Abstract
Marijuana smoking is associated with a number of abnormal findings in the lungs of habitual smokers. Previous studies revealed that Δ9-tetrahydrocannabinol (THC) caused mitochondrial injury in primary lung epithelial cells and in the cell line, A549 (Sarafian et al., 2003; Sarafian et al., 2005). The role of cannabinoid receptors in this injury was unclear, as was the potential impact on cell function. In order to investigate these questions, A549 cells were engineered to over-express the type 2 cannabinoid receptor (CB2R) using a self-inactivating lentiviral vector. This transduction resulted in a 60-fold increase in CB2R mRNA relative to cells transduced with a control vector. Transduced cell lines were used to study the effects of THC on chemotactic activity and mitochondrial function. Chemotaxis in response to a 10 % serum gradient was suppressed in a concentration-dependent manner by exposure to THC. CB2R-transduced cells exhibited less intrinsic chemotactic activity (p < 0.05) and were 80- to 100-fold more sensitive to the inhibitory effects of THC. Studies using SR144528, a selective CB2R antagonist, verified that these effects were mediated by the CB2R. Marijuana smoke extract, but not smoke extracts from tobacco or placebo marijuana cigarettes, reproduced these effects (p < 0.05). THC decreased ATP level and mitochondrial membrane potential (Ψm) in both control and CB2R-transduced cells. However, these decreases did not play a significant role in chemotaxis inhibition since cyclosporine A, which protected against ATP loss, did not increase cell migration. Moreover, CB2R-transduced cells displayed higher Ψm than did control cells. Since both Ψm and chemotaxis are regulated by intracellular signaling, we investigated the effects of THC on the activation of multiple signaling pathways. Serum exposure activated several signaling events of which phosphorylation of IκB-α and JNK were regulated in a CB2R-and THC-dependent manner. We conclude that airway epithelial cells are sensitive to both CB2R-dependent and independent effects mediated by THC.
Keywords: Tetrahydrocannabinol (THC), Cannabinoid receptor, Chemotaxis, Mitochondria, Lentiviral vector, MAPK signaling
INTRODUCTION
Marijuana is one of the most frequently used illicit substances in the U.S. with approximately 5 % of high school seniors reporting that they smoke marijuana daily (Johnston, 2006) . In addition, 11 states have legalized the use of marijuana for medicinal purposes, which may further increase its use as the medicinal applications of cannabinoids continue to be developed and refined (Klein, 2005; Marx, 2006; Seamon et al., 2007). However, there is still little information about the biologic consequences of marijuana smoke on the lung and the mechanisms that mediate them. Marijuana smoke contains a large number of toxic compounds in amounts similar to that of tobacco smoke, as well as a variety of cannabinoids (Novotny et al., 1982; Moir et al., 2007). Δ9-tetrahydrocannabinol (THC) is the predominant cannabinoid and may constitute 20 % or more of the tar phase of marijuana smoke (Roth et al., 2001). Cannabinoids can exert a wide range of effects mediated through their interaction with conventional type 1 and type 2 cannabinoid receptors (CB1R and CB2R); recently described alternative receptors such as the aryl-hydrocarbon receptor, and vanilloid receptors; and through so-called receptor-independent mechanisms (Adams and Martin, 1996; Roth et al., 2001; Maccarrone, 2006; Pacher et al., 2006). The expression of CB1R and CB2R vary widely between different tissues and cells, but the lack of monoclonal antibody reagents has hindered a detailed description of functional protein expression. While a variety of cannabinoid receptor agonists and antagonists are available, most of the studies defining their activity have focused on binding and/or signaling, rather than on function, and have been carried out with rodent or other non-human cells (Pertwee, 2006). The specificity and potency of these reagents remain to be defined when studied in other settings. All of these factors have complicated attempts to understand the acute and long-term effects of marijuana use on the lung.
The epithelial surface of the lung may be particularly vulnerable to injury from inhalation of marijuana smoke as it is directly exposed to the highest concentrations of cannabinoids and other smoke toxins. In a small animal smoke inhalation model we recently demonstrated that cannabinoid levels are 5- to 10-fold higher in lung tissue as compared to the levels measured in blood (Sarafian et al., 2006). Habitual marijuana smokers frequently complain of chronic cough and sputum production and their airways appear grossly-inflamed and edematous (Roth et al., 1998; Aldington et al., 2007). Epithelial biopsies demonstrate goblet cell hyperplasia, squamous metaplasia, cellular atypia and disorganization, and molecular dysregulation (Barsky et al., 1998). Given these changes, there has been a significant concern that marijuana smoking predisposes to lung, head, and neck cancer. However, there have been conflicting reports of cancer risk associated with marijuana smoking and further controlled large-scale studies are needed to characterize this relationship (Sidney et al., 1997; Zhang et al., 1999; Hashibe et al., 2005; Aldington et al., 2007).
We have shown previously that THC and marijuana smoke extract compromise cell energetics in lung epithelial cells in vitro (Sarafian et al., 2003; Sarafian et al., 2005). In both primary human lung epithelial cells and the lung tumor cell line, A549, mitochondrial membrane potential (Ψm) is diminished along with cellular ATP level. We have also performed in vivo smoke inhalation studies with rats exposed to marijuana smoke and found reduced mitochondrial-pattern staining using the potentiometric probe, JC-1, suggesting that marijuana smoke may impair epithelial cell energetics in humans (Sarafian et al., 2006). These events do not occur upon exposure to tobacco smoke suggesting that marijuana smokers may be at risk of physiological impairment distinct from that of tobacco smokers. One potential adverse consequence of energy depletion in the epithelium could be the impairment of cell migration. Cell migration, measured by chemotaxis assays, is fundamental to the process of airway remodeling and wound repair (Wang et al., 2001; Christensen et al., 2004). It is likely that cell migration is dependent on mitochondrial ATP-dependent mechanisms (Phillips et al., 2005; Wu et al., 2005; Campello et al., 2006; Chen et al., 2006).
In the present studies, we have taken a novel approach to study the action of marijuana smoke and purified THC on the chemotactic activity of pulmonary epithelial cells. With the aide of self-inactivating lentiviral vectors that express either a marker gene (green fluorescent protein; GFP) alone or in combination with the human CB2R gene, we constructed a pair of cell lines based on a human lung epithelial tumor cell line, A549. A549 cells have been extensively characterized in our laboratory, display many properties of epithelial cells and are readily amenable to stable gene transduction. This genetic manipulation resulted in a high level of constitutive overexpression of CB2R mRNA and heightened the responsiveness of these cells to some of the effects of marijuana smoke extract and THC, but not to others, allowing us to define the pathways involved in the regulatory effects of marijuana smoke on chemotaxis.
METHODS
Materials and Reagents
Corning Transwell permeable insert supports (8.0 µm pore) and HEMA 3 Fixative were purchased from Fisher Scientific (Kalamazoo, MI). The A549 cell line was obtained from American Type Culture Collection (CCL-185, Bethesda, MD). JC-1 was purchased from Molecular Probes (Eugene, OR). Marijuana cigarettes (2.9 % THC), placebo marijuana cigarettes (0 %), THC, and SR144528 were obtained from National Institute on Drug Abuse (NIDA, Bethesda, MD). Marlboro Red 100’s cigarettes were purchased commercially (Phillip Morris Inc., Richmond, VA). Diff-Quick Solutions I and II were obtained from Dade Behring Inc., (Newark, DE). Cyclosporin A, oligomycin, FCCP, and soybean trypsin inhibitor were purchased from Sigma-Aldrich (St. Louis, MO). Cell Titer-Glo Luminescent Viability Assay was purchased from Promega (Madison, WI).
Preparation of THC and Smoke Extracts
Stock THC (50 mg/ml in ethanol) was serially diluted in ethanol and then RPMI-1 % BSA so that the final concentration of ethanol was 0.2 %. Control media was prepared with the same concentrations of ethanol but lacked THC. Cigarette smoke extracts were prepared from marijuana (3 % THC), placebo marijuana (0 % THC), and tobacco (Marlboro Red) cigarettes as previously described (Sarafian et al., 2001). In brief, lit cigarettes were placed into holders and manually puffed through Tygon tubing connected to a 3-way stop-cock and syringe. Smoke particulates were captured using an in-line Cambridge filter (Performance Systematic, Caledonia, MI) and then extracted by drop-wise application of 2 ml DMSO followed by centrifugation at 2000 rpm for 4 min. Tar concentrations in these extracts were normalized by measuring absorbance at 400 nm using appropriate standard curves as described previously (Sarafian et al., 2001).
Preparation and Characterization of Genetically Modified A549 Cell Lines
A plasmid expressing full-length human CB2R cDNA (pCB2R; Gene ACC#AY242132) was obtained from the University of Missouri-Rolla (UMRcDNA Resource Center, Rolla, MO). A bi-cistronic lentiviral expression plasmid containing both the CB2R and GFP genes was produced according to established techniques by introducing the human CB2R cDNA into the pRRL-sin.-cPPT-hCMV-MCS-IRES-GFP-pre backbone so that the bicistronic mRNA transcribed by a CMV promoter was translated through a cap-dependent (CB2R) and internal initiation (GFP) mechanisms (Stripecke et al., 2003). Large-scale stocks of the final LV/CB2-GFP vector were produced by co-transfecting 293T cells with the expression plasmid, 2 packaging plasmids (to provide gag, pol and rev in trans) and a capsid-encoding plasmid (to provide a VSV-G capsid in trans). The final self-inactivating lentivirus (LV/CB2R-GFP) was harvested from cell supernatants, concentrated by ultracentrifugation, and titered by standard p24 ELISA (Stripecke et al., 2003). The control vector, expressing only GFP (LV/GFP), was prepared in a similar manner using the pRRL-sin.-cPPT-hCMV-GFP gene transfer plasmid during the co-transfection step. Both vectors were adjusted to approximately 5 µg/ml p24 equivalents (roughly 1×108 infective viral particles/ml), aliquoted and cryopreserved. To produce the genetically modified A549/CB2R-GFP and A549/GFP cell lines, adherent A549 cells were transduced with 1 µg/ml p24 equivalents of the LV/CB2R-GFP or LV/GFP vectors, respectively, in RPMI-1640 supplemented with 10 % fetal bovine serum (FBS) and antibiotics for 24 hr at 37°C in a CO2 incubator. Cells were then washed, expanded in fresh media for 3 passages and analyzed by flow cytometry (FACScalibur cytometer, Becton Dickinson, San Jose, CA) for their level of GFP expression (FCS Express V3, De Novo Software, Ontario, Canada). Cell sorting (FACSVantage SE cell sorter, Becton Dikinson) was used to select for transduced cells with similar levels of GFP and the A549/CB2R-GFP cells repeatedly transduced with the LV/CB2R-GFP vector until the level of GFP expression matched that of the A549/GFP line. Comparable GFP levels precluded variable fluorescence interference in studies using fluorescent mitochondrial probes. Matching GFP levels allowed the use of fluorescent mitochondrial probes without having to consider fluorescence interference as a significant factor.
Quantitative real-time QT-PCR was performed to simultaneously assess the level of CB2R and β-actin gene expression using an ABI Prism770 multidetector cycler (PE Applied Biosystems, FosterCity, CA) and Clonetech Qzyme Custom Duplex assay. The primer sequence for CB2R was forward: ATTGGCAGCGTGACTATGACC; reverse: CGGGTGAGGAGAGCTTTG, and for β-Actin; forward: GTCCCTTGCCATCCTAAA reverse: ACGAAGGCTCATCATTCAA. PCR was performed with 1 cycle at 95°C for 3 min followed by 45 cycles of 95°C, 15 sec and 56°C 1 min.
Chemotaxis Assays
Chemotaxis assays were performed with A549, A549/GFP, or A549/CB2R-GFP cells that had been split and sub-cultured within 3 days and grown to < 90 % confluence. Cells were washed twice with PBS, serum-starved 1 hr in a CO2 incubator in RPMI containing 1 % BSA, and then dissociated with 0.1 % trypsin and 0.2 mg/ml EDTA. Cells were recovered and immediately diluted in RPMI-1 % BSA containing 0.04 mg/ml soybean trypsin inhibitor. After washing and resuspension in RPMI-1 % BSA, cells were adjusted to 6.6 × 105/ml with media containing THC, smoke extracts or other reagents for 20 min as indicated for the given assay. 150 µl of the treated cell suspension was then applied to Corning Transwell culture inserts containing 8 µm pores that had been precoated overnight with 5 µg/ml fibronectin and dried for 20 min. Inserts that had been loaded with cells were placed into 24-well plates containing 0.6 ml RPMI and 10 % FBS in the bottom wells. Transwell cultures were incubated undisturbed for 6 hrs, at which time inserts were removed and cells on the upper surface removed by scraping for 1 min with cotton-covered Q-tips. Cells on the lower surface were fixed in methanol for 5 min, followed by HEMA3 fixative, and then stained with Diff-Quick according to the manufacturer’s protocol. Ten microscope fields were counted and the sum total of migrated cells reported as the measure of chemotaxis.
Mitochondrial Membrane Potential and Cellular ATP Assay
The fluorescent probe JC-1 (5,5’6,6’-tetrachloro-1,1’,3,3’-tetraethylbenzimidazolyl-carbocyanine iodide) was used to measure Ψm. JC-1 produces green fluorescence in the cytoplasm and red-orange fluorescence when concentrated in actively-respiring mitochondria that maintain a negative internal potential. A549/GFP and A549/CB2R-GFP cells were cultured for 48 hrs in 96-well plates at 1 × 105 cells/well. Media was then removed and cells exposed to media containing the test reagents and pre-warmed JC-1 solution (1 µg/ml) in a final volume of 100 µl. A Cytofluor 2300 fluorescence plate reader (PerSeptive Biosystems, Framingham, MA) was used to take fluorescence measurements at 2, 30, and 60 min of culture using settings of 530 excitation (Ex)/590 emission (Em) for red fluorescence and 485 Ex/530 Em for green fluorescence. Plates were incubated in a 37° C CO2 incubator between measurements. Red/green fluorescence ratios were calculated after subtracting respective background fluorescence values from a well containing JC-1 but no cells.
ATP levels in the A549/GFP and A549/CB2R-GFP cells that had been treated with control media or test reagents for 6 hrs at 37°C in a CO2 incubator were measured using the Promega Cell Titre-Glo Luminescent assay kit according to the manufacturer’s directions. After being treated with buffer and Cell Titre-Glo suspension for 15 min at room temperature, chemoluminescence was measured and ATP values determined from an ATP standard curve.
Assay for Cell Signaling Pathways
A549/GFP and A549/CB2R-GFP cells were cultured in 24-well plates at a seeding density of 105 cells/well. Two days after plating, cells were washed two times with PBS and incubated in 0.5 % BSA in RPMI 1640 for 1 hr at 37° C in 5 % CO2. Following this serum starvation, cells were re-exposed to 10 % FBS in RPMI 1640 with or without 1 µg/ml THC at 37° C in 5 % CO2 for 0, 2, 5, 10, 30, or 60 min. Cells were washed once with cold TBS, lysed, and analyzed for multipathway signaling activities by measuring phosphorylated forms of CREB, Erk/MAP kinase, IκB-α, JNK, p38, p70 S6 kinase, STAT3, and STAT5 using a Beadlyte 8-plex signaling kit (Millipore, Danvers, MA) according to the manufacturer’s directions. Lysates were centrifuged at 8,000 g for 3 min to remove insoluble material. Samples were analyzed using a Luminex 100 fluorescence analyzer. A mixture of HeLa cell lysates prepared from cells stimulated with 50 ng/ml EGF, 100 ng/ml TNF-α, or heat shock followed by 200 nM arsenite was used as a positive control for detection of all 8 signaling pathways.
Statistical Analysis
Data were analyzed using one- or two-way Analysis of Variance (ANOVA) . Data were checked for conformation to model assumptions. Based on density histograms, log transformations for cell counts were done to fit model assumptions. For clarity, the figures are presented in the native scale. Individual comparisons were made using t-tests with a Bonferroni correction for multiple comparisons. In select experiments comparisons were made using a paired t-test. p < 0.05 was considered significant. Statistical analyses were performed using SAS version 9.1 (SAS Cary, NC).
RESULTS
In previous studies using lung epithelial cells we observed that THC disrupted mitochondrial membrane potential and lowered ATP levels, an effect also observed with marijuana smoke extract but not with tobacco or placebo marijuana smoke extract (Sarafian et al., 2003; Sarafian et al., 2005). Among the questions raised in these studies was the role of cannabinoid receptors in mediating the effects on cell energetics. In the present study we sought to produce an epithelial cell model which would allow characterization of the impact of CB2 receptor activation on cell function and which could also be tested for the role of changes in cell energetics as the mechanism underlying the modulation of cell function.
Characterization of the A549/GFP and A549/CB2R-GFP Cell Lines
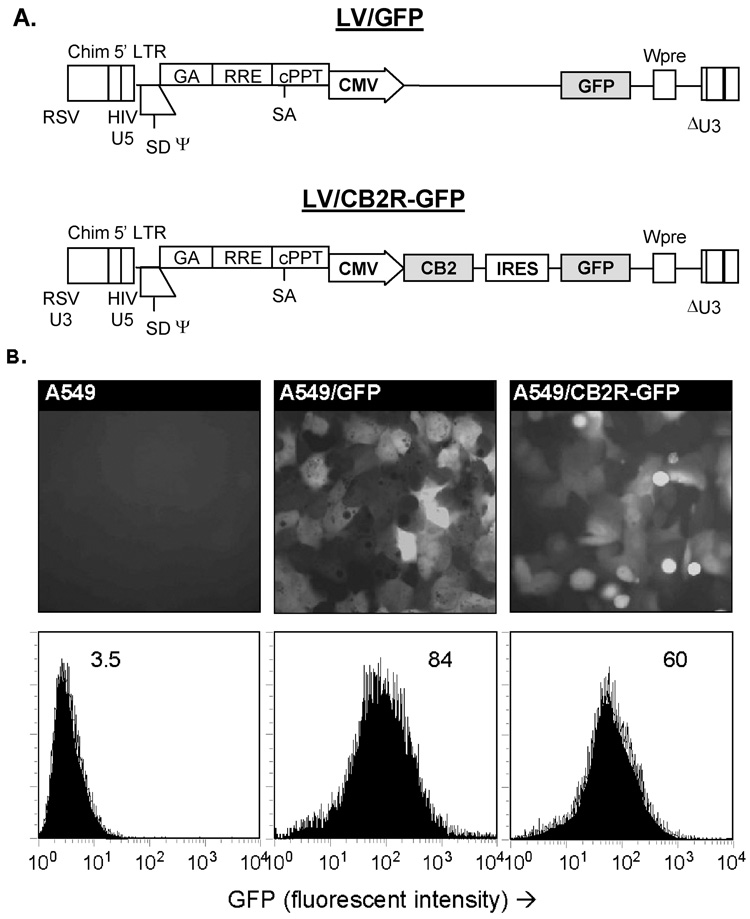
Self-inactivating lentiviral vectors expressing GFP alone (LV/GFP) or in combination with the CB2R (LV/CB2R-GFP) were developed as an efficient means for producing cell lines with matching expression of the GFP marker gene but different expression of the CB2R (Figure 1A). Transgenes expressed by these vectors were integrated into the host DNA to produce stable expression without production of viral gene products. Expression of GFP as the marker gene allowed rapid identification and quantification of the transduced cells by both fluorescent microscopy and standard flow cytometry (Figure 1B). While GFP levels were relatively well-matched, quantitative real-time RT-PCR performed on the cell lines at multiple times demonstrated 60-fold higher levels of CB2R mRNA expression in A549/CB2R-GFP cells compared to A549/GFP cells.
Figure 1.
A) Maps of the lentiviral vector constructs encoding for GFP alone (LV/GFP) or in combination with human CB2R (LV/CB2R-GFP). For the bicistronic LV/CB2R-GFP construct, CB2R expression was under the control of the CMV promoter while GFP expression was directed by an internal ribosome entry site (IRES). B) Stable cell lines A549/GFP and A549/CB2R-GFP were derived from the human A549 airway epithelial cell tumor line by infecting cells with the corresponding lentiviral vectors, followed by fluorescent cell sorting and additional transfection as needed to produce cells with similar levels of GFP expression. GFP expression of parental and transduced A549 lines were assessed by fluorescence microscopy (top row) and flow cytometry (bottom row, values indicate geometric mean fluorescent intensity). Representative experiment.
Over-expression of the CB2R Suppresses Intrinsic Chemotactic Activity
A standard chemotaxis assay was established using A549 cells and a 10 % FBS gradient as the chemotactic stimulus (Di Luozzo et al., 2005; Phillips et al., 2005). In preliminary assays we confirmed that A549 cells migrated through fibronectin-coated Transwell membranes in response to the serum gradient, but not in response to serum-free medium or when serum-containing medium was added to both sides of the Transwell (data not shown).
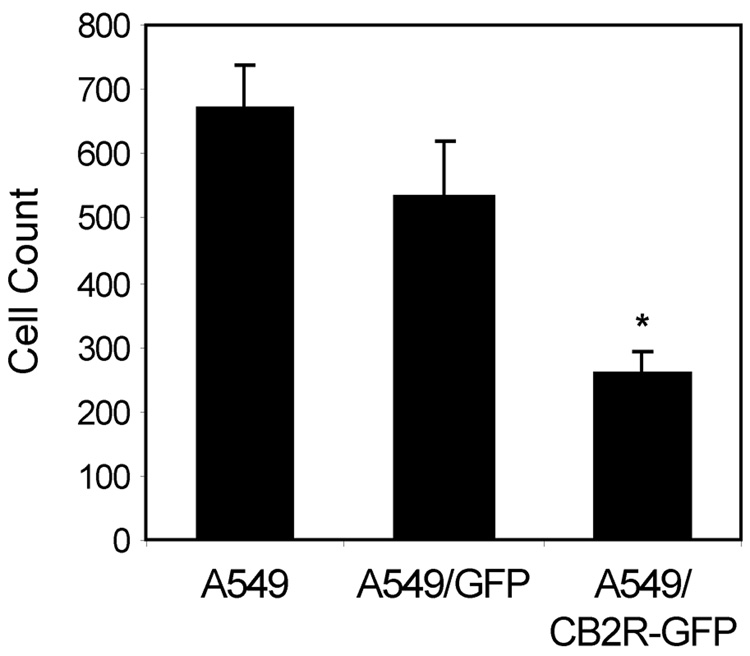
Prior to assessing the effects of THC on chemotaxis, baseline chemotactic activity of the A549/GFP and A549/CB2-GFP cell lines was assessed. There was no significant difference in the chemotactic activity of A549/GFP cells when compared to parental A549 cells (552 ± 87 vs. 667 ± 70 cells/10 HPF, respectively) (Figure 2). However, in side-by-side comparisons, the chemotactic activity of A549/CB2R-GFP cells was always significantly less than that of A549/GFP cells, with over-expression of the CB2R resulting in a 62 % reduction in the number of migrating cells (256 ± 39 versus 552 ± 87 cells/10 HPF, p < 0.05).
Figure 2.
Baseline migration of A549/CB2R-GFP cells is attenuated when compared to A549/GFP and parental A549 cells. Cell lines were plated onto the apical surface of fibronectin-coated Transwell inserts (8 µm pores) and 10 % FBS added to the lower wells as the chemotactic stimulus. Cells were incubated 6 hrs and, following wiping and removal of cells on the apical surface, transwell membranes were fixed, stained, and counted for cells present on the basal surface. Chemotaxis values represent number of cells counted per 10 high-power fields (200x). Mean ± SEM from 15–18 experiments. * There was a significant difference between the groups (p < 0.0001) as assessed by ANOVA. Post-hoc tests showed that CB2R was significantly lower than A549 or GFP cells using t-tests with a Bonferroni multiple comparisons adjustment. p < 0.02 for both comparisons.
Increased Sensitivity of A549/CB2R-GFP Cells to the Suppressive Effects of THC
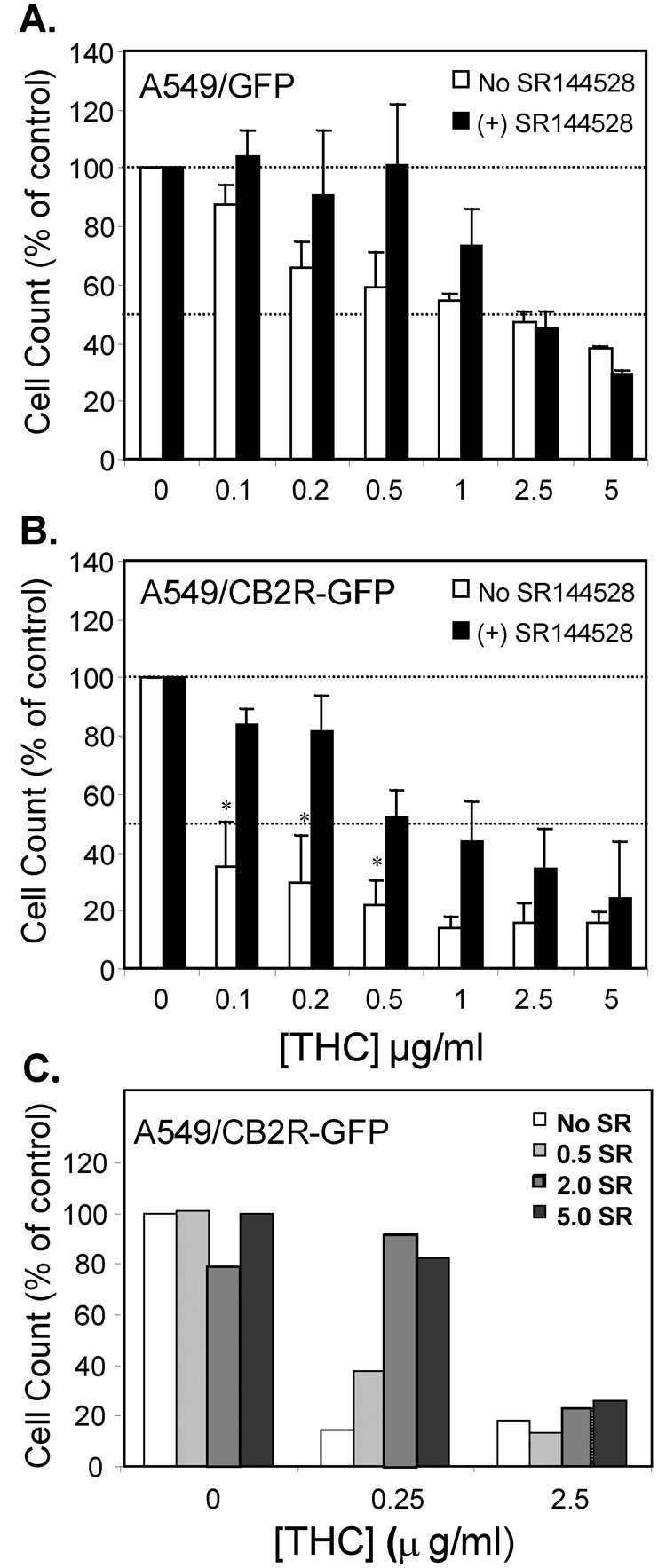
Dose-response studies were carried out to examine the relative effects of THC on the chemotactic activity of A549/GFP and A549/CB2R-GFP cells (Figure 3). The IC50 for THC was calculated by regression analysis to be ~ 1.8 µg/ml when measured using the A549/GFP cells (Figure 3a). In contrast, increased expression of the CB2R by A549/CB2R-GFP cells was associated with a leftward shift in the THC dose-response curve (Figure 3b). With a calculated IC50 of ~ 0.02 µg/ml THC, A549/CB2R-GFP cells were 80- to 100-fold more sensitive to the suppressive effects of THC. A checkerboard analysis with THC added to either the apical, basal, or both sides of the chemotaxis chambers was carried out to assess whether THC was acting as an independent chemotactic agent or as an inhibitor of chemotaxis. THC inhibited serum-induced chemotaxis only when added to the apical or both sides of the transwell and had no effect when only added to the basilar compartment, confirming that its suppressive effects required direct cell contact and were independent of a THC gradient (results not shown). Given all of these results, we hypothesized that the effects of THC on chemotaxis were mediated through activation of CB2R. SR144528, described as a selective CB2R antagonist with a 100- fold higher affinity for the CB2R compared with THC, was therefore evaluated for its ability to block the effects of THC. When A549/GFP cells were pre-treated with 1 µM SR144528, it blocked the suppressive effects of THC on chemotaxis only with low THC concentrations, with little if any effect at THC concentrations > 0.5 µg/ml (IC50 ~ 1.5 µM). However, the antagonistic effects of SR144528 were much more obvious and convincing when tested in combination with A549/CB2R-GFP relative to A549/GFP cells resulting in significant restoration of chemotaxis with 0.1, 0.2, and 0.5 µg/ml THC. As with A549/GFP cells, a progressive loss of antagonist activity was observed with THC concentrations ≥ 0.5 µg/ml for both cell types (Figure 3b). Two possible explanations may account for this loss of potency; either the antagonistic effect of SR144528 in this biologic assay was much less than anticipated based on its known binding affinity for the CB2R, or a second pathway (other than the CB2R) mediated the effects of THC at higher concentrations. We therefore pre-treated A549/CB2R-GFP cells with different concentrations of SR144528 (0 to 5.0 µM) prior to testing the effect of 0, 0.25, or 2.5 µg/ml THC ( 0, 0.8, 8.0 µM) on chemotactic activity (Figure 3c). With 0.25 µg/ml THC there was a clear dose-response to SR144528 suggesting that it’s concentration was the limiting factor in abrogating the actions of THC. However, with 2.5 µg/ml THC, SR144528 had no effect.
Figure 3.
THC induces a concentration-dependent suppression of chemotactic activity in A549/GFP cells (A) which is enhanced in A549/CB2R-GFP cells (B) and sensitive to antagonism by 1 µM of the CB2R-selective antagonist SR144528 at THC concentrations < 0.5 µg/ml. Control conditions were carried out with adjusted amounts of ethanol as diluents in the absence of cannabinoids. Results are presented as a percentage of the chemotactic activity measured in the control conditions (0 THC) for each experiment. Values represent means of 4–9 experiments ± SEM. Control values in panel A were 485 ± 106 and 507 ± 147 for SR144528-unteated and –treated cells, respectively, and in panel B were 263 ± 51 and 353 ± 105, respectively. C) Suppression of chemotactic activity by THC was antagonized by pre-treatment with SR144528 (SR), with the lower concentration of THC but not with a higher concentration of THC. SR144528 concentrations shown are in µM units. Results are presented as a percentage of the chemotactic activity measured in the control condition (i.e. no THC/ no SR144528). Representative experiment demonstrating mean values of replicate wells.
Data for A and B were log-transformed and analyzed by two-way ANOVA. Post-hoc individual comparisons were done using t-tests with a Bonferroni correction for multiple comparisons. In A, there was a significant concentration effect (p < 0.001), a SR144528 group effect (p = 0.017) but a non-significant interaction between the SR144528 group and THC concentration (p = 0.13). In B, there was a significant THC concentration effect (p < 0.001), a significant SR144528 group effect (p = 0.002) and a significant SR144528 group and THC concentration interaction. (p = 0.031). On average, the p values for group differences between A549/GFP and A549/CB2R-GFP cells were < 0.0001 and 0.0168 for SR144528-treated and –untreated cells, respectively. We then used a post-hoc t-test with Bonferroni correction for multiple comparisons to analyze differences between SR144528-treated and –untreated cells for THC concentrations of 0.1, 0.2, and 0.5 mg/ml. All these p values were > 0.05 in panel A and < 0.05 in panel B.
Marijuana Smoke Extract Suppresses the Chemotactic Activity of A549 Cells
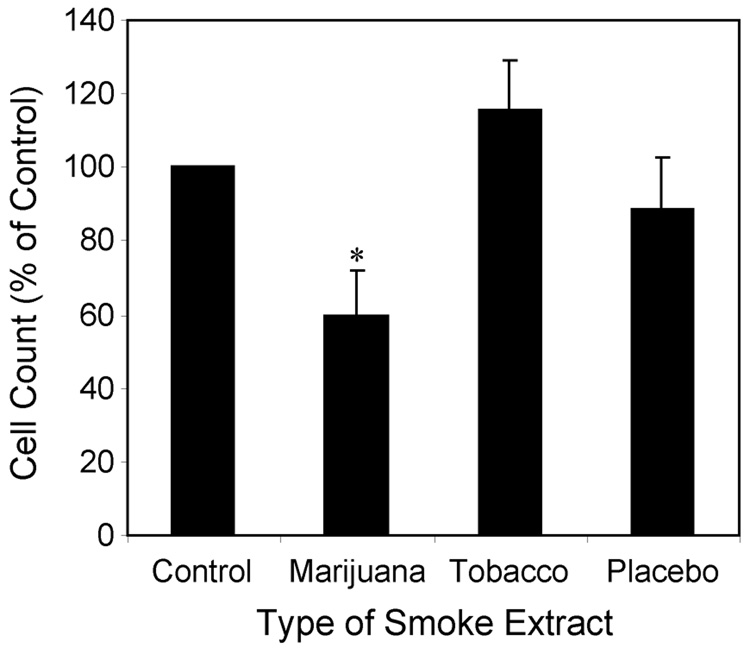
To determine if the THC contained in marijuana smoke can also suppress chemotaxis, smoke extracts were prepared from marijuana cigarettes (3% THC) as well as from tobacco and placebo marijuana cigarettes (0% THC). The particulate phase of each type of smoke was captured using a Cambridge filter and then eluted using DMSO. This approach trapped THC, which is contained in the particulate phase of the smoke generated from marijuana cigarettes, while allowing gaseous components of the smoke to be eliminated. The effects of these different cigarette smoke extracts on chemotaxis were compared using medium containing the DMSO diluent alone as control. At equivalent concentrations of tar (15 µg/ml) only particulate extracts prepared from marijuana smoke significantly and reproducibly suppressed chemotactic activity as demonstrated in Figure 4 (41% suppression, p < 0.05). Given that THC is the primary component of marijuana tar that is missing from the other types of smoke extracts, we hypothesized that THC was the active agent modulating chemotactic activity.
Figure 4.
Marijuana smoke extract suppresses the chemotactic activity of A549 cells. Smoke extracts prepared from the particulate phase of marijuana, placebo marijuana or tobacco cigarette smoke were added to A549 cells at a final concentration of 15 µg of tar/ml 5 min prior to initiating the chemotaxis assay. A549 cells in serum-free medium were plated onto the apical surface of fibronectin-coated Transwell inserts (8 µm pores) and 10 % FBS added to the lower wells as the chemotactic stimulus. Control cells received diluted DMSO in the absence of tar extracts. Cells were incubated 6 hrs, Transwell membranes fixed and stained, and chemotaxis determined by summing the number of migrated cells counted from ten microscope fields (200x). Results are normalized as a percent of chemotactic activity in control wells containing diluent alone. Mean ± SEM from 4 experiments. Control value was 425 ± 58. Group differences were assessed using ANOVA (p < 0.0001). *p < 0.025 compared with control, tobacco, or placebo using t-test and a Bonferroni correction for multiple comparisons.
Role of Mitochondrial Injury in THC-Induced Chemotaxis Inhibition
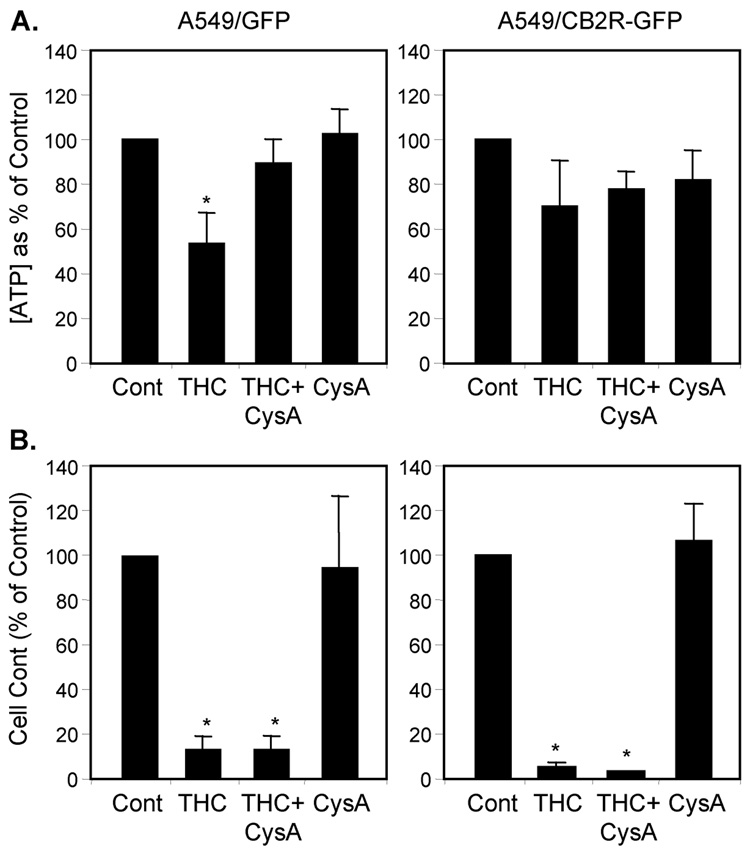
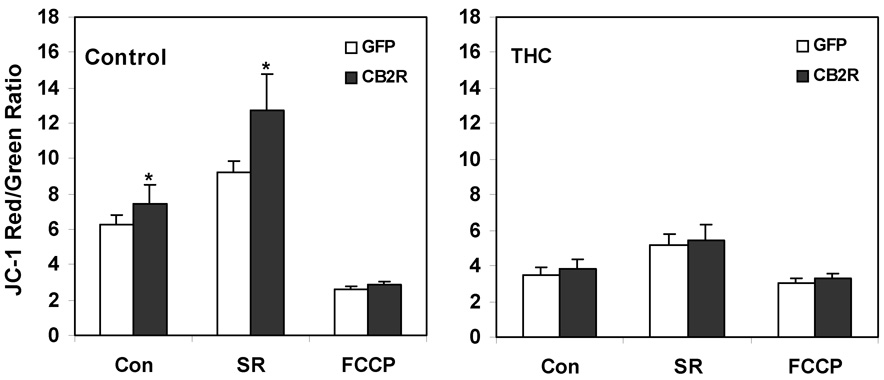
The pattern of selective response to marijuana smoke extract relative to tobacco or placebo extracts was similar to the pattern observed previously for loss of Ψm in A549 cells and in an in vivo smoke inhalation model (Sarafian et al., 2003; Sarafian et al., 2006). This similarity raised the possibility of a causal relationship between THC-mediated disruption of mitochondrial energetics and its effects on chemotaxis. Comparative studies with the A549/GFP and A549/CB2R-GFP cell lines allowed us to directly assess this relationship as well as the role of CB2R expression in regulating mitochondrial function. THC, at a concentration of 10 µg/ml, decreased ATP levels significantly (by 50 %, p < 0.05) in A549/GFP cells, but to a lesser degree (30 % inhibition) in A549/CB2R-GFP cells (Figure 5A). These effects on cellular energetics coincided with inhibition of chemotaxis by 90 % and 95 %, respectively (Figure 5B). Pretreatment with cyclosporin A, which blocks the mitochondrial permeability transition, protected against THC-induced ATP loss, but had no effect on THC-induced inhibition of chemotaxis. Furthermore, over-expression of the CB2R in A549/CB2R-GFP cells was associated with a significant baseline increase in Ψm as measured by the potentiometric dye, JC-1 (Figure 6A). Pretreatment with SR144528 enhanced Ψm even further and did so to a greater extent in A549/CB2R-GFP than A549/GFP cells (70% increase, p < 0.001, data not shown). The mitochondrial electron transport uncoupler, FCCP, lowered Ψm by 58 and 77 %, respectively, for A549/GFP and A549/CB2R-GFP cells. THC treatment (2.5 µg/ml) lowered Ψm in both cell types and eliminated differences between the cell types (Figure 6B). SR144528 did not protect against the THC-induced decrease in Ψm.
Figure 5.
THC mediates effects on cell energetics that are independent of the effects of THC on chemotaxis. A549/GFP and A549/CB2R-GFP cells were treated with THC alone, Cyclosporin A (CysA) alone, or both in combination and examined for the effects on their generation of ATP (A) and on chemotaxis (B). Cyclosporin A (3 µM) was added to cells alone or 5 min prior to exposing the cells to THC (10 µg/ml). Cells were evaluated 6 hrs later for intracellular ATP levels, using the Promega Cell Titre-Glo Luminescent assay kit according to the manufacturer’s directions, or for cell migration across fibronectin-coated Transwell inserts (8 µm pores) in response to a 10 % FBS chemotactic stimulus. Values from each experiment were expressed as the percent of control (Cont) and represent means of 3–5 experiments ± SEM. Control values for chemotaxis were 540 migrated cells/10 HPF for A549/GFP cells and 176 cells/10 HPF for A549/CB2R-GFP cells. Control ATP levels were 181 pmole/ µg protein for A549/GFP cells and 159 pmole/ µg protein for A549/CB2R-GFP cells. Group differences were assessed using ANOVA (p < 0.0001). * p < 0.02 compared with control using t-tests and adjusting for multiple comparisons using a Bonferroni correction.
Figure 6.
Mitochondrial membrane potential changes in A549/GFP and A549/CB2R-GFP cells in response to mitochondrial regulators and a CB2R antagonist. A549 cell lines were cultured in 96-well plates at 5 × 104 cells/well for 1–2 days prior to treatment with either diluent (untreated) or THC (2.5 µg/ml) and then exposed to SR144528 (SR, 1 µM); or FCCP (25 μM).. Cells were then labeled with JC-1 (1 µg/ml) and red (ex = 530 nm, em = 590 nm) and green (ex = 485 nm, em = 525 nm) fluorescence measured using a Cytofluor 2300 fluorescence plate reader after a 1 hr incubation at 37° C in a CO2 incubator. Red/Green fluorescence ratios were determined following subtraction of respective background values from a well that contained JC-1 but lacked cells. Values represent means of 12 determinations ± SEM. * p < 0.05 comparing GFP with respective CB2R cells using paired t-tests.
THC Alters Intracellular Signaling Patterns in a CB2R-dependent Manner
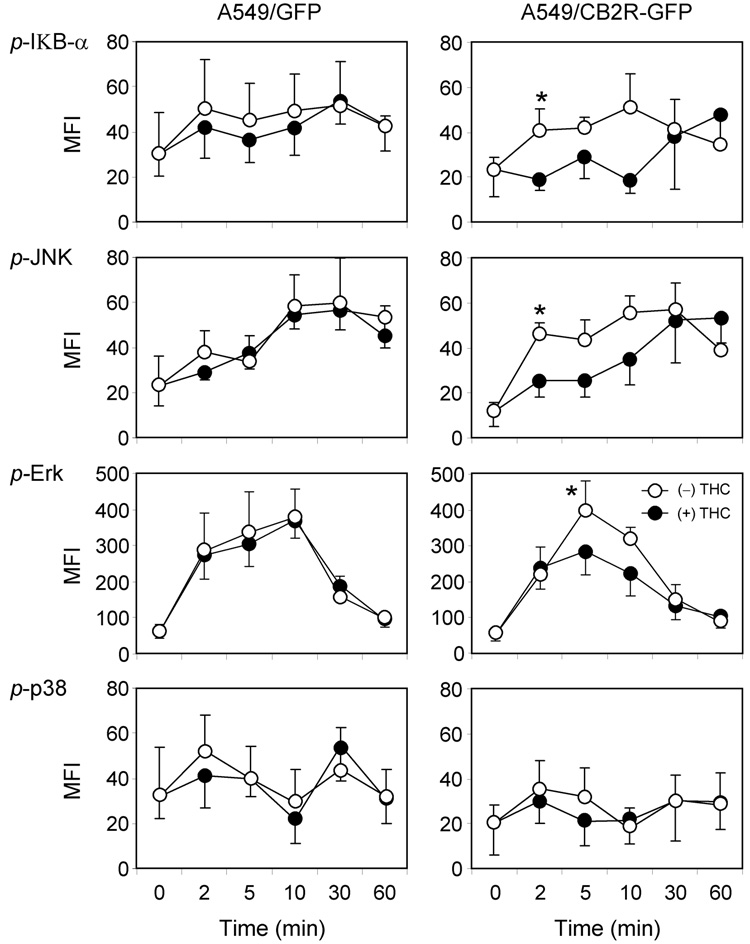
The finding that mitochondrial membrane potential was altered in cells overexpressing CB2R raised the possibility that patterns of intracellular signaling had been altered in these cells. The CB2R is a G protein-coupled receptor and activation by cannabinoids can alter a variety of intracellular signaling events (Demuth and Molleman, 2006) . The A549/GFP and A549/CB2R-GFP cell lines were used to evaluate the effects of serum exposure alone and in combination with THC on time-dependent phosphorylation of eight different intracellular signaling proteins. The exposure paradigms were analogous to those used in the chemotaxis assays. Of the eight signaling proteins investigated, seven demonstrated increased phosphorylation during the first hour following serum exposure with a similar magnitude and time course observed for both the A549/GFP and A549/CB2R-GFP cell lines (Figure 7). Pretreatment with 1 µg/ml THC had no reproducible effect on protein phosphorylation when examined using A549/GFP cells. However, phosphorylation of IkB-a, JNK, Erk-MAPK, p70S6K, STAT3, and STAT5 were all inhibited to some degree when A549/CB2-GFP cells were exposed to THC. Of these, THC-induced effects on phosphorylation of IkB-a and JNK occurred at the earliest time points (2 min) and were of the most significant magnitude (greater than 50% inhibition, p < 0.05). At the time of peak activation, THC inhibited Erk MAPK phosphorylation by 30 % (p < 0.025). The p38 pathway was not activated in response to serum exposure.
Figure 7.
Signaling protein phosphorylation measured using fluorescent immunobeads and a Luminex detector. Assays for phosphorylated forms of IkB-α, JNK, Erk-MAP Kinase, and p38 were performed using an 8-plex cell signaling pathway kit according to the manufacture’s directions. A549/GFP and A549/CB2R-GFP cells were cultured in 24 well plates for 1–2 days. At 60–80% confluence cells were washed with PBS and incubated 1 hr in 0.5% BSA in RPMI 1640. Then RPMI 1640 media containing 10% fetal calf serum and either 0.002 % ethanol vehicle or 1 µg/ml THC was applied and incubated at 37°C for the indicated length of time prior to cell lysis. Lysates were centrifuged at 8000g for 30min and supernatants were used for phosphoprotein measurement. MFI: median fluorescence intensity.
* p < 0.05 compared with THC-treated cells using paired t-test. ANOVA with Bonferroni correction for multiple comparisons revealed group between THC-treated and –untreated cells with p values < 0.01 for IκB-α and JNK. p values for group differences for Erk and p38 were > 0.05
DISCUSSION
The epithelial lining of the lung in smokers of marijuana is exposed to high concentrations of cannabinoids in addition to numerous gaseous and particulate toxicants present in the smoke (Novotny et al., 1982; Roth et al., 2001; Moir et al., 2007). Bronchoscopic visualization and microscopic analysis of airways of habitual marijuana smokers reveals several histopathological abnormalities including squamous cell metaplasia, goblet cell hyperplasia, inflammation, cellular atypic and loss of epithelial microvilli (Tashkin et al., 1997). These abnormalities are also known to occur in tobacco smokers and are associated with increased symptoms of bronchitis and chronic cough (Roth et al., 1998).
We have previously shown that THC, alone and in the presence of other smoke components, can regulate gene expression in human lung epithelial cells (Sarafian et al., 2005), disrupt mitochondrial function and cellular energetics (Sarafian et al., 2003) modulate cytokine and immune function (Baldwin et al., 1997; Yuan et al., 2002), and regulate the balance between an apoptotic and necrotic response to injury (Sarafian et al., 2001). The chemotactic activity of lung epithelial cells, the primary focus of this report, is felt to play an important role in lung remodeling, host protection and lung repair (Rennard et al., 2006; Shahabuddin et al., 2006). In particular, following lung injury from toxins or airborne particulates, wound healing requires a complex multi-step repair process including cell proliferation and migration. This airway remodeling involves dynamic interactions between epithelial cells, fibroblasts and extracellular matrix proteins and impaired epithelial responses may play a role in chronic pulmonary diseases (Cantral et al., 1995; Wang et al., 2001; Puchelle et al., 2006; Rennard et al., 2006). Chemotaxis also plays a fundamental role in the development and metastasis of non-small cell lung cancer (Phillips et al., 2003). THC was recently shown to suppress the chemotactic activity of lymphocytes and macrophages (Sacerdote et al., 2000; Ghosh et al., 2006) and we hypothesized that it may similarly impact on this important function of pulmonary epithelial cells.
Using A549 cells as a model for pulmonary epithelium, we determined that THC and marijuana smoke extract suppressed chemotactic activity whereas tobacco or placebo smoke extract at equivalent tar concentrations did not. This pattern of selective responsiveness to marijuana smoke extract, and to purified THC alone, suggested that THC was the primary active agent. Since both marijuana smoke and THC had previously been shown to disrupt Ψm (Sarafian et al., 2003), we hypothesized that mitochondrial injury might play a role in the suppression of chemotaxis. However, over-expression of the CB2R in A549 cells increased the susceptibility of these cells to the effects of THC on chemotaxis but not to the effects on cellular energetics. If anything, mitochondrial function was enhanced in A549 cells that over-expressed CB2R. Furthermore, while the addition of cyclosporine A prevented the ATP loss that normally occurs when cells are exposed to THC, this treatment had no impact on the capacity for THC to suppress chemotaxis. It is clear from these studies that THC can affect A549 cells through multiple pathways and that its impact on cellular energetics is not directly linked to its suppressive effects on chemotactic activity. Moreover, these findings indicate that the mitochondrial injury caused by THC is not mediated by the CB2R while activation of the CB2R plays a central role regulating chemotaxis.
We were interested to determine the effects of THC on intracellular signaling mediated through the phosphorylation of protein intermediates. These signaling events can be altered by THC (Howlett, 2005; Demuth and Molleman, 2006) and can be involved in both the mitochondrial effects of toxicants (Moor et al., 2005; Chen et al., 2006; Oh et al., 2006; Zhou et al., 2006; Zhang et al., 2007) and in the regulation of chemotaxis (Phillips et al., 2003). Using the same serum stimulation conditions as those employed in our chemotaxis assays, time-dependent phosphorylation of the IkB-a, Erk-MAPK, p70S6K, CREB, STAT3, STAT5, and JNK signaling proteins was observed with only p38 failing to show exposure-dependent phosphorylation. The phosphorylation pattern was similar for both the A549/GFP and A549/CB2R-GFP cells, suggesting that early signaling events mediated by these pathways was not responsible for the decrease in intrinsic chemotactic activity caused by CB2R overexpression. However, exposure of the A549/CB2R-GFP cells, but not the A549/GFP cells, to THC suppressed serum-induced phosphorylation of six of the eight proteins analyzed, raising the possibility that one or more of these signaling pathways may underlie the enhanced sensitivity of CB2R overexpressing cells to THC-induced chemotaxis inhibition. Of these six proteins, inhibition of IkB-a and JNK were the most robust. The observed inhibition of JNK phosphorylation is consistent with a recent report that THC inhibited chemotaxis in EGF-stimulated A549 (Preet et al., 2008). In that study, THC-dependent suppression of JNK phosphorylation was demonstrated in both in vitro and the in vivo models. Together with our data, these results suggest that disruption of intracellular signaling events underlie abnormalities in lung epithelial cell chemotaxis caused by THC and that these events involve the CB2R.
There have been several recent reports linking cannabinoids with chemotaxis; some suggesting that endocannabinoids are functional chemokines (Song and Zhong, 2000; Jorda et al., 2002; Kishimoto et al., 2005), but the majority suggesting that stimulation of the CB2R suppresses chemotactic responses to other agents/ligands (Rinaldi-Carmona et al., 1998; Sacerdote et al., 2000; Joseph et al., 2004; Ghosh et al., 2006; Nilsson et al., 2006). Cannabinoid receptors are members of the larger family of seven transmembrane spanning G-protein-coupled receptors (GPCR). GPCRs can form homodimers as well as heterodimers with other GPCRs (Shapira et al., 2003; Kearn et al., 2005). Heterodimerization has been shown to result in heterologous desensitization in many systems and prolonged cannabiniod exposure has been shown to down regulate expression of both opioid and dopamine receptors by this pathway. Dimerization can also modulate and redirect the interaction of GPCRs with different downstream G-protein family members and thereby influence resulting signaling pathways (Kearn et al., 2005). Thus, expression of CB2R has the potential to impact a variety of cell properties that are mediated by other GPCRs. In this setting, the altered cell migration exhibited by A549/CB2R-GFP cells in the absence of exogenous THC could represent an increased sensitivity to endogenous ligands acting in an autocrine manner (i.e. due to the production of endocannaboinoids) or could result from changes in receptor signaling due to dimerization. Given that chemokine receptors are also GPCR proteins, the response of A549 cells to CB2R over-expression or to the addition of THC could be mediated by heterologous desensitization of CXC receptors. In this respect, activation of the CB2R by THC was recently shown to inhibit CXCL12-induced chemotaxis, suggesting a direct biologic interaction between cannabinoid and chemotaxis receptor pathways (Rinaldi-Carmona et al., 1998; Sacerdote et al., 2000; Joseph et al., 2004; Ghosh et al., 2006; Nilsson et al., 2006; Coopman et al., 2007).
The study of cannabinoid receptor biology has been limited by a lack of specific high affinity monoclonal antibodies; making it difficult to detect, quantitate and specifically block either CB1R or CB2R. For the current studies we developed a self-inactivating lentiviral vector expressing the human CB2R as an alternative approach. This form of lentiviral vector, pseudotyped with a vesicular stomatitis virus (VSV-g) capsid, results in stable integration and expression of the target transgene(s) in a wide range of primary mammalian cells and cell lines with minimal cytotoxicity (Stripecke et al., 2003). By employing a bisicstronic construct expressing both the CB2R and the GFP gene, we were able to produce a matched pair of cell lines with one expressing both CB2R and GFP, the other GFP alone. A549/CB2R-GFP cells expressed 60-fold higher levels of CB2R mRNA than the A549/GFP cells. This increase in CB2R enhanced the sensitivity of A549/CB2R-GFP cells to the suppressive effects of THC on chemotaxis by 80- to 100-fold, suggesting a CB2R-dependent mechanism of action. In addition, the A549/CB2R-GFP cell line provided a more sensitive tool for studying the antagonistic effects of SR144528. Much of the research defining the potency of SR144528 has been done with rodent cells. CB2R has 91% amino acid homology comparing rat and human proteins and binding affinities for SR144528 are comparable (Ki ~ 1 nm) (Rinaldi-Carmona et al., 1998; Griffin et al., 2000). While binding studies suggest that SR144528 has a 100-fold higher binding affinity for the CB2R than does THC (Portier et al., 1999; Griffin et al., 2000), its effectiveness as a functional antagonist in our assays was significantly less. This weaker effectiveness was increasingly apparent as [THC] increased above 0.5 µg/ml. Similar results have been reported in in vivo studies with mice, which display THC-induced immune suppression (Lu et al., 2006). The ability of SR144528 to attenuate this immune suppression was substantially limited as THC dose increased. These results may also suggest that non-CB receptor mechanisms play more prominent roles as [THC] is increased. Such non-CB receptor mechanisms have also recently been implicated in cannabiniod-mediated inhibition of human neutrophil chemotaxis (McHugh et al., 2008).
From the present results we draw the following conclusions: 1) Expressions of CB2R mRNA in A549 lung epithelial cells was stably increased by 60-fold using a lentiviral vector construct. 2) CB2R transduced cells displayed attenuated chemotactic activity and increased sensitivity to THC-induced, receptor-mediated inhibition relative to control cells. 3) At higher concentrations of THC, SR144528 lost efficacy in reversing chemotaxis inhibition, implying weaker than expected antagonistic activity for SR144528 in this biological assay and/or CB2R-independent actions of THC. 4) Although mitochondrial properties were altered by CB2R-overexpression and by THC exposure, THC-induced inhibition of A549 chemotaxis occurred independently of mitochondrial injury. 5) CB2R overexpression and THC exposure are associated with changes in phosphorylation of IκB-α, JNK and other intracellular signaling proteins which may play a role in the observed inhibition of both chemotaxis and mitochondrial function. The capacity for marijuana smoke to deliver THC to the airway epithelial cells of habitual smokers raises concern for the impact of this smoke exposure on lung function and the normal host responses to lung injury. Furthermore, the regulation of CB2R expression by physiological or pharmacological means could have therapeutic value.
ACKNOWLEDGEMENTS
The authors are grateful to Alisa Zhukhovitskaya for technical assistance and manuscript preparation. We wish to thank Dr. Erin Hsu for assistance with initiating and guiding chemotaxis studies and Dr. Suzanne Henning for allowing use of the luminescence plate reader for ATP measurements. We also thank Hiromi Nakamura and Kara Karibian for assistance with PCR analysis studies. The lentiviral vectors were generated at the UCLA Molecular Vector Core facility. This work was supported by NIH grant R21-DA21580 (T.S.), R21-DA021813 (M.D.R.) and R01-DA03018 (M.D.R.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
37-131 CHS
REFERENCES
- Adams IB, Martin BR. Cannabis: pharmacology and toxicology in animals and humans. Addiction. 1996;91:1585–1614. [PubMed] [Google Scholar]
- Aldington S, Williams M, Nowitz M, Weatherall M, Pritchard A, McNaughton A, Robinson G, Beasley R. The Effects of Cannabis on Pulmonary Structure, Function and Symptoms. Thorax. 2007 doi: 10.1136/thx.2006.077081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin GC, Tashkin DP, Buckley DM, Park AN, Dubinett SM, Roth MD. Marijuana and cocaine impair alveolar macrophage function and cytokine production. Am J Respir Crit Care Med. 1997;156:1606–1613. doi: 10.1164/ajrccm.156.5.9704146. [DOI] [PubMed] [Google Scholar]
- Barsky SH, Roth MD, Kleerup EC, Simmons M, Tashkin DP. Histopathologic and molecular alterations in bronchial epithelium in habitual smokers of marijuana, cocaine, and/or tobacco. J Natl Cancer Inst. 1998;90:1198–1205. doi: 10.1093/jnci/90.16.1198. [DOI] [PubMed] [Google Scholar]
- Campello S, Lacalle RA, Bettella M, Manes S, Scorrano L, Viola A. Orchestration of lymphocyte chemotaxis by mitochondrial dynamics. J Exp Med. 2006;203:2879–2886. doi: 10.1084/jem.20061877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantral DE, Sisson JH, Veys T, Rennard SI, Spurzem JR. Effects of cigarette smoke extract on bovine bronchial epithelial cell attachment and migration. Am J Physiol. 1995;268:L723–L728. doi: 10.1152/ajplung.1995.268.5.L723. [DOI] [PubMed] [Google Scholar]
- Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- Christensen PJ, Du M, Moore B, Morris S, Toews GB, Paine R., 3rd Expression and functional implications of CCR2 expression on murine alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2004;286:L68–L72. doi: 10.1152/ajplung.00079.2003. [DOI] [PubMed] [Google Scholar]
- Coopman K, Smith LD, Wright KL, Ward SG. Temporal variation in CB2R levels following T lymphocyte activation: evidence that cannabinoids modulate CXCL12-induced chemotaxis. Int Immunopharmacol. 2007;7:360–371. doi: 10.1016/j.intimp.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Demuth DG, Molleman A. Cannabinoid signalling. Life Sci. 2006;78:549–563. doi: 10.1016/j.lfs.2005.05.055. [DOI] [PubMed] [Google Scholar]
- Di Luozzo G, Pradhan S, Dhadwal AK, Chen A, Ueno H, Sumpio BE. Nicotine induces mitogen-activated protein kinase dependent vascular smooth muscle cell migration. Atherosclerosis. 2005;178:271–277. doi: 10.1016/j.atherosclerosis.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Preet A, Groopman JE, Ganju RK. Cannabinoid receptor CB2 modulates the CXCL12/CXCR4-mediated chemotaxis of T lymphocytes. Mol Immunol. 2006;43:2169–2179. doi: 10.1016/j.molimm.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Griffin G, Tao Q, Abood ME. Cloning and pharmacological characterization of the rat CB(2) cannabinoid receptor. J Pharmacol Exp Ther. 2000;292:886–894. [PubMed] [Google Scholar]
- Hashibe M, Straif K, Tashkin DP, Morgenstern H, Greenland S, Zhang ZF. Epidemiologic review of marijuana use and cancer risk. Alcohol. 2005;35:265–275. doi: 10.1016/j.alcohol.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Howlett AC. Cannabinoid receptor signaling. Handb Exp Pharmacol. 2005:53–79. doi: 10.1007/3-540-26573-2_2. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O'Malley P, Bachman JG, Schulenberg JE. Monitoring the Future: National Survey Results on Drug Use, 1975–2001. U.S. Department of Health and Human Services I. 2006 [Google Scholar]
- Jorda MA, Verbakel SE, Valk PJ, Vankan-Berkhoudt YV, Maccarrone M, Finazzi-Agro A, Lowenberg B, Delwel R. Hematopoietic cells expressing the peripheral cannabinoid receptor migrate in response to the endocannabinoid 2-arachidonoylglycerol. Blood. 2002;99:2786–2793. doi: 10.1182/blood.v99.8.2786. [DOI] [PubMed] [Google Scholar]
- Joseph J, Niggemann B, Zaenker KS, Entschladen F. Anandamide is an endogenous inhibitor for the migration of tumor cells and T lymphocytes. Cancer Immunol Immunother. 2004;53:723–728. doi: 10.1007/s00262-004-0509-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearn CS, Blake-Palmer K, Daniel E, Mackie K, Glass M. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors enhances heterodimer formation: a mechanism for receptor cross-talk? Mol Pharmacol. 2005;67:1697–1704. doi: 10.1124/mol.104.006882. [DOI] [PubMed] [Google Scholar]
- Kishimoto S, Muramatsu M, Gokoh M, Oka S, Waku K, Sugiura T. Endogenous cannabinoid receptor ligand induces the migration of human natural killer cells. J Biochem (Tokyo) 2005;137:217–223. doi: 10.1093/jb/mvi021. [DOI] [PubMed] [Google Scholar]
- Klein TW. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat Rev Immunol. 2005;5:400–411. doi: 10.1038/nri1602. [DOI] [PubMed] [Google Scholar]
- Lu T, Newton C, Perkins I, Friedman H, Klein TW. Role of cannabinoid receptors in Delta-9-tetrahydrocannabinol suppression of IL-12p40 in mouse bone marrow-derived dendritic cells infected with Legionella pneumophila. Eur J Pharmacol. 2006;532:170–177. doi: 10.1016/j.ejphar.2005.12.040. [DOI] [PubMed] [Google Scholar]
- Maccarrone M. Fatty acid amide hydrolase: a potential target for next generation therapeutics. Curr Pharm Des. 2006;12:759–772. doi: 10.2174/138161206775474279. [DOI] [PubMed] [Google Scholar]
- Marx J. Drug development. Drugs inspired by a drug. Science. 2006;311:322–325. doi: 10.1126/science.311.5759.322. [DOI] [PubMed] [Google Scholar]
- McHugh D, Tanner C, Mechoulam R, Pertwee RG, Ross RA. Inhibition of human neutrophil chemotaxis by endogenous cannabinoids and phytocannabinoids:evidence for a site distinct from CB1 and CB2. Mol Pharmacol. 2008;73:441–450. doi: 10.1124/mol.107.041863. [DOI] [PubMed] [Google Scholar]
- Moir D, Rickert WS, Levasseur G, Larose Y, Maertens R, White P, Desjardins S. A Comparison of Mainstream and Sidestream Marijuana and Tobacco Cigarette Smoke Produced under Two Machine Smoking Conditions. Chem Res Toxicol. 2007 doi: 10.1021/tx700275p. [DOI] [PubMed] [Google Scholar]
- Moor AN, Flynn JM, Gottipati S, Giblin FJ, Cammarata PR. 17beta-estradiol stimulates MAPK signaling pathway in human lens epithelial cell cultures preventing collapse of mitochondrial membrane potential during acute oxidative stress. Mitochondrion. 2005;5:235–247. doi: 10.1016/j.mito.2005.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson O, Fowler CJ, Jacobsson SO. The cannabinoid agonist WIN 55,212-2 inhibits TNF-alpha-induced neutrophil transmigration across ECV304 cells. Eur J Pharmacol. 2006;547:165–173. doi: 10.1016/j.ejphar.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Novotny M, Merli F, Weisler D, Fencl M, Saeed T. Fractionation and capillary gas chromatographic mass spectrometric characterization of the neutral components in marijuana and tobacco smoke concentrates. Journal of Chromatography. 1982;238:141–150. [Google Scholar]
- Oh HL, Seok JY, Kwon CH, Kang SK, Kim YK. Role of MAPK in ceramide-induced cell death in primary cultured astrocytes from mouse embryonic brain. Neurotoxicology. 2006;27:31–38. doi: 10.1016/j.neuro.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. The pharmacology of cannabinoid receptors and their ligands: an overview. Int J Obes (Lond) 2006;30 Suppl 1:S13–S18. doi: 10.1038/sj.ijo.0803272. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Burdick MD, Lutz M, Belperio JA, Keane MP, Strieter RM. The stromal derived factor-1/CXCL12-CXC chemokine receptor 4 biological axis in non-small cell lung cancer metastases. Am J Respir Crit Care Med. 2003;167:1676–1686. doi: 10.1164/rccm.200301-071OC. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Mestas J, Gharaee-Kermani M, Burdick MD, Sica A, Belperio JA, Keane MP, Strieter RM. Epidermal growth factor and hypoxia-induced expression of CXC chemokine receptor 4 on non-small cell lung cancer cells is regulated by the phosphatidylinositol 3-kinase/PTEN/AKT/mammalian target of rapamycin signaling pathway and activation of hypoxia inducible factor-1alpha. J Biol Chem. 2005;280:22473–22481. doi: 10.1074/jbc.M500963200. [DOI] [PubMed] [Google Scholar]
- Portier M, Rinaldi-Carmona M, Pecceu F, Combes T, Poinot-Chazel C, Calandra B, Barth F, le Fur G, Casellas P. SR 144528, an antagonist for the peripheral cannabinoid receptor that behaves as an inverse agonist. J Pharmacol Exp Ther. 1999;288:582–589. [PubMed] [Google Scholar]
- Preet A, Ganju RK, Groopman JE. Delta9-Tetrahydrocannabinol inhibits epithelial growth factor-induced lung cancer cell migration in vitro as well as its growth and metastasis in vivo. Oncogene. 2008;27:339–346. doi: 10.1038/sj.onc.1210641. [DOI] [PubMed] [Google Scholar]
- Puchelle E, Zahm JM, Tournier JM, Coraux C. Airway epithelial repair, regeneration, and remodeling after injury in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3:726–733. doi: 10.1513/pats.200605-126SF. [DOI] [PubMed] [Google Scholar]
- Rennard SI, Togo S, Holz O. Cigarette smoke inhibits alveolar repair: a mechanism for the development of emphysema. Proc Am Thorac Soc. 2006;3:703–708. doi: 10.1513/pats.200605-121SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Millan J, Derocq JM, Casellas P, Congy C, Oustric D, Sarran M, Bouaboula M, Calandra B, Portier M, Shire D, Breliere JC, Le Fur GL. SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J Pharmacol Exp Ther. 1998;284:644–650. [PubMed] [Google Scholar]
- Roth MD, Arora A, Barsky SH, Kleerup EC, Simmons M, Tashkin DP. Airway inflammation in young marijuana and tobacco smokers. Am J Respir Crit Care Med. 1998;157:928–937. doi: 10.1164/ajrccm.157.3.9701026. [DOI] [PubMed] [Google Scholar]
- Roth MD, Marques-Magallanes JA, Yuan M, Sun W, Tashkin DP, Hankinson O. Induction and regulation of the carcinogen-metabolizing enzyme CYP1A1 by marijuana smoke and delta (9)-tetrahydrocannabinol. Am J Respir Cell Mol Biol. 2001;24:339–344. doi: 10.1165/ajrcmb.24.3.4252. [DOI] [PubMed] [Google Scholar]
- Sacerdote P, Massi P, Panerai AE, Parolaro D. In vivo and in vitro treatment with the synthetic cannabinoid CP55, 940 decreases the in vitro migration of macrophages in the rat: involvement of both CB1 and CB2 receptors. J Neuroimmunol. 2000;109:155–163. doi: 10.1016/s0165-5728(00)00307-6. [DOI] [PubMed] [Google Scholar]
- Sarafian T, Habib N, Mao JT, Tsu IH, Yamamoto ML, Hsu E, Tashkin DP, Roth MD. Gene expression changes in human small airway epithelial cells exposed to Delta9-tetrahydrocannabinol. Toxicol Lett. 2005;158:95–107. doi: 10.1016/j.toxlet.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Sarafian TA, Habib N, Oldham M, Seeram N, Lee RP, Lin L, Tashkin DP, Roth MD. Inhaled marijuana smoke disrupts mitochondrial energetics in pulmonary epithelial cells in vivo. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1202–L1209. doi: 10.1152/ajplung.00371.2005. [DOI] [PubMed] [Google Scholar]
- Sarafian TA, Kouyoumjian S, Khoshaghideh F, Tashkin DP, Roth MD. Delta 9-tetrahydrocannabinol disrupts mitochondrial function and cell energetics. Am J Physiol Lung Cell Mol Physiol. 2003;284:L298–L306. doi: 10.1152/ajplung.00157.2002. [DOI] [PubMed] [Google Scholar]
- Sarafian TA, Tashkin DP, Roth MD. Marijuana smoke and Delta(9)-tetrahydrocannabinol promote necrotic cell death but inhibit Fas-mediated apoptosis. Toxicol Appl Pharmacol. 2001;174:264–272. doi: 10.1006/taap.2001.9224. [DOI] [PubMed] [Google Scholar]
- Seamon MJ, Fass JA, Maniscalco-Feichtl M, Abu-Shraie NA. Medical marijuana and the developing role of the pharmacist. Am J Health Syst Pharm. 2007;64:1037–1044. doi: 10.2146/ajhp060471. [DOI] [PubMed] [Google Scholar]
- Shahabuddin S, Ji R, Wang P, Brailoiu E, Dun N, Yang Y, Aksoy MO, Kelsen SG. CXCR3 chemokine receptor-induced chemotaxis in human airway epithelial cells: role of p38 MAPK and PI3K signaling pathways. Am J Physiol Cell Physiol. 2006;291:C34–C39. doi: 10.1152/ajpcell.00441.2005. [DOI] [PubMed] [Google Scholar]
- Shapira M, Gafni M, Sarne Y. Long-term interactions between opioid and cannabinoid agonists at the cellular level: cross-desensitization and downregulation. Brain Res. 2003;960:190–200. doi: 10.1016/s0006-8993(02)03842-8. [DOI] [PubMed] [Google Scholar]
- Sidney S, Quesenberry CP, Jr, Friedman GD, Tekawa IS. Marijuana use and cancer incidence (California, United States) Cancer Causes Control. 1997;8:722–728. doi: 10.1023/a:1018427320658. [DOI] [PubMed] [Google Scholar]
- Song ZH, Zhong M. CB1 cannabinoid receptor-mediated cell migration. J Pharmacol Exp Ther. 2000;294:204–209. [PubMed] [Google Scholar]
- Stripecke R, Koya RC, Ta HQ, Kasahara N, Levine AM. The use of lentiviral vectors in gene therapy of leukemia: combinatorial gene delivery of immunomodulators into leukemia cells by state-of-the-art vectors. Blood Cells Mol Dis. 2003;31:28–37. doi: 10.1016/s1079-9796(03)00062-7. [DOI] [PubMed] [Google Scholar]
- Tashkin DP, Simmons MS, Sherrill DL, Coulson AH. Heavy habitual marijuana smoking does not cause an accelerated decline in FEV1 with age. Am J Respir Crit Care Med. 1997;155:141–148. doi: 10.1164/ajrccm.155.1.9001303. [DOI] [PubMed] [Google Scholar]
- Wang H, Liu X, Umino T, Skold CM, Zhu Y, Kohyama T, Spurzem JR, Romberger DJ, Rennard SI. Cigarette smoke inhibits human bronchial epithelial cell repair processes. Am J Respir Cell Mol Biol. 2001;25:772–779. doi: 10.1165/ajrcmb.25.6.4458. [DOI] [PubMed] [Google Scholar]
- Wu GJ, Tai YT, Chen TL, Lin LL, Ueng YF, Chen RM. Propofol specifically inhibits mitochondrial membrane potential but not complex I NADH dehydrogenase activity, thus reducing cellular ATP biosynthesis and migration of macrophages. Ann N Y Acad Sci. 2005;1042:168–176. doi: 10.1196/annals.1338.019. [DOI] [PubMed] [Google Scholar]
- Yuan M, Kiertscher SM, Cheng Q, Zoumalan R, Tashkin DP, Roth MD. Delta 9-Tetrahydrocannabinol regulates Th1/Th2 cytokine balance in activated human T cells. J Neuroimmunol. 2002;133:124–131. doi: 10.1016/s0165-5728(02)00370-3. [DOI] [PubMed] [Google Scholar]
- Zhang S, Zhou F, Ding JH, Zhou XQ, Sun XL, Hu G. ATP-sensitive potassium channel opener iptakalim protects against MPP-induced astrocytic apoptosis via mitochondria and mitogen-activated protein kinase signal pathways. J Neurochem. 2007;103:569–579. doi: 10.1111/j.1471-4159.2007.04775.x. [DOI] [PubMed] [Google Scholar]
- Zhang ZF, Morgenstern H, Spitz MR, Tashkin DP, Yu GP, Marshall JR, Hsu TC, Schantz SP. Marijuana use and increased risk of squamous cell carcinoma of the head and neck. Cancer Epidemiol Biomarkers Prev. 1999;8:1071–1078. [PubMed] [Google Scholar]
- Zhou Y, Wang Q, Mark Evers B, Chung DH. Oxidative stress-induced intestinal epithelial cell apoptosis is mediated by p38 MAPK. Biochem Biophys Res Commun. 2006;350:860–865. doi: 10.1016/j.bbrc.2006.09.103. [DOI] [PMC free article] [PubMed] [Google Scholar]