Abstract
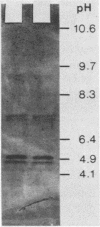
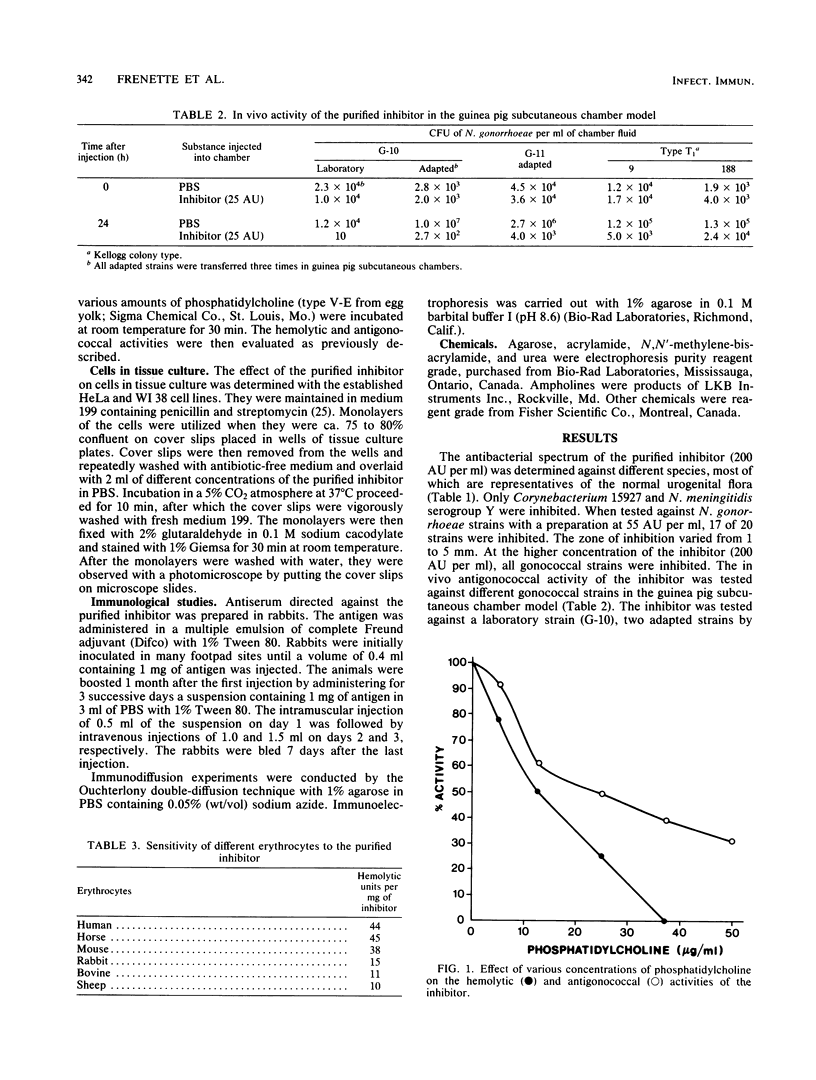
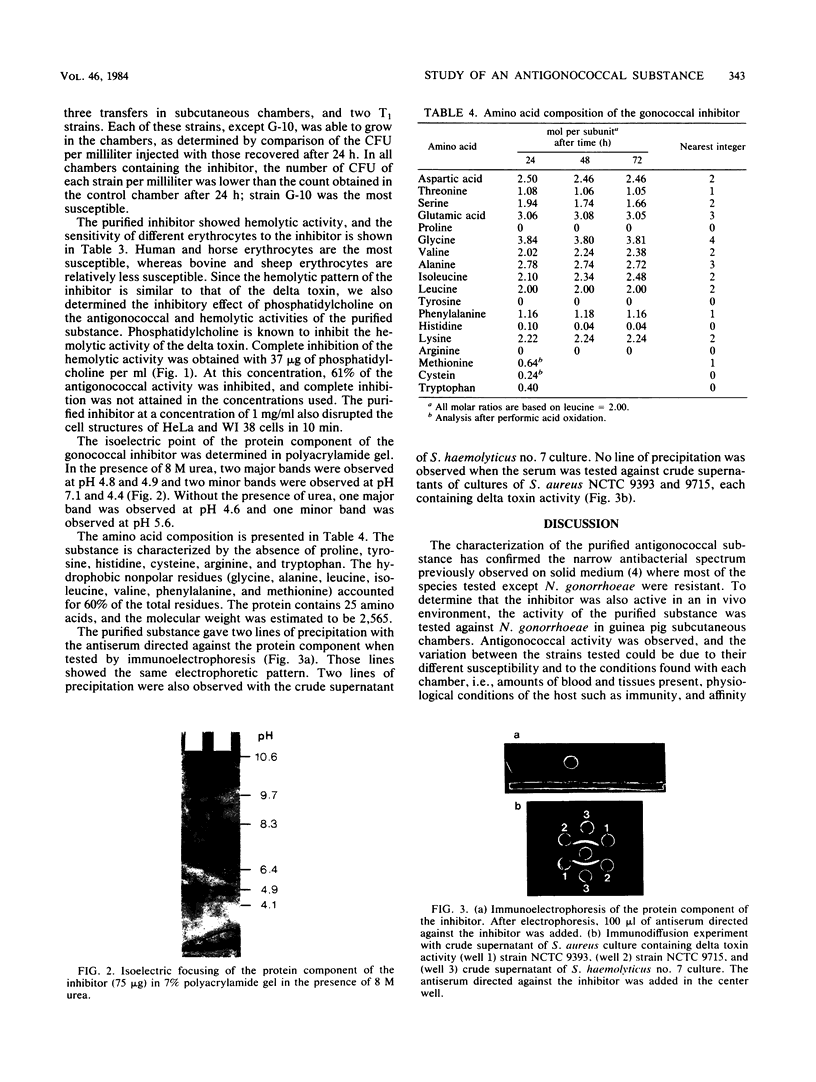
The purified antigonococcal substance produced by Staphylococcus haemolyticus no. 7 has shown a broad antigonococcal spectrum and a narrow antibacterial spectrum. The inhibitor produced in vitro was also active in the guinea pig subcutaneous chamber. The inhibitor has shown hemolytic activity; the human, horse, and mouse erythrocytes were the most susceptible. Hemolytic and antigonococcal activities were inhibited in the presence of phosphatidylcholine. The amino acid composition of the antigonococcal substance was characterized by the absence of proline, tyrosine, histidine, cysteine, and tryptophan. The molecular weight was found to be 2,565, and the major isoelectric points were 4.8 and 4.9 in the presence of 8 M urea and 4.6 without urea. The inhibitor has some properties similar to those of the delta toxin of Staphylococcus aureus, although the two substances are different based mainly on their chemical characteristics. Also an antiserum directed against the gonococcal inhibitor did not give a precipitation line with the delta toxin, indicating that the two substances are antigenically unrelated.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali M. I., Haque R. Identification of staphylococcal epsilon hemolysin. Can J Microbiol. 1972 Apr;18(4):535–536. doi: 10.1139/m72-082. [DOI] [PubMed] [Google Scholar]
- BERNHEIMER A. W., SCHWARTZ L. L. Isolation and composition of staphylococcal alpha toxin. J Gen Microbiol. 1963 Mar;30:455–468. doi: 10.1099/00221287-30-3-455. [DOI] [PubMed] [Google Scholar]
- Beaudet R., Bisaillon J. G., Saheb S. A., Sylvestre M. Production, purification, and preliminary characterization of a gonococcal growth inhibitor produced by a coagulase-negative staphylococcus isolated from the urogenital flora. Antimicrob Agents Chemother. 1982 Aug;22(2):277–283. doi: 10.1128/aac.22.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisaillon J. G., Beaudet R., Lafond L., Saheb S. A., Sylvestre M. Antigonococcal and antibacterial spectra of some bacterial isolates of the urogenital flora. Rev Can Biol. 1981 Jun;40(2):215–227. [PubMed] [Google Scholar]
- Bisaillon J. G., Beaudet R., Saheb S. A., Morisset R. Interference of Neisseria gonorrhoeae growth by aerobic bacterial representatives of the urogenital flora. Rev Can Biol. 1980 Dec;39(4):201–208. [PubMed] [Google Scholar]
- Chow A. W., Gribble M. J., Bartlett K. H. Characterization of the hemolytic activity of Staphylococcus aureus strains associated with toxic shock syndrome. J Clin Microbiol. 1983 Mar;17(3):524–528. doi: 10.1128/jcm.17.3.524-528.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajani A. S., Wannamaker L. W. Demonstration of a bactericidal substance against beta-hemolytic streptococci in supernatant fluids of staphylococcal cultures. J Bacteriol. 1969 Mar;97(3):985–991. doi: 10.1128/jb.97.3.985-991.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitton J. E., Dell A., Shaw W. V. The amino acid sequence of the delta haemolysin of Staphylococcus aureus. FEBS Lett. 1980 Jun 30;115(2):209–212. doi: 10.1016/0014-5793(80)81170-7. [DOI] [PubMed] [Google Scholar]
- Gagliano V. J., Hinsdill R. D. Characterization of a Staphylococcus aureus bacteriocin. J Bacteriol. 1970 Oct;104(1):117–125. doi: 10.1128/jb.104.1.117-125.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C., Wiseman G. M. The nature of epidermidins, new antibiotics from staphylococci. Can J Microbiol. 1972 Feb;18(2):121–125. doi: 10.1139/m72-021. [DOI] [PubMed] [Google Scholar]
- Kaye D., Levison M. E. In vitro inhibition of growth of neisseria gonorrhoeae by genital microorganisms. Sex Transm Dis. 1977 Jan-Mar;4(1):1–3. [PubMed] [Google Scholar]
- Kraus S. J., Ellison N. Resistance to gonorrhea possibly mediated by bacterial interference. Appl Microbiol. 1974 Jun;27(6):1014–1016. doi: 10.1128/am.27.6.1014-1016.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus S. J., Geller R. C., Perkins G. H., Rhoden D. L. Interference by Neisseria gonorrhoeae growth by other bacterial species. J Clin Microbiol. 1976 Sep;4(3):288–295. doi: 10.1128/jcm.4.3.288-295.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafond L., Bisaillon J. G., Saheb S. A., Beaudet R., Sylvestre M. Bactericidal and bacteriostatic antigonococcal activities produced by urogenital staphylococci. Microbios. 1982;33(131):27–34. [PubMed] [Google Scholar]
- Lafond L., Bisaillon J. G., Saheb S. A., Beaudet R., Sylvestre M. In vitro antigonococcal activity of urogenital coagulase-negative staphylococci. Can J Microbiol. 1981 Jan;27(1):138–141. doi: 10.1139/m81-021. [DOI] [PubMed] [Google Scholar]
- Morin A., Saheb S. A., Bisaillon J. G., Beaudet R., Sylvestre M. In vitro inhibition of Neisseria gonorrhoeae growth by strict anaerobes. Infect Immun. 1980 Jun;28(3):766–770. doi: 10.1128/iai.28.3.766-770.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morriss D. M., Lawson J. W., Rogolsky M. Effect of a staphylococcin on Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1978 Aug;14(2):218–223. doi: 10.1128/aac.14.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Yamazaki N., Taniguchi H., Fujimura S. Production, purification, and properties of a bacteriocin from Staphylococcus aureus isolated from saliva. Infect Immun. 1983 Feb;39(2):609–614. doi: 10.1128/iai.39.2.609-614.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn C. W., Sen D., Veale D. R., Parsons N. J., Smith H. Morphological, biological and antigenic properties of Neisseria gonorrhoeae adapted to growth in guinea-pig subcutaneous chambers. J Gen Microbiol. 1976 Nov;97(1):35–43. doi: 10.1099/00221287-97-1-35. [DOI] [PubMed] [Google Scholar]
- Rogolsky M. Nonenteric toxins of Staphylococcus aureus. Microbiol Rev. 1979 Sep;43(3):320–360. doi: 10.1128/mr.43.3.320-360.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHINDLER C. A., SCHUHARDT V. T. LYSOSTAPHIN: A NEW BACTERIOLYTIC AGENT FOR THE STAPHYLOCOCCUS. Proc Natl Acad Sci U S A. 1964 Mar;51:414–421. doi: 10.1073/pnas.51.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saigh J. H., Sanders C. C., Sanders W. E., Jr Inhibition of Neisseria gonorrhoeae by aerobic and facultatively anaerobic components of the endocervical flora: evidence for a protective effect against infection. Infect Immun. 1978 Feb;19(2):704–710. doi: 10.1128/iai.19.2.704-710.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. IV. Pili: their role in attachment of gonococci to tissue culture cells. J Exp Med. 1973 Mar 1;137(3):571–589. doi: 10.1084/jem.137.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk J., Kraus S. J. Asymptomatic meningococcal urethritis. Possible protective value against gonococcal infection by bacteriocin production. Br J Vener Dis. 1973 Dec;49(6):511–512. doi: 10.1136/sti.49.6.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman G. M. The hemolysins of Staphylococcus aureus. Bacteriol Rev. 1975 Dec;39(4):317–344. doi: 10.1128/br.39.4.317-344.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]