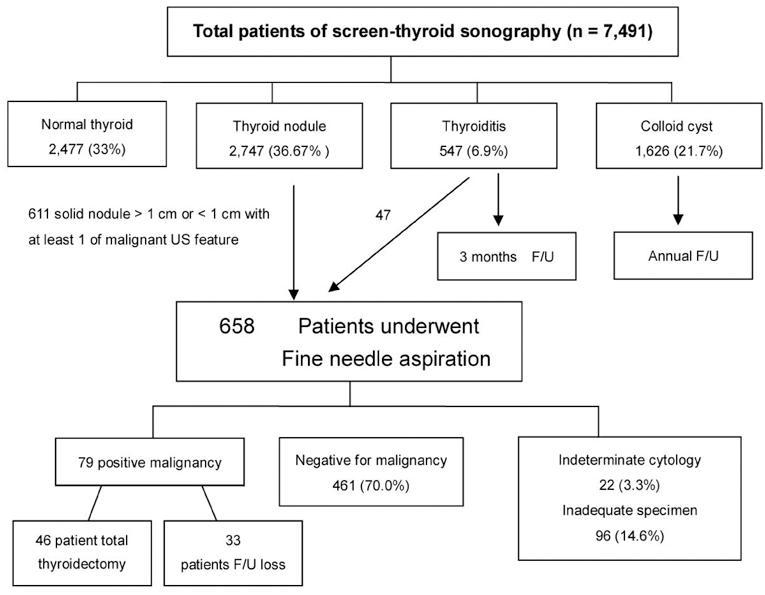
Flow chart of patients undergoing high resolution ultrasound at a medical center for thyroid cancer screening. US, ultrasound; F/U, follow up.
During the same period, 287 referred patients underwent the FNAB procedure at the Department of Radiology, and 254 patients underwent a surgical procedure. Patients (n = 157) who had at least 1 of the following symptoms or had overlapping symptoms, including a palpable neck lesion (n = 89), voice change (n = 21), swallowing difficulty (n = 6) and neck pain (n = 54), were included as representing patients with symptomatic thyroid cancer. When there was more than one cancer nodule confirmed, the largest major lesion was considered for evaluation, whereas ipsilateral smaller lesions were disregarded for the pathological analysis. For statistical purposes of comparison, bilateral symptomatic thyroid cancer (n = 2) cases were excluded from the study.
US imaging
We used an HDI 5000 (Advanced Technology Laboratories, Bothell, WA, USA), IU22 (Philips Medical Systems, Bothell, WA, USA), or LOGIQ 700 ultrasound scanner (GE Medical Systems, Milwaukee, WI, USA) equipped with a 5 - 12 MHz linear-array transducer. Board-certified radiologists with at least 5 years of experience in thyroid US performed all of the sonographic examinations.
Indications for US guided FNAB for nodules that were larger than 1 cm were based primarily on the internal content of the lesions. A predominantly cystic nodule (cyst portion more than 50%) and nodules with an internal spongiform appearance were not candidates for FNAB.15 Candidates for FNAB for nodules less than 1 cm on the longest diameter on a longitudinal scan were nodules with at least 1 finding of microcalcifications, an irregular shape, an ill defined margin and a solid nodule with echogenicity that was lower than the echogenicity of strap muscle.16
Three board certified radiologists performed US-FNAB using a 23-gauge needle attached to a 10 cc syringe with an aspirator. Movement of the needle tip toward and into the target was visualized by real time-US. Based on a cytological evaluation, nodules were classified as malignant, indeterminant, benign, or an inadequate sample. A positive malignant cell based on an FNAB cytology finding was considered as a screen-detected thyroid cancer.
We compared 46 screen-detected thyroid cancers to 157 symptomatic thyroid cancers to determine if there were any existing differences in age, gender, size of the cancer, peri-thyroidal extension, lympho-vascular invasion, underlying pathology, stage, and lymph node invasion status and pathological type. The underlying pathologies were evaluated and compared to clinically overt tumors. We also compared the above-mentioned features of micropapillary carcinomas (MPCs) (n = 111) to papillary thyroid carcinomas (PTCs) (n = 92) to evaluate different findings.
Statistical analysis was performed by use of chi-squared test or Fisher's exact test to compare categorical variables, and by Student's t-test to compare continuous variables between groups using SPSS version 10.0 (Chicago, IL USA). Probability levels less than 0.05 were considered as statistically significant.
RESULTS
Thyroid HRS performed as screening for thyroid cancer
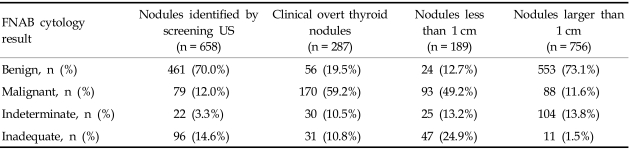
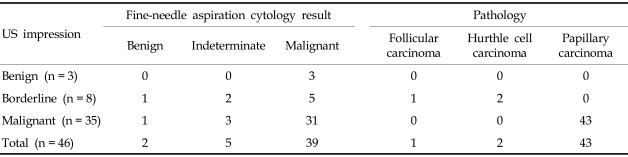
For two consecutive years, 7,491 subjects underwent screening of thyroid HRS. Among the subjects screened, 2,477 (33%) participants had a normal thyroid and 1,626 participants (21.7%) presented with a colloid cyst. Approximately 76 subjects (1%) had lesions that were suspicious for diffuse thyroid infiltrative disease with heterogenous coarse mixed parenchymal echogenicity with nodular enlargement, and 547 subjects (6.9%) were diagnosed with a US feature that was suspicious of thyroiditis. There were 2,747 subjects (36.7%) with thyroid nodules. Fig. 1 demonstrates the flow chart of nodule evaluation. Among subjects that had a thyroid nodule or nodules (n = 2,747), 658 patients (23.95%) underwent FNAB for presenting with a solid nodule larger than 1 cm or a nodule less than 1 cm, however, showed at least one US feature suggestive of a malignancy. Table 1 shows the cytology results of FNAB for the screened patients, symptomatic patients and nodules according to sizes. Of the subjects screened, 461 subjects (70.0%) were negative for a malignancy, based on a cytology finding, 79 subjects (12.0%) were positive for a malignancy, 22 (3.3%) subjects had an indeterminate cytology, and 96 subjects (14.6%) had an inadequate cellular sample for diagnosis. In symptomatic referred patients, malignant cell cytology was identified in 59.2% of patients. When categorized according to size, 24.9% of inadequate specimens consisted of a nodule less than 1 cm in size and only 1.5% of inadequate specimens consisted of nodules larger than 1 cm. Table 2 depicts the cytology results of 46 patients whose nodules were identified by screening US at the medical screening center. Having an underlying pathology did not influence the preoperative diagnostic rate by FNAB for screen-identified cancers and clinical overt thyroid cancers (p = 0.811).
Table 1.
Fine-needle Aspiration Results in Screen-identified Nodules and Clinically Overt Nodules
FNAB, fine-needle aspiration biopsy; US, ultrasound.
Table 2.
Surgically Confirmed Thyroid Malignant Nodules on Screening Ultrasound at a Medical Screening Center
US, ultrasound.
Comparison of clinically overt and occult PMCs
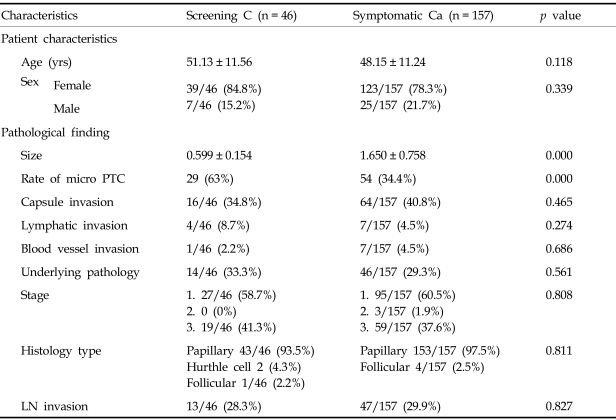
Table 3 summarizes and compares the clinicopathological features of patients with screen-detected cancers and symptomatic thyroid cancers. The size of symptomatic cancers was significantly larger than the screen-detected tumors (1.141 ± 0.796 cm versus 0.852 ± 0.439 cm; p = 0.002) and was statistically significant. Out of 46 (65%) surgically confirmed screen-identified cancers, 30 cancers were identified as microcarcinomas. Thyroid cancers were found more frequently in women in screened subjects as well as symptomatic subjects (p = 0.339), and age was not a statistically significant factor. Differences in extrathyroidal extension (34.8% vs. 40.8%), vascular invasion (8.7% vs. 4.5%), and cervical lymph node invasion (28.3% versus 29.9%) were not statistically significant between the screened subjects and symptomatic subjects (p < 0.005). Of 46 patients with occult tumors, 19 patients (41.3%) were in stage 3, and they underwent a bilateral thyroidectomy and central neck node dissection with lateral lymph node dissection, whereas 59 of 157 patients (37.6%) with clinically overt PTCs were diagnosed as stage 3 and received a bilateral thyroidectomy (p = 0.808). The histological type was mainly well-differentiated type papillary carcinomas in both screened subjects as well as in the symptomatic subjects. For screen-identified cancers, 93.5% of the cancers were papillary, 2 cancers were Hurthle cell carcinomas and 1 patient was diagnosed with a follicular carcinoma. For the symptomatic patients, 97.5% were identified with papillary cancers and 4 patients had a follicular carcinoma.
Table 3.
Patients and Pathological Features of Thyroid Cancer
LN, lymph nodes; PTC, papillary thyroid carcinoma; C, indicates screen detected cancer; Ca, cancer.
Comparison of PMCs and PTCs
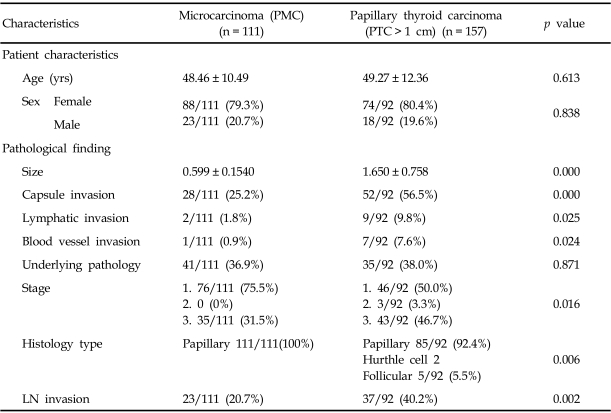
When PMCs and PTCs were compared and analyzed (Table 4), extrathyoidal extension (25.2% versus 56.5%), vascular invasion (0.9% versus 7.6%), lymphatic invasion (1.8% vs. 9.8%) and cervical lymph node invasion (20.7% vs. 40.2%) occurred more frequently in papillary carcinomas larger than 1 cm in the longest diameter and with a higher stage (stage 3, 31.5% vs. 46.7%). These findings were statistically significant (p < 0.005).
Table 4.
Patient and Pathological Features of Papillary Microcarcinomas (Longest Diameter < 1 cm) and Papillary Thyroid Carcinomas (> 1 cm)
LN, lymph nodes; PTC, papillary thyroid carcinoma; PMC, papillary microcarcinoma.
DISCUSSION
Rate of malignancy in thyroid nodules detected at a medical screening center
Thyroid nodules are found by palpation in 4 - 8% of the general population in adults,17 and are more frequently detected at a rate of 30 - 50% on sonography9,18,19 and 50% in autopsy cases.20 The malignancy rate has been reported at 7 - 15%;8,21 the malignancy rate within thyroid incidentalomas was 12 - 28.8%.6,8 The malignancy rate is increasing due to wide use of highly sensitive US for routine health examinations.22 The rate of thyroid incidentalomas at our institute was 36.67%, with a malignancy rate of 12%. The malignancy rates for thyroid incidentaloma, however, differ among institutes, and this difference may be due to a selection bias of candidates selected for FNAB that is different among various institutes. In our institution, solid nodules larger than 1 cm are considered as candidates for FNAB. Nodules less than 1 cm in the longest diameter with at least 1 US feature of suspicious malignant finding are also considered as candidates for FNAB. When nodules are depicted as purely cystic nodules, nodules are not considered as candidates for FNAB, regardless of size. There are no US characteristics of a nodule that can be used to prove that a nodule is benign 4. If FNABs are performed in all patients who had nodules despite the US features, incidence of malignancies in the screening population would better be depicted. However, performance of extraneous FNABs increases the cost of health care. There are US features of thyroid malignancies that distinguish nodules from benign nodules to suspicious of malignancy nodules, but some of the features overlap. Further studies are needed to obtain a consensus of FNAB indications for screen-detected nodules and US features of benign nodules with high diagnostic accuracy to preclude the undertaking of an FNAB. The rate of malignancy based on the cytology results is higher in nodules less than 1 cm (49.2% vs. 11.6%) because of the FNAB indications employed.
Wide use of screening thyroid US at medical centers in Korea to detect thyroid cancer is difficult because of limited evidence of cost effectiveness along with controversies remaining in management of the disease. However, screening with FNAB detects early cancers that present with a smaller size, and patients can undergo treatment at an earlier stage to reduce possible morbidity and mortality. In addition, screen-detected cancers do not behave like "occult" or indolent cancers, but the pathological characteristics are more similar to the characteristics of symptomatic clinical overt cancers, indicating that there are benefits of screening thyroid US. When a self-referred patient demands screening HRS, there is no reason to counter the demand or decision to undertake HRS, because of high malignancy rate observed with screen-detected thyroid nodules and early detection of smaller sized cancers.
Differences between screen-detected cancers and symptomatic thyroid cancers
The gender distribution of thyroid cancers was not different between subjects with screen-detected and clinically overt thyroid cancers. More females than males presented with cancers, as has been reported previously.6,23 Age is an important prognostic factor relative to mortality in thyroid cancer. In our study, however no statistical age differences existed between screen-detected thyroid cancers and clinically overt cancers. Age as a risk factor should be evaluated with a prospective study of thyroid cancer prognosis, but not with a cross sectional study. Most screen-detected thyroid cancers were well-differentiated thyroid cancers, and no difference in the rate was seen between screen-detected cancers and symptomatic thyroid cancers. This may be a reason for a stable mortality despite the increasing prevalence of thyroid cancer.24
Approximately 65% of surgically confirmed screen found cancers were microcarcinomas, a cancer measuring less than 1 cm in the longest diameter.25 The management of microcarcinomas is still controversial. An observational trial of papillary microcarcinomas in 162 patient without surgical procedures by Ito et al in Japan26 showed that more than 70% of tumors either did not change or decreased in size, or enlarged by more than 10 mm in 10.2% of cases; lymph node metastasis in the lateral compartments appeared in only 1.2% of patients during follow-up. In addition, a metastasis was confirmed histologically in 50.5% of cases and multiple tumor formation was seen in 42.8% of patients with a rate of recurrence of 2.7% at 5 years and 5.0% at 8 years after surgery. Investigators suggested that papillary microcarcinomas frequently did not become clinically apparent, therefore, patients can wait while tumors are not progressing, although lesions are pathologically multifocal and involve lymph nodes at an high incidence. According to our present data, performing screening thyroid US can lead to excessive and unnecessary surgical procedures. We cannot differentiate which nodule progresses aggressively and which nodule stays indolent or occult. Biological behavior of microcarcinomas is currently not clear. Many published studies described an aggressive clinical course of micropapillary carcinomas, showing high lymph node invasion, therefore, there would be a very limited number of patients who would choose follow-up rather than a surgical procedure if a treatment option is given. In a study of prognostic factors and extension of thyroid microcarcinomas in Korean patients, about 29.0% of patients presented with lymph node metastasis and extrathyroidal extension.27 Similar findings were also found in the present study, with 28/111 patients (25.2%) presented with capsule invasion and 23/111 patients (20.7%) presented with lymph node invasion. Thyroid microcarcinomas in Korean patients may be associated with poor prognostic factors, and appears to exist at relatively higher cancer stages but with a relatively low mortality rate. Therefore, the value of thyroid cancer screening should further be studied and management of micropapillary carcinomas should further be investigated, because most cancers found at screening centers appeared to be microcarcinomas.
When comparing the histopathology features in the screened-subjects and symptomatic subjects, clinically overt thyroid cancers presented with a larger size, whereas, the other clinical and pathological features were not statistically different, thus suggesting that screen found thyroid cancer and clinically overt symptomatic thyroid cancer should equally be treated. Kasai et al. reported that occult thyroid cancer follows a rather benign clinical course, suggesting a less aggressive treatment, however, also found that lymph node metastasis and extrathyroidal extension increase as the size of malignant nodules increase,28 which is concordant with our present results (Table 4). Earlier diagnosis with proper surgical removal of the tumor before distant metastasis may help improve prognosis of thyroid cancer patients. Radiological or cytological evaluation cannot determine cancer behavior, and it is standard practice in Korea to perform a thyroidectomy once a thyroid carcinoma is identified. Therefore, further prospective study on the natural history of occult thyroid carcinomas is needed. We attempted to identify any different clinical and pathological features between the screen-identified cancers and symptomatic cancers to provide evidence to justify less aggressive treatment, however, our results showed no statistical significant difference. Our findings support that screen-detected thyroid malignancies require investigation and treatment similar to those of clinically overt thyroid cancers. Since HRS detects too many small, non-palpable thyroid nodules which are mostly benign, a simple follow-up neck palpation has been suggested to be sufficient,29 however, we strongly believe that it should strictly be evaluated based on US characteristics and FNAB cytology findings once an incidentaloma is identified.
The primary limitation of this study is that the prevalence of thyroid nodules and cancer is based on a population that requested thyroid screening ultrasound. The medical faculty did not interfere in a decision of a patient, however, this particular population of subjects may not represent a general population, because of specific age and economic status differences of patients that seek health care. Second, FNAB was not performed on every single nodule found at the medical screening center, but was performed mainly based on the presence of specific US features. No definite US feature for a benign nodule exists. Consequently the FNAB indication used can generate false negative cases, providing a selection bias. A further investigation is needed to evaluate the value of screening thyroid US and strict FNAB guidelines for screen-identified nodules. Third, this study is a retrospective study. We hypothesized that screen-identified cancers behave in a manner similar to clinically occult thyroid cancers, however, which cancer stays occult while which cancer manifests as overt disease is not known.
Although many investigators have extensively described the approach to treatment of palpable or symptomatic thyroid nodules,20,30-32 there still remains disagreement about the accepted evaluation and treatment of incidental thyroid nodules.33,34 We think that the current system of wide use of screening for thyroid cancer is difficult to justify with such limited evidence of cost effectiveness of screening and the controversies still remaining for the management of thyroid incidentalomas and microcarcinomas. However, when a self-referred patient demands screening HRS, there is no reason to interfere with the demand or decision to undergo HRS. Thus, if the medical center performs screening thyroid ultrasound, nodules found in a screening setting should strictly be managed, based on US characteristics and FNAB cytology findings, and screen-identified cancers should be treated in the same way as clinically symptomatic thyroid nodules.
References
- 1.Woolner LB, Lemmon ML, Beahrs OH, Black BM, Keating FR., Jr Occult papillary carcinoma of the thyroid gland: a study of 140 cases observed in a 30-year period. J Clin Endocrinol Metab. 1960;20:89–105. doi: 10.1210/jcem-20-1-89. [DOI] [PubMed] [Google Scholar]
- 2.Kim JY, Lee CH, Kim SY, Jeon WK, Kang JH, An SK, et al. Radiologic and pathologic findings of nonpalpable thyroid carcinomas detected by ultrasonography in a medical screening center. J Ultrasound Med. 2008;27:215–223. doi: 10.7863/jum.2008.27.2.215. [DOI] [PubMed] [Google Scholar]
- 3.Steele SR, Martin MJ, Mullenix PS, Azarow KS, Andersen CA. The significance of incidental thyroid abnormalities identified during carotid duplex ultrasonography. Arch Surg. 2005;140:981–985. doi: 10.1001/archsurg.140.10.981. [DOI] [PubMed] [Google Scholar]
- 4.Frates MC, Benson CB, Charboneau JW, Cibas ES, Clark OH, Coleman BG, et al. Society of Radiologists in Ultrasound. Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Radiology. 2005;237:794–800. doi: 10.1148/radiol.2373050220. [DOI] [PubMed] [Google Scholar]
- 5.Kim TY, Kim WB, Ryu JS, Gong G, Hong SJ, Shong YK. 18F-fluorodeoxyglucose uptake in thyroid from positron emission tomogram (PET) for evaluation in cancer patients: high prevalence of malignancy in thyroid PET incidentaloma. Laryngoscope. 2005;115:1074–1078. doi: 10.1097/01.MLG.0000163098.01398.79. [DOI] [PubMed] [Google Scholar]
- 6.Kang HW, No JH, Chung JH, Min YK, Lee MS, Lee MK, et al. Prevalence, clinical and ultrasonographic characteristics of thyroid incidentalomas. Thyroid. 2004;14:29–33. doi: 10.1089/105072504322783812. [DOI] [PubMed] [Google Scholar]
- 7.Lee HK, Hur MH, Ahn SM. Diagnosis of occult thyroid carcinoma by ultrasonography. Yonsei Med J. 2003;44:1040–1044. doi: 10.3349/ymj.2003.44.6.1040. [DOI] [PubMed] [Google Scholar]
- 8.Nam-Goong IS, Kim HY, Gong G, Lee HK, Hong SJ, Kim WB, et al. Ultrasonography-guided fine-needle aspiration of thyroid incidentaloma: correlation with pathological findings. Clin Endocrinol (Oxf) 2004;60:21–28. doi: 10.1046/j.1365-2265.2003.01912.x. [DOI] [PubMed] [Google Scholar]
- 9.Brander A, Viikinkoski P, Nickels J, Kivisaari L. Thyroid gland: US screening in middle-aged women with no previous thyroid disease. Radiology. 1989;173:507–510. doi: 10.1148/radiology.173.2.2678263. [DOI] [PubMed] [Google Scholar]
- 10.Lang W, Borrusch H, Bauer L. Occult carcinomas of the thyroid. Evaluation of 1,020 sequential autopsies. Am J Clin Pathol. 1988;90:72–76. doi: 10.1093/ajcp/90.1.72. [DOI] [PubMed] [Google Scholar]
- 11.Ottino A, Pianzola HM, Castelletto RH. Occult papillary thyroid carcinoma at autopsy in La Plata, Argentina. Cancer. 1989;64:547–551. doi: 10.1002/1097-0142(19890715)64:2<547::aid-cncr2820640232>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 12.Solares CA, Penalonzo MA, Xu M, Orellana E. Occult papillary thyroid carcinoma in postmortem species: prevalence at autopsy. Am J Otolaryngol. 2005;26:87–90. doi: 10.1016/j.amjoto.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Neuhold N, Kaiser H, Kaserer K. Latent carcinoma of the thyroid in Austria: a systematic autopsy study. Endocr Pathol. 2001;12:23–31. doi: 10.1385/ep:12:1:23. [DOI] [PubMed] [Google Scholar]
- 14.Piersanti M, Ezzat S, Asa SL. Controversies in papillary microcarcinoma of the thyroid. Endocr Pathol. 2003;14:183–191. doi: 10.1007/s12022-003-0011-5. [DOI] [PubMed] [Google Scholar]
- 15.Leenhardt L, Hejblum G, Franc B, Fediaevsky LD, Delbot T, Le Guillouzic D, et al. Indications and limits of ultrasound-guided cytology in the management of nonpalpable thyroid nodules. J Clin Endocrinol Metab. 1999;84:24–28. doi: 10.1210/jcem.84.1.5418. [DOI] [PubMed] [Google Scholar]
- 16.Kim EK, Park CS, Chung WY, Oh KK, Kim DI, Lee JT, et al. New sonographic criteria for recommending fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. AJR Am J Roentgenol. 2002;178:687–691. doi: 10.2214/ajr.178.3.1780687. [DOI] [PubMed] [Google Scholar]
- 17.Wiest PW, Hartshorne MF, Inskip PD, Crooks LA, Vela BS, Telepak RJ, et al. Thyroid palpation versus high-resolution thyroid ultrasonography in the detection of nodules. J Ultrasound Med. 1998;17:487–496. doi: 10.7863/jum.1998.17.8.487. [DOI] [PubMed] [Google Scholar]
- 18.Carroll BA. Asymptomatic thyroid nodules: incidental sonographic detection. AJR Am J Roentgenol. 1982;138:499–501. doi: 10.2214/ajr.138.3.499. [DOI] [PubMed] [Google Scholar]
- 19.Naik KS, Bury RF. Imaging the thyroid. Clin Radiol. 1998;53:630–639. doi: 10.1016/s0009-9260(98)80289-4. [DOI] [PubMed] [Google Scholar]
- 20.Bennedbaek FN, Hegedüs L. Management of the solitary thyroid nodule: results of a North American survey. J Clin Endocrinol Metab. 2000;85:2493–2498. doi: 10.1210/jcem.85.7.6672. [DOI] [PubMed] [Google Scholar]
- 21.Frates MC, Benson CB, Doubilet PM, Kunreuther E, Contreras M, Cibas ES, et al. Prevalence and distribution of carcinoma in patients with solitary and multiple thyroid nodules on sonography. J Clin Endocrinol Metab. 2006;91:3411–3417. doi: 10.1210/jc.2006-0690. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell J, Parangi S. The thyroid incidentaloma: an increasingly frequent consequence of radiologic imaging. Semin Ultrasound CT MR. 2005;26:37–46. doi: 10.1053/j.sult.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Lin JD, Chao TC, Weng HF, Huang HS, Ho YS. Clinical presentations and treatment for 74 occult thyroid carcinoma. Comparison with nonoccult thyroid carcinoma in Taiwan. Am J Clin Oncol. 1996;19:504–508. doi: 10.1097/00000421-199610000-00015. [DOI] [PubMed] [Google Scholar]
- 24.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 25.Baloch ZW, LiVolsi VA. Microcarcinoma of the thyroid. Adv Anat Pathol. 2006;13:69–75. doi: 10.1097/01.pap.0000213006.10362.17. [DOI] [PubMed] [Google Scholar]
- 26.Ito Y, Uruno T, Nakano K, Takamura Y, Miya A, Kobayashi K, et al. An observation trial without surgical treatment in patients with papillary microcarcinoma of the thyroid. Thyroid. 2003;13:381–387. doi: 10.1089/105072503321669875. [DOI] [PubMed] [Google Scholar]
- 27.Lee J, Rhee Y, Lee S, Ahn CW, Cha BS, Kim KR, et al. Frequent, aggressive behaviors of thyroid microcarcinomas in Korean patients. Endocr J. 2006;53:627–632. doi: 10.1507/endocrj.k06-013. [DOI] [PubMed] [Google Scholar]
- 28.Kasai N, Sakamoto A. New subgrouping of small thyroid carcinomas. Cancer. 1987;60:1767–1770. doi: 10.1002/1097-0142(19871015)60:8<1767::aid-cncr2820600816>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 29.Tan GH, Gharib H. Thyroid incidentalomas: management approaches to nonpalpable nodules discovered incidentally on thyroid imaging. Ann Intern Med. 1997;126:226–231. doi: 10.7326/0003-4819-126-3-199702010-00009. [DOI] [PubMed] [Google Scholar]
- 30.Gharib H. Changing concepts in the diagnosis and management of thyroid nodules. Endocrinol Metab Clin North Am. 1997;26:777–800. doi: 10.1016/s0889-8529(05)70282-6. [DOI] [PubMed] [Google Scholar]
- 31.Burguera B, Gharib H. Thyroid incidentalomas. Prevalence, diagnosis, significance, and management. Endocrinol Metab Clin North Am. 2000;29:187–203. doi: 10.1016/s0889-8529(05)70123-7. [DOI] [PubMed] [Google Scholar]
- 32.Miki H, Oshimo K, Inoue H, Kawano M, Tanaka K, Komaki K, et al. Incidence of ultrasonographically-detected thyroid nodules in healthy adults. Tokushima J Exp Med. 1993;40:43–46. [PubMed] [Google Scholar]
- 33.Papini E, Guglielmi R, Bianchini A, Crescenzi A, Taccogna S, Nardi F, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002;87:1941–1946. doi: 10.1210/jcem.87.5.8504. [DOI] [PubMed] [Google Scholar]
- 34.Silver RJ, Parangi S. Management of thyroid incidentalomas. Surg Clin North Am. 2004;84:907–919. doi: 10.1016/j.suc.2004.02.002. [DOI] [PubMed] [Google Scholar]