Abstract
Purpose
The Behçet's Disease Quality of Life (BD-QoL) is a BD-specific measure developed in the UK. The aim of this study was to adapt the BD-QoL for use in Korea.
Patients and Methods
The translation was based on the guidelines for cross-cultural adaptation. A total of 201 Korean patients with BD participated in this study. To evaluate the psychometric properties, internal consistency and test-retest reliability were used. Factor analysis was performed to examine the construct validity. To provide further evidence for validity, the correlation of BD-QoL with the Clinical Activity Form for Korean Patients with BD (BDCAF-K) and the Center for Epidemiologic Studies-Depression (CES-D) scales was assessed.
Results
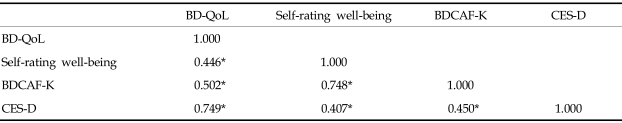
The Korean version had high internal consistency (Cronbach's alpha, 0.93) and test-retest reliability (r = 0.835). Factor analysis of the questionnaire revealed one interpretable factor as a general health-related quality of life factor. The Korean version significantly correlated with scores of CES-D (r = 0.749, p= 0.000), self-rating scale of well-being over the past 28 days (r = 0.446, p= 0.000), and BDCAF-K score (r = 0.502, p = 0.000).
Conclusion
Adaptation of the BD-QoL for use in Korea was successful. Together with the BDCAF-K, it may be a valuable tool for assessing the influence of interventions in BD patients and outcome in clinical trials.
Keywords: Adaptation, Behçet's Disease, quality of life, reliability, validity
INTRODUCTION
Behçet's Disease (BD) is a multisystemic disorder of recurrent chronic inflammation characterized by major symptoms of oral aphthous ulcer, uveitis, skin lesions and genital ulcers.1,2 Although the most common symptom of BD is related to mucosal ulceration, the disorder can affect virtually every organ system and cause various clinical problems, such as arthritis, neurologic impairment, and gastrointestinal symptoms. Because of such complex signs and symptoms, a variety of activity limitations (disability) and restriction of participation in many areas of life (handicap) occur in BD.3
To date, the measurement of outcome in BD has mainly focused on impairment. For assessment of disease activity, the BD Current Activity Form (BDCAF) was developed.4 However, it took no consideration of the wider impact of the condition on the individual's lifestyle.3 Chronic disabling diseases such as BD limit basicdaily activities and affect the QoL of patients. Moreover, choice of treatment modalities might influence patients' lives.5 Therefore, an assessment of the medical status of patients and clinical outcome should include an appropriate measure of QoL.
There have been a few studies of QoL in BD.6-8 These studies investigated the influence of BD on QoL, physical activity, and social relationships of patients as well as psychosomatic illnesses such as depression and anxiety. However, no study with a BD-specific instrument, which is more sensitive to primary interest, has been carried out.
Recently, Gilworth and colleagues3 developed a BD-specific self-reporting questionnaire (The Leeds BD-QoL). It is composed of 30 items (answered true or not true) and each item is scored 0 or 1 (scoring range from 0 to 30). It has shown sufficient reliability and validity for assessing QoL of BD patients. However, a BD-specific QoL measure has not yet been developed in Korea. We thought that the Leeds BD-QoL Measure may be a relevant tool for assessing the influence of BD on QoL. The aim of the present study was to adapt the BD-QoL questionnaire for the use in Korea by testing the reliability and validity of the Korean version.
PATIENTS AND METHODS
Participants
From July 2005 to February 2006, 201 Korean patients with BD who satisfied the International Classification Criteria for BD9 participated in this study. They were recruited from 2 hospitals - 117 patients from the Department of Dermatology, Ajou University Hospital (Suwon, Korea) and 84 from Behçet's Disease Specialty Clinic, Yonsei University Severance Hospital (Seoul, Korea). All questionnaires were filled out by patients. The prevalence of symptoms during the progression of the disease and at the time of measurement of QoL was recorded by 1 physician after reviewing medical records. Ethical approval was granted by the Institutional Review Board of Ajou University Hospital.
Measures
The Leeds Behçet's Disease Quality of Life Measure
Before initiating the present study, permission to use the Leeds Behçet's Disease Quality of Life Measure was granted by the developer. The translation and adaptation processes were carried out at the Department of Dermatology, Ajou University School of Medicine (Suwon, Korea). For the translation process, the guidelines for cross-cultural adaptation10 were used. Stage I was forward translation, in which 2 different translators, independently translated the same version. Stage II was the synthesis of the translations. The "true" and "not true" response format of the original version was transformed to a "yes" and "no" format, which is a more suitable response format in Korean. Stage III was back translations; the synthesized version was back-translated into English by 2 translators. Then, the 2 back-translated questionnaires were posted to the original developer, who replied that 4 of 30 items in the back translations did not express her primary intent. For item 3, she indicated that the concept of the effort involved in getting out seemed to have been lost in translation. Item 5 was originally intended to apply to people both in paid work or staying at home. She pointed out, however, that both translators used the word "work" in English and that readers would usually think of paid work. The translations of item 7 seemed too extreme and she recommended that we make it less extreme by using any alternative word. Finally, she indicated that the concept of "coping" in item 26 seemed to have been lost in translation. The 4 items were processed from stage I-III again. The revised Korean version and 2 back-translations were sent back to the developer and she replied that all 30 items were compatible with her intent. After reviewed by an expert committee, a field test with 30 BD patients was performed to assure that all statements were easily understood. All patients completed the questionnaire within 4 - 6 minutes, and responded that the Korean version was easy to understand. The final version was then ready for further evaluation of reliability and validity.
Self-rating well being
Patients were asked to assess self-perceived well-being on an 11-point scale between 0 and 10 points. Zero points meant "no suffering from the disease" and 10 points meant "I would rather die than suffer from the disease."11
BDCAF-K
The BDCAF-K is a self-reporting instrument developed by Lee et al.11 Scoring of this instrument is based on the history of clinical symptoms in the 4 weeks prior to assessment. Scoring was weighted to 4 clinical features: oral and genital ulcerations, skin lesions, and ocular symptoms. Evaluation of these 4 organ systems accounts for 80% of total score (each organ has 20%). Also, epididymitis, and activities of vascular, gastrointestinal, arthritic, and neurologic symptoms, and other symptoms related with BD have 20% of total score. Scores range from 0 to 100.
Korean Version of CES-D Scale
The CES-D is one of the most widely used self-reporting questionnaires, specifically designed for epidemiological investigations of depressive symptoms in the general population. It is composed of 20 items and depressive symptoms are grouped into 4 degrees of severity (0, 1, 2, or 3) according to the frequency of symptoms over the previous week. Scores range from 0 to 60. The Korean version was validated by Cho and Kim.12 Twenty-one points are the optimal cutoff as a primary screening tool for identifying subjects with depressive symptoms and 25 is optimal for detecting possible cases of major depression in Koreans.12
Data analysis
The data were entered into a computer database and analyzed by SPSS statistical package program (ver. 10.0, SPSS Inc., Chicago, IL, USA). For test of reliability, test-retest reliability and internal consistency were calculated. Test-retest reliability was assessed by comparing instrument scores in 30 patients at 4-week intervals. Scores at each administration were correlated. Pearson's product moment correlation coefficient of at least 0.7 was considered acceptable. Cronbach's α coefficient was computed as a measure of internal consistency. Alpha coefficient of 0.7 or higher was considered acceptable. For construct validity, factor analysis of BD-QoL items, applying principal axis factoring with oblimin rotation was performed to identify the factor structure underlying a patient's response. For determining the number of extracted factors, Scree test was performed. Finally, we also tested for correlation between the Korean version of BD-QoL and other measures. Pearson's correlation coefficients among the scores of the Korean version of BD-QoL, the BD Clinical Activity Form for Korean Patients (BDCAF-K), and the Korean version of Center for Epidemiologic Studies Depression (CES-D) Scale were computed. The t-test was used for comparison of the mean scores of the 4 measurements according to gender.
RESULTS
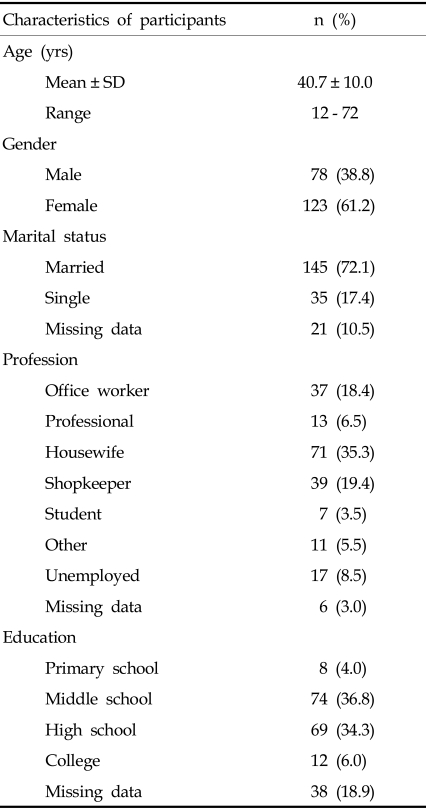
Characteristics of participants
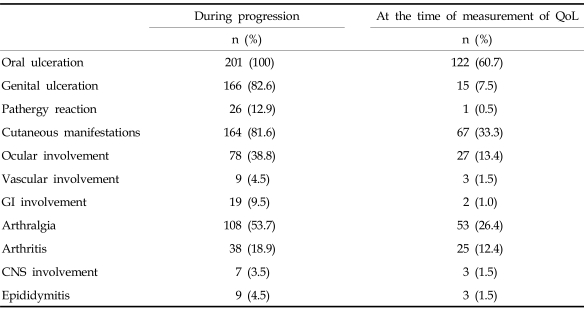
The mean age of 201 patients was 40.7 ± 10.0 (range, 12 - 72 years). Male to female ratio was 1 : 1.58. About 60% of participants were female. Other sociodemographic characteristics of participants are summarized in Table 1. The prevalence of clinical manifestations in participants is summarized in Table 2. All patients had oral ulcerations, and 38.8% had ocular involvement. However, many symptoms were attenuated at the time of measurement, since all patients had been treated.
Table 1.
SociodemographicCharacteristics of Participants
Table 2.
Prevalence of Clinical Manifestations in Participants
GI, gastrointestinal; CNS, central nervous system; QoL, Quality of Life.
Reliability
Test-retest reliability was good with a high correlation between the 2 time points (Pearson's correlation coefficient of 0.835). The internal consistency was excellent with Cronbach's α coefficient of 0.93. Alpha coefficients, if an item was deleted, ranged from 0.92 to 0.932. The item-to-total correlations ranged from 0.34 to 0.68.
Validity
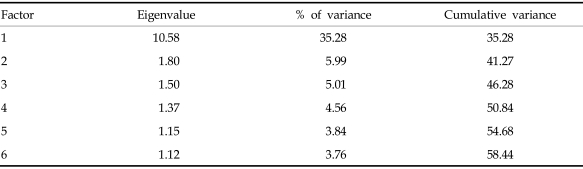
For factor analysis, 5 or 10 cases per item are required.13 The sample size in the present study was 201, exceeding the minimal number of cases for factor analysis of the 30-item BD-QoL. To determine the number of factors, 2 widely used guidelines are the eigenvalue rule and scree test.14 The principal axis factoring with oblimin rotation for the Korean version extracted 6 factors that had eigenvalue greater than 1.0. These factors accounted for 58.4% of the total variance (Table 3). However, the eigenvalue rule often leads to extracting too many factors or overfactoring. Examination of the scree plot provides a reasonably accurate indication of the number of factors.15 The result of the Scree test indicated that only 1 factor was valuable (Fig. 1). Therefore, the 1 factor solution is clearly optimal. The ratio of the first to second eigenvalue was 5.88, which exceeded the strict criterion of a ratio greater than 4.0 for evidence of unidimensionality.13
Table 3.
Number of Extracted Factor by Principal Axis Factoring with Oblimin Rotation
Fig. 1.
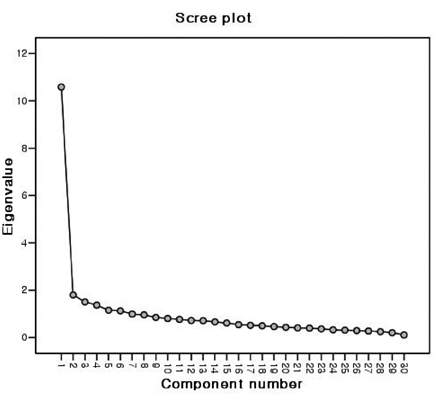
Scree plot of eigenvalues obtained in the factor analysis plotted against their associated components.
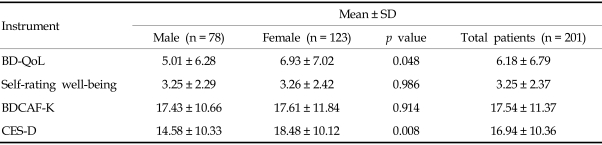
The mean and comparison of mean according to gender of the BD-QoL, self-rating well-being, BDCAF-K, and CES-D scores are described in Table 4. The mean scores of the Korean version of BD-QoL and CES-D were significantly increased in the female group. There were significant correlations between the Korean version of BD-QoL score and self-rating well-being, BDCAF-K score, and CES-D score (Table 5). The Korean version revealed a high correlation with CES-D score, moderate correlation with self-rating well-being and BDCAF-K score, and low correlation with the number of symptoms during progression of the disease.
Table 4.
Mean, Standardization, and Group Differences in BD-QoL, Self-rating Well-being, BDCAF-K, and CES-D Score
BD-QoL, Korean version of the Leeds BD QoL measure; BDCAF-K, Behçet's Disease Clinical Activity Form for Korean Patients; CES-D, Center for Epidemiologic Studies Depression.
Table 5.
Correlations among BD-QoL, Self-rating Well-being, BDCAF-K, and CES-D
BD-QoL, Korean version of the Leeds BD QoL measure; BDCAF-K, Behçet's Disease Clinical Activity Form for Korean Patients; CES-D, Center for Epidemiologic Studies Depression.
*p = 0.000.
DISCUSSION
The purpose of this study was to adapt the Leeds BD-QoL questionnaire for Korean patients with BD by testing the reliability and validity of the Korean version. The results of reliability and validity testing indicated a successful adaptation of the Leeds BD-QoL into the Korean language.
The result of test-retest reliability was good. Pearson's correlation coefficient (0.835) indicated a high correlation between 2 time points. The internal consistency of the Korean version was excellent (Cronbach's α coefficient of 0.93). The results of reliability testing were comparable to those of the original version (0.84 and 0.94, respectively).3
Although 6 factors with eigenvalue greater than 1.0 were extracted, Scree test demonstrated that only 1 factor was valuable. It was interpreted as a general health-related QoL factor. The first factor had an eigenvalue of 10.58 and explained 35.3% of the total valiance in participant's responses. Also, the ratio of the first to second eigenvalue demonstrated that the Korean version of BD-QoL was unidimensional. The original version was based on the Rasch model. One advantage of the Rasch model is unidimensionality. Although the Rasch analysis was not conducted in this study, unidimensionality of the Korean version was confirmed by factor analysis.
When no difference of disease activity was observed between male and female groups, the BD-QoL and CES-D scores were significantly increased in the female group. It is well known that the rate of depression is higher in women than men.16 Also, the Korean version of BD-QoL revealed a high correlation with CES-D score. This result suggests that the BD-QoL well reflects depressive symptoms and mood of patients with BD. However, self-rating well-being score showed a moderate correlation with the Korean version of BD-QoL, and we postulate that patients tend to estimate mainly physical pain and discomfort. Similarly, clinical activity showed a moderate correlation with QoL. As mentioned by Gilworth et al,3 these results suggest that the BD-QoL complements information obtained through the BD clinical activity scale.
Both oral and general QoL are impaired in BD and associated with disease activity and treatment modalities.17 Oral ulceration is the most common clinical manifestation in BD. Therefore, in addition to general QoL, it may be necessary to assess necessarily oral health-related QoL as well. BD is a multisystemic disorder, which can involve virtually every organ system. All affected organs can influence QoL of patients. When used with the BD-QoL questionnaire, the questionnaires about specificorgan involvement such as oral ulcer or ocular involvement, may provide complementary information for evaluating influence of the disease on the life of patients.
In conclusion, the BD-QoL was successfully adapted into the Korean language. This is the first adaptation of the BD-QoL into a non-English language. Together with the BDCAF-K, the Korean version of the BD-QoL may be a valuable tool for assessing the influence of interventions in patients with BD and the outcome in clinical trials. Further studies are required to examine the correlation between the Korean version of BD-QoL and genericinstruments.
References
- 1.Suzuki Kurokawa M, Suzuki N. Behçet's disease. Clin Exp Med. 2004;4:10–20. doi: 10.1007/s10238-004-0033-4. [DOI] [PubMed] [Google Scholar]
- 2.Alpsoy E, Zouboulis CC, Ehrlich GE. Mucocutaneous lesions of Behçet's disease. Yonsei Med J. 2007;48:573–585. doi: 10.3349/ymj.2007.48.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilworth G, Chamberlain MA, Bhakta B, Haskard D, Silman A, Tennant A. Development of the BD-QoL: a quality of life measure specificto Behçet's disease. J Rheumatol. 2004;31:931–937. [PubMed] [Google Scholar]
- 4.Bhakta BB, Brennan P, James TE, Chamberlain MA, Noble BA, Silman AJ. Behçet's disease: evaluation of a new instrument to measure clinical activity. Rheumatology (Oxford) 1999;38:728–733. doi: 10.1093/rheumatology/38.8.728. [DOI] [PubMed] [Google Scholar]
- 5.Emery P, Kosinski M, Li T, Martin M, Williams GR, Becker JC, et al. Treatment of rheumatoid arthritis patients with abatacept and methotrexate significantly improved health-related quality of life. J Rheumatol. 2006;33:681–689. [PubMed] [Google Scholar]
- 6.Blackford S, Finlay AY, Roberts DL. Quality of life in Behçet's syndrome: 335 patients surveyed. Br J Dermatol. 1997;136:293. doi: 10.1111/j.1365-2133.1997.tb14924.x. [DOI] [PubMed] [Google Scholar]
- 7.Bodur H, Borman P, Özdemir Y, Atan Ç, Kural G. Quality of life and life satisfaction in patients with Behçet's disease: relationship with disease activity. Clin Rheumatol. 2006;25:329–333. doi: 10.1007/s10067-005-0046-8. [DOI] [PubMed] [Google Scholar]
- 8.Gur A, Sarac AJ, Burkan YK, Nas K, Cevik R. Arthropathy, quality of life, depression, and anxiety in Behçet's disease: relationship between arthritis and these factors. Clin Rheumatol. 2006;25:524–531. doi: 10.1007/s10067-005-0100-6. [DOI] [PubMed] [Google Scholar]
- 9.International Study Group for Behçet's Disease (ISGBD) Criteria for diagnosis of Behçet's disease. Lancet. 1990;335:1078–1080. [PubMed] [Google Scholar]
- 10.Beaton DE, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine. 2000;25:3186–3191. doi: 10.1097/00007632-200012150-00014. [DOI] [PubMed] [Google Scholar]
- 11.Lee ES, Kim HS, Bang D, Yu HG, Chung H, Shin DH, et al. Development of clinical activity form for Korean patients with Behçet's disease. Adv Exp Med Biol. 2003;528:153–156. doi: 10.1007/0-306-48382-3_31. [DOI] [PubMed] [Google Scholar]
- 12.Cho MJ, Kim KH. Use of the Center for Epidemiologic Studies Depression (CES-D) Scale in Korea. J Nerv Ment Dis. 1998;186:304–310. doi: 10.1097/00005053-199805000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Hubley AM, Russell LB, Palepu A. Injection Drug Use Quality of Life Scale (IDUQOL): a validation study. Health Qual Life Outcomes. 2005;3:43. doi: 10.1186/1477-7525-3-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeVellis RF. Factor analysis. In: DeVellis RF, editor. Scale development: Theory and applications (applied Social Research Methods) 2nd ed. Thousand Oaks: Sage Publications; 2003. pp. 102–137. [Google Scholar]
- 15.Russell DW. In search of underlying dimensions: The use (and abuse) of factor analysis in personality and social psychology bulletin. Pers Soc Psychol Bull. 2002;28:1629–1646. [Google Scholar]
- 16.Weissman MM, Bland R, Joyce PR, Newman S, Wells JE, Wittchen HU. Sex differences in rates of depression: cross-national perspectives. J Affect Disord. 1993;29:77–84. doi: 10.1016/0165-0327(93)90025-f. [DOI] [PubMed] [Google Scholar]
- 17.Mumcu G, Inanc N, Ergun T, Ikiz K, Gunes M, Islek U, et al. Oral health related quality of life is affected by disease activity in Behçet's disease. Oral Dis. 2006;12:145–151. doi: 10.1111/j.1601-0825.2005.01173.x. [DOI] [PubMed] [Google Scholar]