Abstract
Purpose
Non-steroidal anti-inflammatory drugs (NSAID) are frequently used in oral surgical procedures in dentistry. The evaluation of the frequency of sister chromatid exchange (SCE) is accepted as a reliable cytogenetic method to assess the genotoxic effects of environmental factors.
Materials and Methods
In this study, the genotoxic effects of various NSAIDs were assessed in 30 patients to who they were administered following encluosed third molar surgery using SCE analysis before and after the operation. The frequency of SCE was evaluated before the operation and after 3 days of etodolac, nimesulid and naproxen use.
Results
There was no statistically significant difference in the frequency of SCE between the preoperative and postoperative states in patients given etodolac, nimesulid or naproxen sodium.
Conclusion
Short term use of selective and non-selective NSAIDs was not associated with a significant genotoxic effect that could be detected using the SCE method in peripheric lymphocytes.
Keywords: Genotoxicity, sister chromatid exchange, oral surgery, non-steroidal anti-inflammatory drugs
INTRODUCTION
Non-steroidal anti-inflammatory drugs (NSAIDs) have a wide range of indications in the treatment of medical and surgical diseases due to their analgesic, antipyretic and anti-inflammatory effects.1,2 They are frequently used in painful and inflammatory conditions such as rheumatologic diseases, orthopedic and gynecologic surgery and in soft tissue traumas. This group of medications is also commonly used in the clinical practice of dentistry, such as in the treatment of oro-facial pain or after oral surgical procedures.3,4
Some NSAIDs are widely used in Turkey, without prescription, for their analgesic and anti-inflammatory properties.
Naproxen, a propionic acid derivative, is a prototypical anti-inflammatory agent which is generally used for the treatment of rheumatoid arthritis, osteoarthritis, primary dysmenorrhea, ankylosing spondylitis, bursitis, tendonitis, juvenile arthritis, acute gout, fever, following dental, obstetric or orthopedic surgery, for prophylaxis and the treatment of vascular headaches. The recommended adult dosage of naproxen sodium for acute pain is 550 - 1,100 mg/day in divided doses. If necessary, the dosage may be increased up to 1,650 mg/day for a short period.5
Etodolac is a pyranocarboxylic acid-derived nonsteroidal anti-inflammatory drug (NSAID) that possesses analgesic activity. Etodolac suppresses the biosynthesis of prostaglandins by inhibition of cyclcoxygenases such as other NSAIDs. Etodolac is used in osteoarthritis, rheumatoid arthritis, tendonitis, bursitis, acute sports injuries, gout, pain of orthopedic pathologies, dysmenorrhea, postoperative pain (surgical and dental procedures) and for pain associated with non-rheumatic inflammatory conditions or vascular headaches. A dosage of 200 - 400 mgr every 6 to 8 hours is recommended for acute pain.3 Earlier studies have reported that etodolac at 50 to 400 mg/day provided dose-related relief of moderate to severe postoperative pain from a variety of surgical and dental procedures.6,7
Nimesulide is a sulfononilide nonsteroidal anti-inflammatory drug with marked anti-inflammatory, antipyretic and analgesic properties and is indicated for osteoarthritis, rheumatoid arthritis, reduction of fever, primary dysmenorrhea and for relief of mild to moderate pain. The recommended adult dosage of nimesulide is 100 mg administered orally as tablets twice daily.8
NSAIDs have severe toxic effects on the gastrointestinal and renal systems; central nervous system, otic, ocular and adverse effects (pruritis, skin eruptions, or rashes, ecchymoses, dermatitis, sweeting and photosensitivity reactions). They can cause gastric mucosal damage, which may result in ulceration and/or bleeding, although they can inhibit platelet aggregation and may prolong the bleeding time but they do not effect the prothrombin time or whole blood clotting time.5 The gastrointestinal and renal side effects of selective COX2 inhibitors are claimed to be fewer than those of the classical NSAIDs.1,2 For that reason their use has become more frequent in recent times.
Studies on the genotoxic potential of these drugs are limited. Various studies have shown that they either have only a weak or no genotoxic effect at all.9-12
Enclosed third molar surgery is one of the most common surgical interventions in oral surgery.13 NSAIDs are given after the surgical operation to prevent complaints and complications. Pharmacologic therapy plays a very important role in acute inflammation. Patients want relief from pain, so the most useful agents are those that provide analgesic as well as anti-inflammatory effects. Various drugs are commonly administered in the postoperative period for the relief of pain and oedema. For these patients, the drugs of choice are NSAIDs which are widely used in routine daily practice.
Analysis of the frequency of Sister Chromatid Exchange (SCE) is a sensitive and reliable technique for the detection of DNA damage caused by various environmental factors, such as various physical and chemical agents and drugs.14-16
In this study, the genotoxic effects of three commonly used NSAIDs were investigated using the frequency of SCEs in peripheral lymphocytes before and after drug use in patients undergoing oral surgery.
MATERIALS AND METHODS
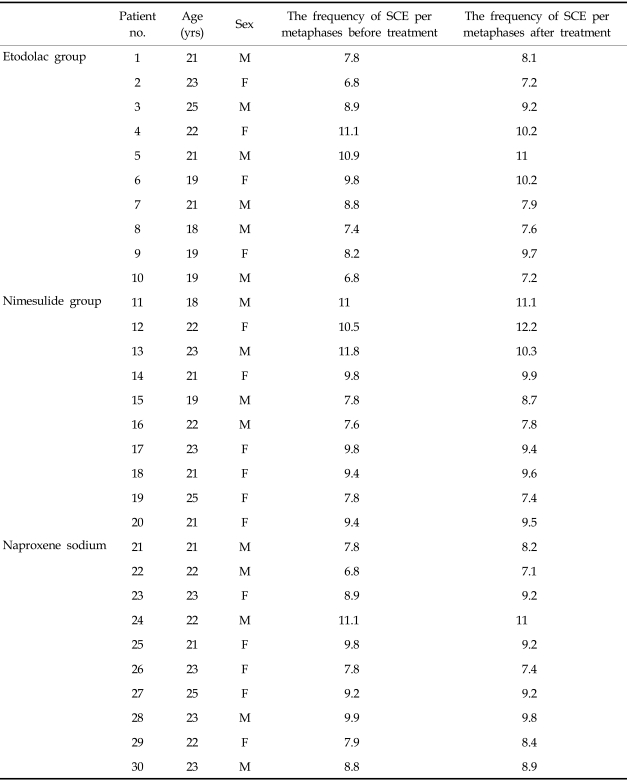
In this prospective study, thirty consequential patients (male/female = 1, age:19 - 25 years, mean age: 21.6 ± 1.90) for whom an enclused third molar operation was planned were randomly and blindly separated into 3 groups (etodolac, naproxen sodium and nimesulid groups). The patients were non-smokers and on no other medications at the time of this study. Written and oral consents were obtained from all of patients. The first peripheric blood samples were obtained prior to commencing therapy and the second after 3 days of NSAID treatment. Patients were given either etodolac 800 mg/day, naproxen sodium 1,100 mg/day or nimesulid 200 mgr/day in two divided doses.
Peripheric lymphocytes were cultured using a modified procedure of the peripheric blood culture method developed by Moorhead et al.17 A stock medium was prepared by the addition of 20% fetal bovine serum, 1% Penicilinle-streptomycine, 1.5% PHA-M (PHA-M, Biochrom KG, Berlin, Germany) and 1% L-glutamine to 100 mL of Ham's F-10 medium (Biochrom KG, Berlin, Germany). In each culture tube, 0.3 mL of the blood sample, with heparin was added using a sterile syringe. At 24 hours, 100 microliters of the stock solution (50 µg) containing 0.5 mg/mL Bromodeoxyuridine (BrdU) (BrdU, Sigma Chemical Company, St. Louis, MO, USA) was added to achieve a final concentration of 0.5 µg/mL. After the BrdU addition the culture tubes were covered with light resistant paper and incubated for 72 hours at 37℃. After 70 hours, colchicine (0.2 µ/mL) was added.Two hours after the addition of colchicine (Colchicine powder, Sigma chemical CO, St. Louis, MO, USA) (at 72nd hours), the culture tubes were removed from the incubator. After standard harvesting procedures, slides were stained by the fluorescence plus Giemsa (FPG) method18 using Hoechst 33258 dye exposure to fluorescent black light and Giemsa staining. Every point of exchanges was counted as a SCE: terminal exchanges were scored as one SCE and interstitial ones as two SCEs (Fig. 1). The frequencies of SCE per metaphases before and after treatment for each individuals were evaluated. In this study, a statistical analysis was performed using the Kruskal Wallis and Wilcoxon tests to compare the percentage of SCE in different sample groups, which were calculated with the Graph Pad Prisma V.3 computer programme.
Fig. 1.
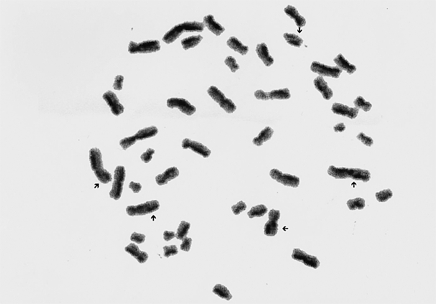
In the normal state, one of the sister chromatids is stained dark, while the other is stained light. When a reciprocal change occurs, a dark segment and its homologue light counterpart change places leading to alternating dark and light regions throughout the chromatids.
RESULTS
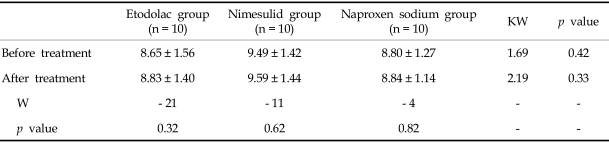
The SCE frequency per metaphase was calculated in all patients and compare within each group before and after 3 days of medication. These findings are shown in Table 1. In the Etol group, the SCE frequencies were 8.65 ± 1.56 and 8.83 ± 1.40 before and after treatment, respectively. The corresponding frequencies for the nimesulid and naproxen groups, were 9.49 ± 1.42 and 9.59 ± 1.44, and 8.8 ± 1.27 and 8.84 ± 1.14 respectively (Fig. 2). When all groups were combined, the SCE frequencies per metaphase were 8.93 ± 1.41 and 9.08 ± 1.33 before and after NSAIDs treatment, respectively. There was no statistically significant difference in the frequencies of SCE between the preoperative and postoperative values of the etodolac (W = - 21; p = 0.32), nimesulid (W = - 11; p = 0.62) and naproxen sodium (W = - 4; p = 0.82) groups. The differences between the groups before the treatment (KW = 1.69; p = 0.42) were found to be statistically insignificant. The difference between the groups (KW = 2.19; p = 0.33) after the treatment was also not significant (Table 2).
Table 1.
Demographic Data and Frequency of Sister Chromatid Exchanges per Metaphases
SCE, sister chromatid exchange; M, male; F, female.
Fig. 2.
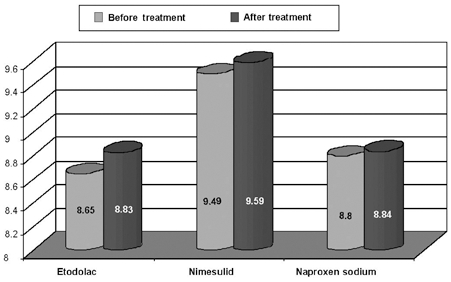
Comparison of sister chromatid exchange frequencies was performed between the preoperative and postoperative values for etodolac, nimesulid and naproxen sodium.
Table 2.
The Differences in the Frequencies of Sister Chromatid Exchange between Preoperative and Postoperative Values of Etodolac, Nimesulid and Naproxen Sodium Were Found to be Statistically Insignificant
W, Wilcoxon test; KW, Kruskal Wallis test.
n : 10 (n : 10 patient).
DISCUSSION
Cytogenetic markers, such as chromosomal aberrations (CAs), and micronuclei (MN), and SCE, are among the most widely used in the indication of the early biological effects of DNA damaging agents. In addition to cytogenetic markers, various molecular genetic techniques, including the Commet assay, have been used in the evaluation of mutagenicity / carcinogencity. The main difference between cytogenetic analysis and the Commet assay is the kind of changes detected. The Commet assay can detect repairable defects or alkali labile sites, whereas cytogenetic analysis can detect only chromosomal aberrations that have occurred at least one cell cycle earlier.19
Sister Chromatid Exchange can be defined as the exchange of parts between sister chromatids.20 Although the mechanism is not well understood,21 SCE is thought to occur during the replication process, and is accepted as a reliable test for the evaluation of DNA damage.22,23 Viral infections,22 cigarette smoking,24 advanced age,25,26 malignant diseases,27 medications10,11,12,28 and UV light20,26 have all been shown to increase the frequency of SCE.
In the present study, the in vivo genotoxicities of three NSAIDs, namely etodolac, nimesulid and naproxen sodium, were evaluated by SCE in peripheral blood samples. Although the genotoxicities of other NSAIDs have been evaluated in various studies,9-12 according to our knowledge, this is the first report of genotoxicity studies for etodolac and nimesulid with the use of an in vivo SCE assay.
Kullich and Klein10 reported no increase in the frequencies of SCE after 2 weeks of treatment with various NSAIDs, including diclofenac, flurbiprofen, ibuprofen, indomethacin, isoxicam, ketoprofen, piroxicam, pirprofen, and tiaprofenic acid. A study on rat bone marrow cells revealed a weak genotoxic effect of the non-selective COX2 inhibitors ibuprofen, ketoprofen and naproxen sodium.11
Özkul et al.12 reported a slight statistically insignificant increase in the frequency of SCE with the use of naproxen sodium.
In our study, the average SCE frequency per metaphase was slightly higher after treatment, without reaching statistical significance. Also, the short term use of selective (etodolac) and non-selective NSAIDs (nimesulid and naproxen sodium) were not associated with any genotoxic effect that could be detected using the SCE method in peripheric lymphocytes.
It is concluded that in the short term therapeutic application of these drugs there are no genotoxic effects on the chromosomes of peripheral blood lymphocytes. However, further studies will be required to understand the possible genotoxic effects of their long-term use.
References
- 1.Roux L, Liang MH. Osteoarthritis. In: Rakel RE, Bope ET, editors. Conn's current therapy 2001. Philadelphia: WB Saunders Company; 2000. pp. 969–970. [Google Scholar]
- 2.Reynolds JEF, editor. Martindle, the extra pharmacopoeia, nonsteroidal anti-inflammatory drugs. 31st ed. London: The Pharmaceutical Press; 1996. pp. 72–75. [Google Scholar]
- 3.Balfour JA, Buckley MM. Etodolac. A reappraisal of its pharmacology and therapeutic use in rheumatic diseases and pain states. Drugs. 1991;42:274–299. doi: 10.2165/00003495-199142020-00008. [DOI] [PubMed] [Google Scholar]
- 4.Thomson PDR, editor. USPDI Drug information for the health care professional. 17th ed. Vol. 1. Massachusetts: 1997. pp. 379–394. [Google Scholar]
- 5.McEvoy GK, editor. American Hospital formulary service drug information 2000. Bethesda, MO: American Society Health-System Pharmacists; 2005. pp. 1854–1860. [Google Scholar]
- 6.Scott R, Ellis E, 3rd, Upton LG. Double-blind evaluation of etodolac (200 mg, 400 mg) compared with zomepirac (100 mg) and placebo on third molar extraction pain. Oral Surg Oral Med Oral Pathol. 1986;62:638–642. doi: 10.1016/0030-4220(86)90255-0. [DOI] [PubMed] [Google Scholar]
- 7.Giglio JA, Campbell RL. Comparison of etodolac, zomepirac, and placebo for relief of pain after oral surgery. J Oral Maxillofac Surg. 1986;44:765–770. doi: 10.1016/0278-2391(86)90150-3. [DOI] [PubMed] [Google Scholar]
- 8.Fusetti G, Magni E, Armandola MC. Tolerability of nimesulide. Epidemiological data. Drugs. 1993;46(Suppl 1):277–280. doi: 10.2165/00003495-199300461-00070. [DOI] [PubMed] [Google Scholar]
- 9.Lee SH, Norppa H. Effects of indomethacin and arachidonic acid on sister chromatid exchange induction by styrene and styrene-7,8-oxide. Mutat Res. 1995;348:175–181. doi: 10.1016/0165-7992(95)90006-3. [DOI] [PubMed] [Google Scholar]
- 10.Kullich W, Klein G. Investigations of the influence of nonsteroidal antirheumatic drugs on the rates of sister-chromatid exchange. Mutat Res. 1986;174:131–134. doi: 10.1016/0165-7992(86)90103-x. [DOI] [PubMed] [Google Scholar]
- 11.Philipose B, Singh R, Khan KA, Giri AK. Comparative mutagenic and genotoxic effects of three propionic acid derivatives ibuprofen, ketoprofen and naproxen. Mutat Res. 1997;393:123–131. doi: 10.1016/s1383-5718(97)00095-8. [DOI] [PubMed] [Google Scholar]
- 12.Özkul Y, Erenmemişoğlu A, Ekecik A, Saatci C, Özdamar S, Demirtaş H. Do non-steroidal anti-inflammatory drugs induce sister chromatid exchanges in T lymphocytes? J Int Med Res. 1996;24:84–87. doi: 10.1177/030006059602400110. [DOI] [PubMed] [Google Scholar]
- 13.Sailer HF, Pajarola GF. Oral surgery for the general dentist. New York: Georg Thieme Verlag; 1999. p. 80. [Google Scholar]
- 14.Latt SA. Sister chromatid exchanges, indices of human chromosome damage and repair: detection by fluorescence and induction by mitomycinc. Proc Natl Acad Sci U S A. 1974;71:3162–3166. doi: 10.1073/pnas.71.8.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carrano AV, Thompson LH, Lindl PA, Minkler JL. Sister chromatid exchange as an indicator for mutagenesis. Nature. 1978;271:551–553. doi: 10.1038/271551a0. [DOI] [PubMed] [Google Scholar]
- 16.De Ferrari M, Artuso M, Bonassi S, Bonatti S, Cavalieri Z, Pescatore D, et al. Cytogenetic biomonitoring of an Italian population exposed to pesticides: chromosome aberration and sister-chromatid exchange analysis in peripheral blood lymphocytes. Mutat Res. 1991;260:105–113. doi: 10.1016/0165-1218(91)90086-2. [DOI] [PubMed] [Google Scholar]
- 17.Moorhead PS, Nowell PC, Mellman WJ, Battips DM, Hungerford DA. Chromosome preparations of leukocytes cultured from human peripheral blood. Exp Cell Res. 1960;20:613–616. doi: 10.1016/0014-4827(60)90138-5. [DOI] [PubMed] [Google Scholar]
- 18.Perry P, Wolff S. New Giemsa method for the differential staining of sister chromatids. Nature. 1974;251:156–158. doi: 10.1038/251156a0. [DOI] [PubMed] [Google Scholar]
- 19.Perera FP, Whyatt RM. Biomarkers and molecular epidemiology in mutation/cancer research. Mutat Res. 1994;313:117–129. doi: 10.1016/0165-1161(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 20.Dean BJ, Danford N. Assays for the detection of chemically induced chromosome damage in cultured mammalian cells. In: Venitt S, Parry JM, editors. Mutagenicity testing: A practical approach. USA: Oxford University Press; 1984. pp. 187–232. [Google Scholar]
- 21.Nakanishi Y, Schneider EL. In vivo sister-chromatid exchange: a sensitive measure of DNA damage. Mutat Res. 1979;60:329–337. doi: 10.1016/0027-5107(79)90023-x. [DOI] [PubMed] [Google Scholar]
- 22.Gebhart E. Sister chromatid exchange (SCE) and structural chromosome aberration in mutagenicity testing. Hum Genet. 1981;58:235–254. doi: 10.1007/BF00294917. [DOI] [PubMed] [Google Scholar]
- 23.Carrano AV, Moore DH. The rationale methodology for quantifying sister-chromatid exchanges in humans. In: Heddle JA, editor. New horizons in genetic toxicology. New York: Academic Press; 1982. pp. 267–304. [Google Scholar]
- 24.Rowland RE, Harding KM. Increased sister chromatid exchange in the peripheral blood lymphocytes of young women who smoke cigarettes. Hereditas. 1999;131:143–146. doi: 10.1111/j.1601-5223.1999.00143.x. [DOI] [PubMed] [Google Scholar]
- 25.Barale R, Chelotti L, Davini T, Del Ry S, Andreassi MG, Ballardin M. Sister chromatid exchange and micronucleus frequency in human lymphocytes of 1,650 subjects in an Italian population: II. Contribution of sex, age and lifestyle. Environ Mol Mutagen. 1998;31:228–242. doi: 10.1002/(sici)1098-2280(1998)31:3<228::aid-em4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 26.Carbonell E, Peris F, Xamena N, Creus A, Marcos R. SCE analysis in human lymphocytes of a Spanish control population. Mutat Res. 1995;335:35–46. doi: 10.1016/0165-1161(95)90062-4. [DOI] [PubMed] [Google Scholar]
- 27.Öztürk Ş, Palanduz Ş, Aktan M, Çefle K, Serakinci N, Perkçelen Y. Sister chromatid exchange frequency in B-cells stimulated by TPA in chronic lymphocytic leukemia. Cancer Genet Cytogenet. 2000;123:49–51. doi: 10.1016/s0165-4608(00)00300-9. [DOI] [PubMed] [Google Scholar]
- 28.Palanduz Ş, Sever MŞ, Öztürk Ş, Taşçıoğlu C, Karan MA, Sönmez G, et al. Genotoxic potential of cyclosporine A in patients with renal transplantation. Cell Biol Toxicol. 1999;15:13–17. doi: 10.1023/a:1007594421458. [DOI] [PubMed] [Google Scholar]