Abstract
Laparoscopic approaches are increasingly used in pancreatic surgery. In the treatment of neuroendocrine tumors (NETs) of the pancreas, enucleation is one of the recommended surgery. Although many clinical experiences have reported the safety and efficacy of laparoscopic enucleation of functioning NETs, such as insulinomas, few reports have explored such treatment for non-functioning NETs. Here, we present a case of 70-year old female patient who underwent successful laparoscopic enucleation of a nonfunctioning NET located in the body of the pancreas.
Keywords: Laparoscopy, enucleation, neuroendocrine tumor, pancreas
INTRODUCTION
Laparoscopic approaches are increasingly used in pancreatic surgery, and have been extended to use in the staging of malignant pancreatic neoplasms, the management of benign inflammatory pancreatic disease, and the resection of benign or borderline malignant cysts and neoplasms of the pancreas.1-5 In the treatment of neuroendocrine tumors (NETs) of the pancreas, enucleation is the recommended surgery, since the tumors are usually benign and pancreatic function must be preserved. Although many researches have described the safety and efficacy of laparoscopic enucleation of functioning NETs, such as insulinomas,5-10 few reports on such treatment for non-functioning NETs of the pancreas have been introduced. Here, we present a case of successful laparoscopic enucleation of a nonfunctioning NET located in the body of the pancreas.
CASE REPORT
Patient
A 70-year-old female patient was admitted to the Department of Surgery at Yonsei Medical Center in Seoul, Korea after a pancreatic mass was incidentally discovered during the evaluation of abdominal discomfort and nausea. She had previously been in good health without any prior medical history. An abdominal CT scan revealed a 1 cm-sized hypervascular mass in the body of the pancreas without any evidence of regional lymph node enlargement or distant metastasis (Fig. 1). An endoscopic ultrasound of the entire pancreas revealed a single 1 cm-sized homogenous, hypoechoic mass in the pancreatic body (Fig. 2) Routine blood chemistry and tumor marker levels (CA 19-9, CEA, and CA 125) were all within normal limits. Because it appeared that the patient had a neuroendocrine tumor of the pancreas, she underwent laparoscopic enucleation.
Fig. 1.
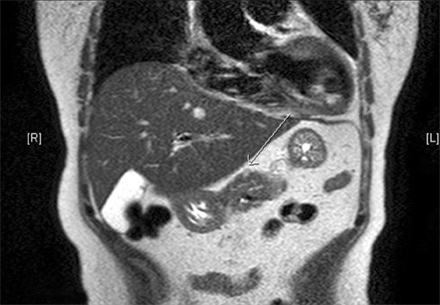
Abdominal CT scan. About 1 cm-sized enhanced mass is noted in the surface of the pancreatic body.
Fig. 2.
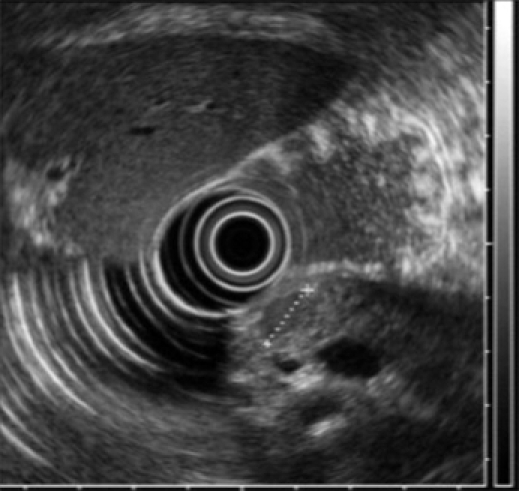
Endoscopoic Ultrasonogram. About 1 cm-sized, single, homogenous, hypoechoic mass was found in the pancreatic body.
Surgery
The patient was placed in the supine position with her legs separated. The surgeon and scrub nurse stood on the right side of the patient, the assistant surgeon navigated the camera and stood between the patient's legs, while another assistant surgeon stood on the left side of the patient. A total of four trocars (one 12-mm trocar, three 5-mm trocars) were used in this surgical procedure (Fig. 3). Two trocars (5-mm) were placed on the right side to pass the instruments, and another trocar (5-mm) was inserted on the left side to retract the lateral segment of the liver. Two monitors, a 30 degree laparoscope, a harmonic scalpel and routine laparoscopic instruments were used, and CO2 pneumoperitoneum (12 mmHg) was maintained throughout the procedure. Initially, the hepatogastric ligament was opened and the pancreatic mass was easily identified. It was superficial and well-demarcated pancreatic mass (Fig. 4A). The entire pancreas was explored with a flexible laparoscopic ultrasound and the lesion was detected at the site previously indicated by CT and EUS. One prolene 5-0 holding suture was placed through the pancreatic mass for appropriate traction. Enucleation of the pancreatic mass was achieved by blunt and sharp dissection of the peripancreatic tissue (Figs. 4B and C). After completion of the surgical procedure, the pancreatic mass was withdrawn from the abdominal cavity with a plastic bag. The surgical procedure was completed without requiring any abdominal drain.
Fig. 3.
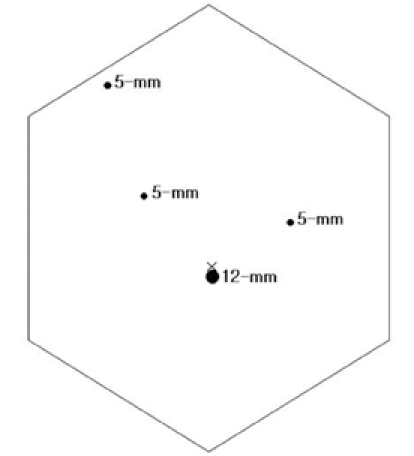
Trocar placement.
Fig. 4.
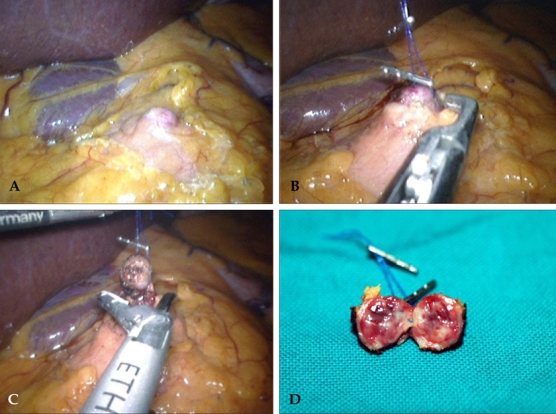
Intraoperative laparoscopic view. Superficial locating, well-demarcated pancreatic mass is noted in the body of pancreas (A). Enucleation of the pancreatic mass is achieved (B and C). Gross pathologic finding. It was about 1cm sized homogenous well demarcating mass (D).
Postoperative course
The patient recovered without complication. She returned to an oral diet immediately and was discharged on the third postoperative day. Pathologic evaluation revealed the resected specimen was found to be a 10 × 12 × 10 mm mass (Fig. 4D), with the histological features of a pancreatic endocrine tumor. The cells stained immunohistochemically with chromogranin A (Figs. 5 and 6). It had no evidence of local invasiveness (no neural invasion, no lymphovascular invasion, no capsular /peripancreatic invasion). Especially, the expression of Ki-67 was less than 5%.
Fig. 5.
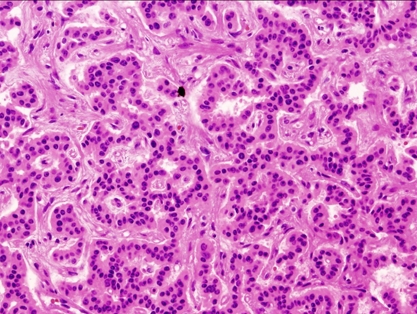
Hematoxylin-Eosin staining (× 200).
Fig. 6.
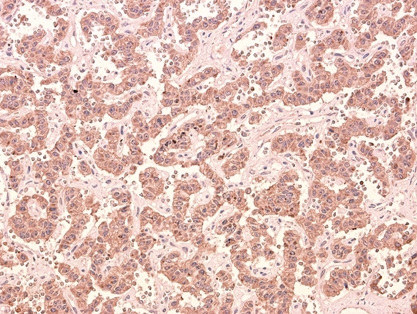
Immunohistochemstry with chromogranin A (× 200).
DISCUSSION
Neuroendocrine (islet cell) tumor (NET) of the pancreas is a relatively rare pathologic condition.5 Most NETs of the pancreas are not associated with the clinical symptoms of hormonal over-secretion and are classified as nonfunctional.
Laparoscopic pancreatic surgery is emerging as a treatment option for nonfunctioning NETs of the pancreas. Based on our surgical experience with nonfunctioning NETs,11 53% of the patients had tumors in distal part of the pancreas and the median tumor size was 3.5 cm in diameter. Theoretically, laparoscopic distal pancreatectomy can be recommended for those neoplasms. However, in our experience, tumors greater than 3 cm in diameter were preoperative risk factors for an increased rate of metastasis and macroinvasion to adjacent organs, and substantially impacted patient survival. Therefore, the laparoscopic approach should be carefully chosen in large nonfunctioning NETs because curative resection is so important for long-term survival.
Laparoscopic distal pancreatectomy with or without splenectomy has been reported in the literature as a treatment for nonfunctioning NETs of the pancreas,1,5,12 but reports of laparoscopic enucleation of nonfunctioning NETs of the pancreas are rare. This relative paucity of data may be related to the asymptomatic nature of these neoplasms. In contrast, laparoscopic enucleation of functioning NETs, such as insulinomas, has been frequently described. The asymptomatic nature of small nonfunctioning NETs is an obstacle to the early discovery of such tumors. However, the frequency of early detection of small lesions in the pancreas is expected to increase due to easy accessibility of abdominal ultrasound and increased patient health awareness. With such early detection, laparoscopic enucleation of small, nonfunctioning NETs of the pancreas could offer a shorter hospital stay, good cosmetic effect, less pain, and an early return to normal social activity.
Only two cases of laparoscopic enucleation for nonfunctioning pancreatic NETs have been reported in the literature (Table 1).13,14 In general, nonfunctioning NETs in the pancreatic head are not recommended for such surgery because of the risk of open conversion due to technical difficulties or an inability to localize the tumor.15 However, successful laparoscopic enucleation of such tumors has been reported, and this procedure appears to be feasible and reproducible in well-selected benign nonfunctioning NENs of the pancreas.
Table 1.
Literature Review of Nonfunctioning Neuroendocrine Tumors of the Pancreas Treated by Laparoscopic Enucleation
M, male; F, female.
Minimally invasive surgery is thought to both reduce surgical damage and minimize the frequency of postoperative complications. Enucleation procedures have the advantage of preserving pancreatic and spleen parenchyma, without the morbidity, mortality, or late sequelae of extensive resection.16 The most common complication is pancreatic fistula.17 Accordingly, patients may be considered candidates for enucleation if the pancreatic neoplasm is free of the pancreatic duct and has no vascular involvement on preoperative imaging studies. To this end, intraoperative ultrasound can aid in identifying the mass and determining its proximity to the pancreatic duct, which can decrease the incidence of the pancreatic fistula.18 In current case, we did not place any type of drain system because the tumor was very small (about 1 cm) and superficial location apart from main pancreatic duct, which may have low risk of postoperative pancreatic fistula. We don't think any drainage sysment is necessary.
In summary, we avoided a long abdominal incision and inflicted less stressful surgical damage by laparoscopically treating the small, nonfunctioning NET of this older patient. Laparoscopic enucleation of well-selected non-functioning NETs of the pancreas, such as a single, small, benign pancreatic mass superficially located in the pancreas, seems to be feasible. However, long-term follow up is mandatory given their likelihood of a more malignant course.
References
- 1.Shimizu S, Tanaka M, Konomi K, Mizumoto K, Yamaguchi K. Laparoscopic pancreatic surgery: current indications and surgical results. Surg Endosc. 2004;18:402–406. doi: 10.1007/s00464-003-8164-3. [DOI] [PubMed] [Google Scholar]
- 2.Park A, Schwartz R, Tandan V, Anvari M. Laparoscopic pancreatic surgery. Am J Surg. 1999;177:158–163. doi: 10.1016/s0002-9610(98)00325-0. [DOI] [PubMed] [Google Scholar]
- 3.Cuschieri A. Laparoscopic surgery of the pancreas. J R Coll Surg Edinb. 1994;39:178–184. [PubMed] [Google Scholar]
- 4.Fernandez-cruz L, Saenza A, Pantoja JP. Laparoscopy in pancreatic surgery. Int J Surg Sci. 2000;7(1):121. [Google Scholar]
- 5.Toniato A, Meduri F, Foletto M, Avogaro A, Pelizzo M. Laparoscopic treatment of benign insulinomas localized in the body and tail of the pancreas: a single-center experience. World J Surg. 2006;30:1916–1919. doi: 10.1007/s00268-005-0645-1. discussion 1920-1. [DOI] [PubMed] [Google Scholar]
- 6.Dakin GF, Inabnet WB. Multimedia article. Laparoscopic enucleation of a pancreatic insulinoma. Surg Endosc. 2004;18:1680. doi: 10.1007/s00464-004-6016-4. [DOI] [PubMed] [Google Scholar]
- 7.Bozbora A, Barbaros U, Erbil Y, Ozarmagan S, Mercan S. Is laparoscopic enucleation the gold standard in selected cases with insulinoma? J Laparoendosc Adv Surg Tech A. 2004;14:230–233. doi: 10.1089/lap.2004.14.230. [DOI] [PubMed] [Google Scholar]
- 8.Lo CY, Chan WF, Lo CM, Fan ST, Tam PK. Surgical treatment of pancreatic insulinoma in the era of laparoscopy. Surg Endosc. 2004;18:297–302. doi: 10.1007/s00464-003-8156-3. [DOI] [PubMed] [Google Scholar]
- 9.Stein DM, Licht AL, Gecelter GR. Laparoscopic enucleation of an insulinoma: advantages of using the curved laparoscopic coagulating shears. Surg Endosc. 2002;16:1365. doi: 10.1007/s00464-001-4128-7. [DOI] [PubMed] [Google Scholar]
- 10.Takamatsu S, Teramoto K, Inoue H, Goseki N, Takahashi S, Baba H, et al. Laparoscopic enucleation of an insulinoma of the pancreas tail. Surg Endosc. 2002;16:217. doi: 10.1007/s004640041025. [DOI] [PubMed] [Google Scholar]
- 11.Kang CM, Kim KS, Choi JS, Lee WJ, Kim BR. Experiences with nonfunctioning neuroendocrine neoplasms of the pancreas. Dig Surg. 2005;22:453–458. doi: 10.1159/000092011. [DOI] [PubMed] [Google Scholar]
- 12.Fernández-Cruz L, Herrera M, Sáenz A, Pantoja JP, Astudillo E, Sierra M. Laparoscopic pancreatic surgery in patients with neuroendocrine tumours: indications and limits. Best Pract Res Clin Endocrinol Metab. 2001;15:161–175. doi: 10.1053/beem.2001.0133. [DOI] [PubMed] [Google Scholar]
- 13.Singh N, Lo CY, Chan WF. Laparoscopic enucleation of a nonfunctioning neuroendocrine tumor at the head of the pancreas. JSLS. 2006;10:259–262. [PMC free article] [PubMed] [Google Scholar]
- 14.Pierce RA, Spitler JA, Hawkins WG, Strasberg SM, Línehan DC, Halpin VJ, et al. Outcomes analysis of laparoscopic resection of pancreatic neoplasms. Surg Endosc. 2006;21:579–586. doi: 10.1007/s00464-006-9022-x. [DOI] [PubMed] [Google Scholar]
- 15.Berends FJ, Cuesta MA, Kazemier G, van Eijck CH, de Herder WW, van Muiswinkel JM, et al. Laparoscopic detection and resection of insulinomas. Surgery. 2000;128:386–391. doi: 10.1067/msy.2000.107413. [DOI] [PubMed] [Google Scholar]
- 16.Aranha GV, Shoup M. Nonstandard pancreatic resections for unusual lesions. Am J Surg. 2005;189:223–228. doi: 10.1016/j.amjsurg.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Ayav A, Bresler L, Brunard L, Boissel P. Laparoscopic approach for solitary insulinoma: a multicenter study. Langenbecks Arch Surg. 2005;390:134–140. doi: 10.1007/s00423-004-0526-3. [DOI] [PubMed] [Google Scholar]
- 18.Giger U, Michel JM, Wiesli P, Schmid C, Krähenbühl L. Laparoscopic surgery for benign lesions of the pancreas. J Laparoendosc Adv Surg Tech. 2006;16:452–457. doi: 10.1089/lap.2006.16.452. [DOI] [PubMed] [Google Scholar]



