Abstract
Endotoxic shock is a systemic inflammatory response that is associated with an increase in nitric oxide production and a decrease in the formation of 20-hydroxyeicosatetraenoic acid (20-HETE), which may contribute to the fall in blood pressure and vascular reactivity. The present study examined the effects of a synthetic analogue of 20-HETE, N-[20-hydroxyeicosa-5(Z),14(Z)-dienoyl]glycine (5,14-HEDGE), on the fall in blood pressure and vascular responsiveness to vasoscontrictors and acetylcholine in rats treated with endotoxin. The MAP fell by 31 mmHg, and the heart rate rose by 90 beats/min in male Wistar rats treated with endotoxin (10 mg/kg, intraperitoneally). The fall in MAP was associated with a decrease in the vasoconstrictor response to norepinephrine in isolated aorta and superior mesenteric artery and increased levels of nitrite in the serum, kidney, heart, and vascular tissues. The effects of endotoxin were prevented by 5,14-HEDGE (30 mg/kg, s.c.) given 1 h after injection of endotoxin. Furthermore, a competitive antagonist of vasoconstrictor effects of 20-HETE, 20-hydroxyeicosa-6(Z),15(Z)-dienoic acid (30 mg/kg, s.c.), prevented the beneficial effects of 5,14-HEDGE on MAP and vascular tone in rats treated with endotoxin. These data are consistent with the view that a fall in the production of 20-HETE contributes to the fall in MAP and vascular reactivity in rats treated with endotoxin, and that 5,14-HEDGE has a beneficial effect.
Keywords: Lipopolysaccharide, blood pressure, 20-hydroxyeicosatetraenoic acid, nitric oxide, vascular hyporeactivity
INTRODUCTION
The expression of iNOS is enhanced in many tissues in response to mediators released by endotoxin. This leads to increased generation of NO, which contributes to fall in blood pressure, vascular hyporeactivity, multiple organ failure, and the high mortality rate that are associated with septic shock. Systemic blockade of NOS opposes the fall in blood pressure in endotoxic shock (1). This is not only caused by withdrawal of vasodilator effects of NO, but also is associated with increased activity of vasoconstrictor pathways including the sympathetic nervous, renin-angiotensin, endothelin, and 20-hydroxyeicosatetraenoic acid (20-HETE) systems (1, 2). It has been reported that NO inhibits renal cytochrome P450 (CYP) ω-hydroxylase activity and the production of 20-HETE (3-5). The 20-HETE is a potent vasoconstrictor that plays an important role in the regulation of vascular tone by inhibiting the activity of calcium-activated potassium channels. Moreover, an NO-induced fall in the endogenous production of 20-HETE has also been found to contribute to the cyclic guanosine 5′-monophosphate–independent vasodilator effects of NO in the renal and cerebral microcirculations (3, 6). These findings suggest that NO induced inhibition of the formation of 20-HETE, and removal of its influence on vascular tone may contribute to the fall in MAP and vascular hyporeactivity in endotoxic shock. Therefore, the present study examined whether systemic administration of a synthetic analogue of 20-HETE, N-[20-hydroxyeicosa-5(Z),14(Z)-dienoyl]glycine (5,14-HEDGE), would prevent the hypotension, vascular hyporeactivity, and the increase in NO production after administration of endotoxin to rats.
MATERIALS AND METHODS
Endotoxic shock model
Experiments were performed on 89 male Wistar rats weighing 250 to 300 g that were fed a standard chow. The rats were housed in an animal facility with a 12-h light and dark cycle. All experiments were carried out in accord with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The protocol was approved by the Ethics Committee of the Mersin University School of Medicine. Endotoxic shock was induced as previously described by Tunctan et al. (7). Briefly, conscious rats received a 10 mg/kg (intraperitoneal [i.p.]) injection of endotoxin (Escherichia coli lipopolysaccharide, 0111:B4; Sigma Chemical Co, St Louis, Mo) or an equivalent volume of saline (4 mL/kg, i.p.) at time 0. The MAP and heart rate (HR) were measured using a tail-cuff device (MAY 9610 Indirect Blood Pressure Recorder System, COMMAT Ltd, Ankara, Turkey) during a control period at time 0 and 1, 2, 3, and 4 h later. Separate groups of endotoxin-treated rats were given a synthetic 20-HETE analogue, 5,14-HEDGE, or a competitive antagonist of the vasoconstrictor effects of 20-HETE, 20-hydroxyeicosa-6(Z),15(Z)-dienoic acid (20-HEDE; WIT002) (8, 9). 5,14-HEDGE and 20-HEDE were synthesized in the Department of Biochemistry University of Texas Southwestern Medical Center, Dallas, Texas. 5,14-HEDGE is an amide analog of 20-hydroxyeicosa-5(Z),14(Z)-dienoic acid (5,14-HEDE) that has been previously shown to be a 20-HETE mimetic both in vivo and in vitro (8, 9). The amide group was added to the C1 position to prevent β oxidation and solubility of this 20-HETE mimetic. Previous studies demonstrated that substitutions at this site have no effect on the vasoconstrictor properties of 20-HETE mimetics (8, 9). 5,14-HEDGE and 20-HEDE were given in an s.c. dose of 30 mg/kg 1 h after injection of saline or endotoxin. The rats were killed 4 h after the administration of endotoxin, and a blood sample and the kidneys, heart, thoracic aorta, and superior mesenteric artery were collected for measurement of levels of nitrite. The tissues were homogenized in 1 mL of ice-cold 20 mM HEPES buffer (pH 7.5) containing 20 mM of β-glycerophosphate, 20 mM of sodium pyrophosphate, 0.2 mM of sodium orthovanadate, 2 mM of EDTA, 20 mM of sodium fluoride, 10 mM of benzamidine 10, 1 mM of dithiothreitol, 20 mM of leupeptin, and 10 mM of aprotinin (7). An aliquot of the supernatant was analyzed for the measurement of total protein using Coomassie blue method (10) and nitrite using the diazotization method (7).
Vascular response to constrictors and dilators
At the end of the experiment, the rats were killed, and the thoracic aortas and superior mesenteric arteries were isolated as previously described (11, 12). The vessels were cleared of adventitial tissue and cut into rings of 2 to 3 mm in length. The rings were mounted on 250-μm-diameter stainless steel wires in a small vessel myograph (6 mL) for measurement of changes in isometric tension (Tissue Bath Myograph, Model 700MO, Danish Myo Technology A/S, Denmark; Commat Ltd, Turkey). The rings were equilibrated in oxygenated (95% O2/5% CO2) Krebs-Henseleit solution (pH 7.4) (for thoracic aorta [mM]: NaCl 118, KCl 4.7, CaCl2 1.2, KH2PO4 1.2, MgSO4 1.2, NaHCO3 15, and glucose 11; for mesenteric artery [mM]: NaCl 119, KCl 4.7, CaCl2 2.5, KH2PO4 1.2, MgSO4 1.2, NaHCO3 25, glucose 5.5, and EDTA 0.03) at a resting tension of 1.5 or 1.0 g for 1.5 h. After the equilibration period, the rings were precontracted with phenylephrine or KCl. In thoracic aorta and superior mesenteric artery isolated from control rats, phenylephrine (2.6 μM)-and KCl (80 mM)-induced tone were 1.38 ± 0.15 g (n = 13) and 1.04 ± 0.08 (n = 6), respectively. To confirm the presence of endothelium and the ability of vascular smooth muscle to relax, the responses to acetylcholine (10 or 100 μM) and glyceryl trinitrate (10 μM) were determined. All rings were relaxed with acetylcholine (67% ± 4%, n = 13, and 40% ± 5%, n = 6, for thoracic aorta and superior mesenteric artery, respectively) and glyceryl trinitrate (91% ± 3%, n = 13, and 96% ± 3%, n = 6, for thoracic aorta and superior mesenteric artery, respectively). After the control responses were determined, the contractile responses to cumulative concentrations of norepinephrine (0.001 – 100 μmol/L) were evaluated.
Statistical analysis
All data were expressed as mean values ± SEM. The effects of acetylcholine and glyceryl trinitrate were calculated as percentage decrease in tension. The changes in isometric contractions induced by norepinephrine were expressed in grams of tension. Nonlinear regression analysis was used to calculate maximum contractile response (Emax) and the concentration of norepinephrine that produced 50% of the maximal contraction (EC50) values. Data were analyzed by 1-way ANOVA followed by Student-Newman-Keuls test for multiple comparisons, Kruskal-Wallis test followed by Dunns test for multiple comparisons, and Student t or Mann-Whitney U tests when appropriate. A P < 0.05 was considered to be statistically significant.
RESULTS
Effect of 5,14-HEDGE on the cardiovascular response to endotoxin
Endotoxin caused a gradual fall in MAP (Fig. 1A) and increase in HR (Fig. 1B) during the 4-h course of the experiment. The change in MAP and HR reached a maximum at 4 h after the administration of endotoxin. Therefore, this time point was chosen for all between-group comparisons. The MAP fell by 31 mmHg (Fig. 1A), and HR rose by 90 beats/min (Fig. 1B) in rats treated with endotoxin. 5,14-HEDGE completely prevented the fall in MAP (Fig. 1A) and the increase in HR (Fig. 1B) in rats given endotoxin. The competitive antagonist of vasoconstrictor effects of 20-HETE, 20-HEDE, prevented the ability of 5,14-HEDGE to oppose the effects of endotoxin on MAP (Fig. 1A) and HR (Fig. 1B). 5,14-HEDGE and 20-HEDE had no effect on MAP (Fig. 1A) or HR (Fig. 1B) when given to rats treated with vehicle.
Fig. 1. Time course of the effects of 5,14-HEDGE (the synthetic 20-HETE mimetic) and 20-HEDE (the competitive antagonist of vasoconstrictor effects of 20-HETE) on (A) MAP and (B) HR after administration of saline (vehicle) (4 mL/kg, i.p.) or endotoxin (10 mg/kg, i.p.) to conscious rats.
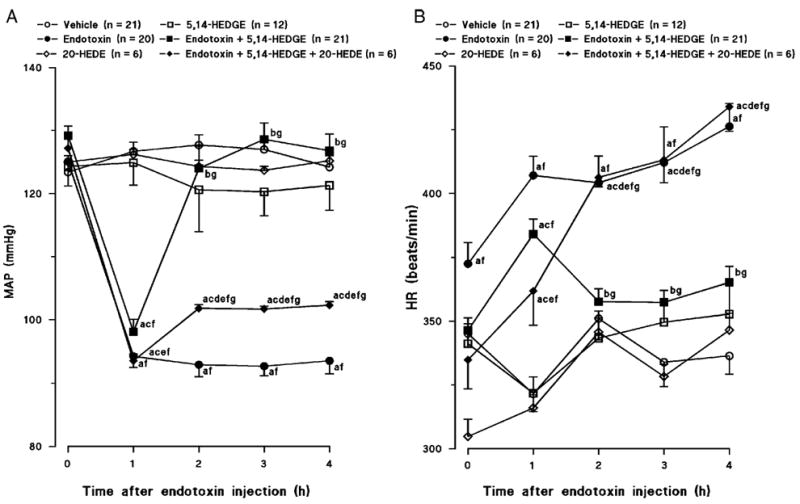
5,14-HEDGE (30 mg/kg, s.c.) or 20-HEDE (30 mg/kg, s.c.) was given 1 h after administration of endotoxin. Mean values ± SEM are presented. Numbers in parentheses indicate the number of animals studied per group. a indicates a significant difference from the corresponding value seen in rats treated with saline (vehicle) (P < 0.05). b indicates a significant difference from the corresponding value seen in the rats treated with vehicle and endotoxin (P < 0.05). c indicates a significant difference from the corresponding value seen in the rats treated with vehicle and 5,14-HEDGE (P < 0.05). d indicates a significant difference from the corresponding value seen in the rats treated with endotoxin and 5,14-HEDGE (P < 0.05). e indicates a significant difference from the corresponding value seen in the rats treated with vehicle and 20-HEDE (P < 0.05). f indicates a significant difference from the time 0 value within a group (P < 0.05). g indicates a significant difference from the time 1 value in each group (P < 0.05).
Effects of 5,14-HEDGE on endotoxin-induced changes in endothelial function
Endotoxin decreased the vasodilator response to acetylcholine in thoracic aorta (Fig. 2A) and superior mesenteric artery (Fig. 2C). Neither 5,14-HEDGE nor 20-HEDE had any effect on the decrease in the vascular response to acetylcholine in rats treated with endotoxin. Endotoxin also decreased the vascular response to the NO donor glyceryl trinitrate in thoracic aorta (Fig. 2B) and superior mesenteric artery (Fig. 2D). The fall in the glyceryl trinitrate–induced relaxations to endotoxin were prevented by 5,14-HEDGE in thoracic aorta (Fig. 2B), but not in superior mesenteric artery (Fig. 2D). 20-HEDE prevented the ability of 5,14-HEDGE to oppose the effects of endotoxin on vasodilator responses in thoracic aorta (Fig. 2B). On the other hand, 20-HEDE increased the response to glyceryl trinitrate in the superior mesenteric artery (Fig. 2D) taken from endotoxemic rats treated with 5,14-HEDGE.
Fig. 2. The effects of 5,14-HEDGE (the synthetic 20-HETE mimetic) and 20-HEDE (the competitive antagonist of vasoconstrictor effects of 20-HETE) on endothelium-dependent (A, C) and endothelium-independent (B, D) relaxations in isolated thoracic aorta (A, B) and isolated superior mesenteric artery (C, D) 4 h after saline (vehicle) (4 mL/kg, i.p.) or endotoxin (10 mg/kg, i.p.) injection to conscious rats.
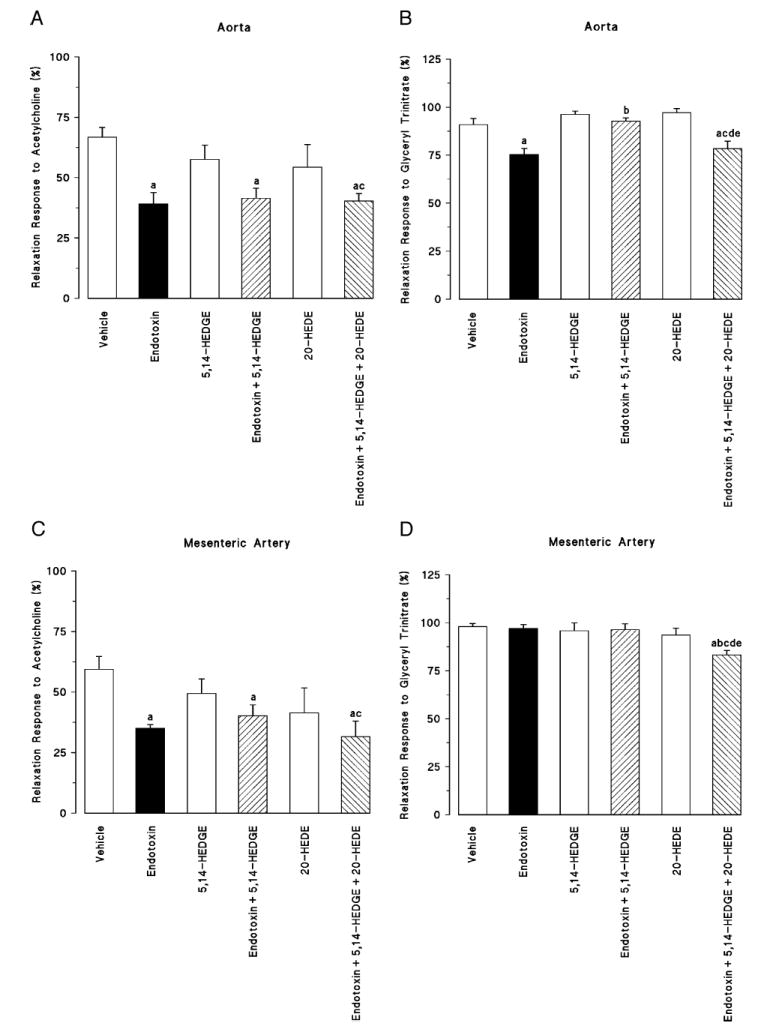
5,14-HEDGE (30 mg/kg, s.c.) or 20-HEDE (30 mg/kg, s.c.) was given 1 h after administration of endotoxin. Values are expressed as means ± SEM from 5 to 13 rats per treatment group. a indicates a significant difference from the corresponding value seen in rats treated with saline (vehicle) (P < 0.05). b indicates a significant difference from the corresponding vale seen in the rats treated with vehicle and endotoxin (P < 0.05). c indicates a significant difference from the corresponding value seen in the rats treated with vehicle and 5,14-HEDGE (P < 0.05). d indicates a significant difference from the corresponding value seen in the rats treated with endotoxin and 5,14-HEDGE (P < 0.05). e indicates a significant difference from the corresponding value seen in the rats treated with vehicle and 20-HEDE (P < 0.05).
Effect of 5,14-HEDGE on the fall in vascular reactivity to norepinephrine in rats treated with endotoxin
Norepinephrine induced a concentration-dependent increase of tension in thoracic aorta (Fig. 3A) and superior mesenteric artery (Fig. 3B) in control rats. Endotoxin shifted the concentration-response curve to norepinephrine in thoracic aorta (Fig. 3A) and superior mesenteric artery to the right (Fig. 3B), and the EC50 values of norepinephrine were increased significantly in both vascular beds (Fig. 4, B and D). 5,14-HEDGE prevented the effects of endotoxin to diminish the vascular response to norepinephrine in thoracic aorta (Figs. 3A and 4A) and superior mesenteric artery (Figs. 3B and 4D). Pretreatment of the animals with the 20-HETE antagonist 20-HEDE prevented the effects of 5,14-HEDGE on the vascular responsiveness to norepinephrine in thoracic aorta (Figs. 3A and 4A) and superior mesenteric artery (Figs. 3B and 4D).
Fig. 3. The effects of 5,14-HEDGE (the synthetic 20-HETE mimetic) and 20-HEDE (the competitive antagonist of vasoconstrictor effects of 20-HETE) on concentration-response curves to norepinephrine (0.001 – 100 μM) in isolated thoracic aorta (A) and superior mesenteric artery (B) 4 h after saline (vehicle) (4 mL/kg, i.p.) or endotoxin (10 mg/kg, i.p.) injection to conscious rats.
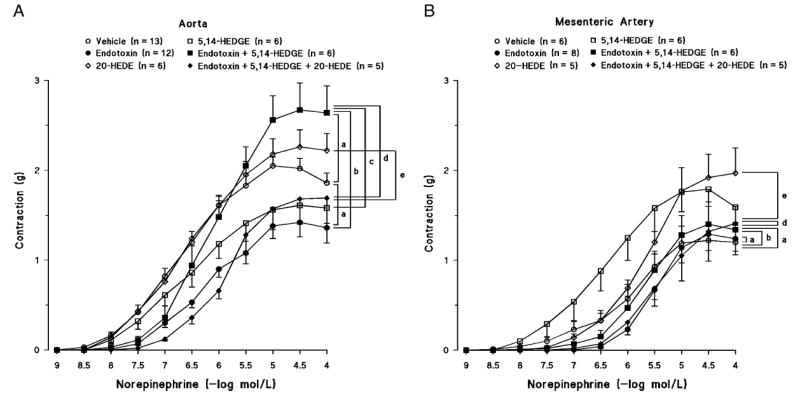
5,14-HEDGE (30 mg/kg, s.c.) or 20-HEDE (30 mg/kg, s.c.) was given 1 h after administration of endotoxin. Mean values ± SEM are presented. Number in parentheses indicate the number of animals studied per group. a indicates a significant difference from the corresponding value seen in rats treated with saline (vehicle) (P < 0.05). b indicates a significant difference from the corresponding value seen in the rats treated with vehicle and endotoxin (P < 0.05). c indicates a significant difference from the corresponding value seen in the rats treated with vehicle and 5,14-HEDGE (P < 0.05). d indicates a significant difference from the corresponding value seen in the rats treated with endotoxin and 5,14-HEDGE (P < 0.05). e indicates a significant difference from the corresponding value seen in the rats treated with vehicle and 20-HEDE (P < 0.05).
Fig. 4. The effects of 5,14-HEDGE (the synthetic 20-HETE mimetic) and 20-HEDE (the competitive antagonist of vasoconstrictor effects of 20-HETE) on vascular hyporeactivity in isolated thoracic aorta (A, B) and superior mesenteric artery (C, D) 4 h after saline (vehicle) (4 mL/kg, i.p.) or endotoxin (10 mg/kg, i.p.) injection to conscious rats.
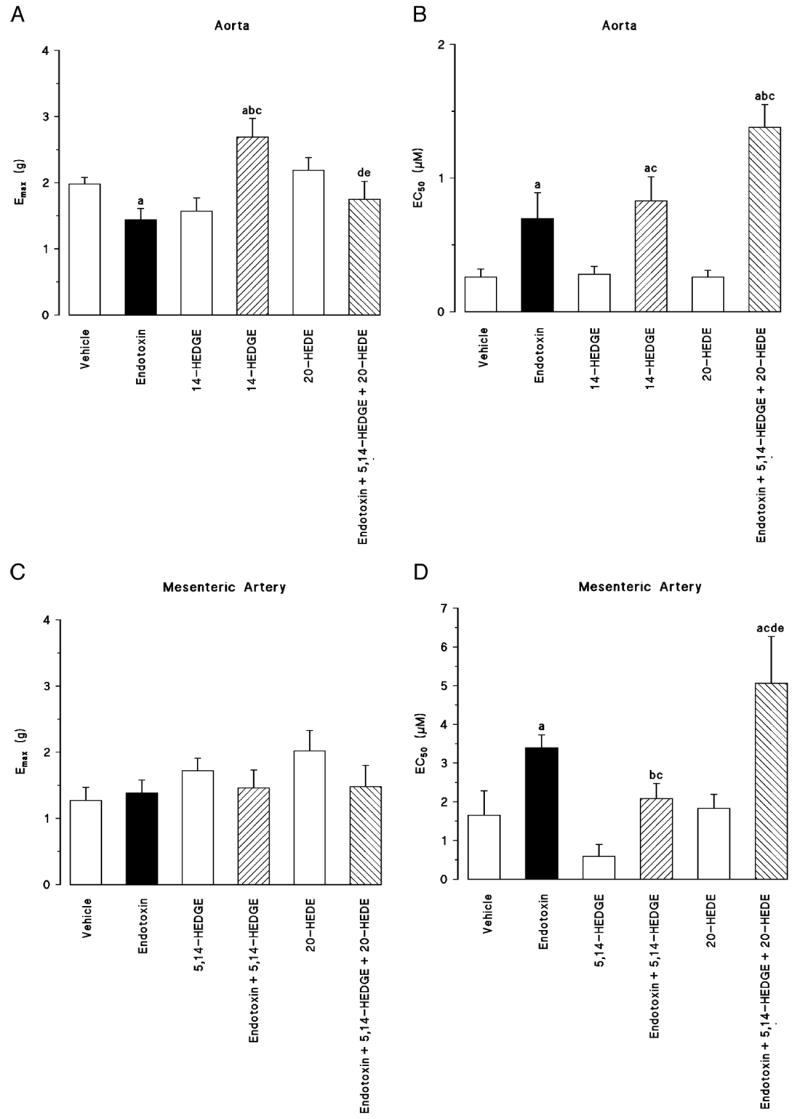
5,14-HEDGE (30 mg/kg, s.c.) or 20-HEDE (30 mg/kg, s.c.) was given 1 h after administration of endotoxin. A and C, Emax. B and D, EC50 values were calculated from cumulative concentration-response curves to norepinephrine (0.001 – 100 μM). Values are expressed as means ± SEM from 5 to 13 rats per treatment group. a indicates a significant difference from the corresponding value seen in rats treated with saline (vehicle) (P < 0.05). b indicates a significant difference from the corresponding value seen in the rats treated with vehicle and endotoxin (P < 0.05). c indicates a significant difference from the corresponding value seen in the rats treated with vehicle and 5,14-HEDGE (P < 0.05). d indicates a significant difference from the corresponding value seen in the rats treated with endotoxin and 5,14-HEDGE (P < 0.05). e indicates a significant difference from the corresponding value seen in the rats treated with vehicle and 20-HEDE (P < 0.05).
Effect of 5,14-HEDGE on endotoxin-induced increases in NO production
Endotoxin increased the levels of nitrite in serum, kidney, heart, thoracic aorta, and superior mesenteric artery (Fig. 5). Administration of 5,14-HEDGE prevented the increase in the levels of nitrite in the serum and tissues of the endotoxin-treated rats. 20-HEDE prevented the beneficial effects of 5,14-HEDGE on the levels of nitrite in endotoxin-treated rats. On the other hand, 20-HEDE alone increased basal levels of nitrite in serum, kidney, heart, thoracic aorta, and superior mesenteric artery in control rats. 20-HEDE also increased the levels of nitrite in thoracic aorta and superior mesenteric artery in rats treated with 5,14-HEDGE and endotoxin.
Fig. 5. The effects of 5,14-HEDGE (the synthetic 20-HETE mimetic) and 20-HEDE (the competitive antagonist of vasoconstrictor effects of 20-HETE) on changes in serum, kidney, heart, thoracic aorta, and superior mesenteric artery nitrite levels measured 4 h after saline (vehicle) (4 mL/kg, i.p.) or endotoxin (10 mg/kg, i.p.) injection to conscious rats.
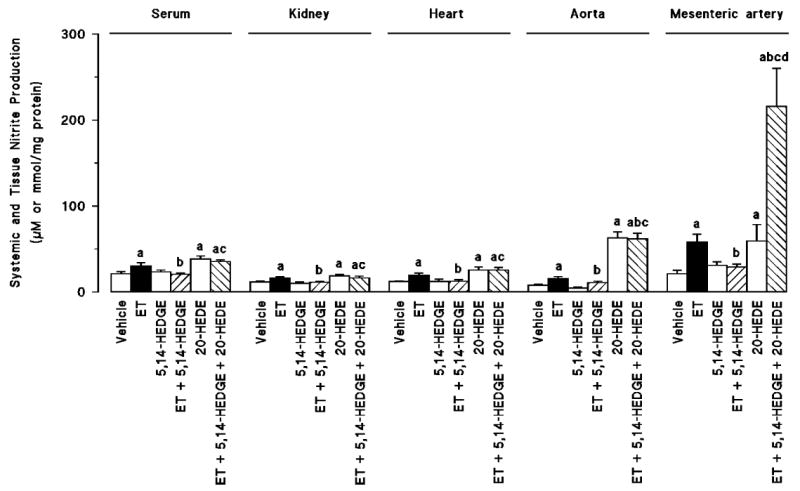
5,14-HEDGE (30 mg/kg, s.c.) or 20-HEDE (30 mg/kg, s.c.) was given 1 h after administration of endotoxin. Values are expressed as means ± SEM from 5 to 22 rats per treatment group. a indicates a significant difference from the corresponding value seen in rats treated with saline (vehicle) (P < 0.05). b indicates a significant difference from the corresponding value seen in the rats treated with vehicle and endotoxin (P < 0.05). c indicates a significant difference from the corresponding value seen in the rats treated with endotoxin and 5,14-HEDGE (P < 0.05). d indicates a significant difference from the corresponding value seen in the rats treated with vehicle and 20-HEDE (P < 0.05).
DISCUSSION
The results of the present study indicate that administration of the synthetic 20-HETE mimetic 5,14-HEDGE prevents the fall in blood pressure and loss of vascular responsiveness to norepinephrine in rats treated with endotoxin. The beneficial effects of 5,14-HEDGE were associated with a reduction in the levels of nitrite. Moreover, a competitive antagonist of vasoconstrictor effects of 20-HETE, 20-HEDE, reversed the beneficial effects of 5,14-HEDGE in endotoxin-treated rats.
Previous studies have indicated that the ω-hydroxylation product of arachidonic acid, 20-HETE, formed by enzymes of the CYP4A pathway, is a potent vasconstrictor that contributes to the regulation of vascular tone and blood pressure (2, 13). NO inhibits the production of 20-HETE (4-6), and NOS inhibitors increase the ω-hydroxylase activity (6), expression of CYP 4A protein (4), and 20-HETE production in the kidney (4, 5). We have also previously demonstrated that the fall in MAP in rats treated with endotoxin is also associated with a decrease in the expression of CYP4A1/A3 protein in the kidney and increased levels of nitrite in serum, kidney, heart, thoracic aorta, and superior mesenteric artery (7, 14-17). These effects were prevented by blockade of iNOS. These observations led us to the present hypothesis that inhibition of CYP4A activity and decreased levels of 20-HETE may contribute to the fall in blood pressure and vascular reactivity in rats treated with endotoxin (7, 14). Supporting this view are the present findings that administration of the 20-HETE agonist 5,14-HEDGE prevented the fall in MAP and increase in HR as well as vascular hyporeactivity to norepinephrine and the increase in the levels of nitrite in serum, kidney, heart, thoracic aorta, and superior mesenteric artery in endotoxin-treated rats. Furthermore, the competitive antagonist of the vasoconstrictor effects 20-HETE, 20-HEDE (8, 9), reversed all of the beneficial effects of 5,14-HEDGE in endotoxin-treated rats, suggesting that the effects of this compound is caused by its 20-HETE agonist activity. These results suggest that besides maintaining the effects of 20-HETE on vascular tone, 5,14-HEDGE may also prevent the fall in MAP after endotoxin by diminishing the production or bioavailability of NO.
Although there is no evidence to date that 20-HETE has any direct effect on the expression or activity of NOS enzymes, it has been shown that increased production of 20-HETE in the vasculature is associated with endothelial dysfunction and increased vascular tone that contributes to the development of hypertension in animal models (13). At least three different pathways have been suggested to play a role in these responses including increased vascular expression of subunits of reduced nicotinamide-adenine dinucleotide phosphate oxidase by 20-HETE, leading to production of superoxide (18), decreased association of endothelial NOS with heat shock protein 90 by 20-HETE, leading to diminished formation of NO and increased formation of superoxide (19, 20), and increased formation of superoxide directly by 20-HETE in endothelial cells (21). All of these changes in the vasculature diminish the bioavailability of NO, leading to endothelial dysfunction and hypertension (13). Therefore, one possible mechanism by which 20-HEDE may increase the basal levels of nitrite in serum and tissues as well as the nitrite production in vascular tissue in rats treated with 5,14-HEDGE and endotoxin may be through antagonizing the stimulatory effect of 20-HETE on the formation of reactive oxygen species to reduce NO production and its bioavailability.
There are conflicting results concerning the effects of endotoxin on the endothelium-dependent and endothelium-independent relaxations in vessels isolated from endotoxemic rats (22, 23). In this study, 5,14-HEDGE did not prevent the endotoxin-induced decrease in endothelium-dependent relaxations in thoracic aorta and superior mesenteric artery, although it reversed the effects of endotoxin on the fall in blood pressure, vascular hyporeactivity, and overproduction of nitrite in these tissues. Moreover, the 20-HETE antagonist 20-HEDE also had no effect on the endothelial dysfunction in the endotoxemic rats treated with 5,4-HEDGE. In contrast to the findings on acetylcholine, 5,14-HEDGE prevented the endotoxin-induced decrease in endothelium-independent relaxations in thoracic aorta, and its effect was reversed by 20-HEDE. Endotoxin had no effect on the endothelium-independent relaxations in superior mesenteric artery; however, 20-HEDE caused a decrease in the relaxation responses in endotoxemic rats treated with 5,14-HEDGE. It has been shown that increased production of 20-HETE leading to diminished formation of NO and increased formation of superoxide in the vasculature is associated with endothelial dysfunction and increased vascular tone (13, 18-21). Therefore, increased production of superoxide in endothelial and/or vascular smooth muscle cells might contribute to the effects of 5,14-HEDGE on the endotoxin-induced decrease in endothelium-dependent and endothelium-independent relaxations in thoracic aorta and superior mesenteric artery.
In conclusion, the present study indicates that administration of the 20-HETE agonist 5,14-HEDGE prevents hypotension and vascular hyporeactivity associated with the changes in NO production in rats treated with endotoxin. Impairment of cardiovascular and renal function is critically involved in the pathophysiological sequela in inflammatory diseases such as septic shock finally resulting in multiorgan failure and death. Restoration of these impaired functions should have therapeutic benefit. These studies suggest that stable analogs of 20-HETE may have therapeutic potential for the treatment of devasting effects of organ hypoperfusion in septic shock.
Acknowledgments
This study was supported by the Research Foundation of Mersin University (Project Code No. BAP ECZF EMB 2006-3 to B.T.), National Institutes of Health (grant no. GM31278), and the Robert A. Welch Foundation.
Footnotes
This study was performed at the Department of Pharmacology, Faculty of Pharmacy, Mersin University, Mersin, Turkey.
References
- 1.Tunctan B, Altug S. The use of nitric oxide synthase inhibitors in inflammatory diseases: a novel class of anti-inflammatory agents. Curr Med Chem. 2004;3:271–301. [Google Scholar]
- 2.Miyata N, Roman RJ. Role of 20-hydroxyeicosatetraenoic acid (20-HETE) in vascular system. J Smooth Muscle Res. 2005;41:175–193. doi: 10.1540/jsmr.41.175. [DOI] [PubMed] [Google Scholar]
- 3.Alonso-Galicia M, Sun CW, Falck JR, Harder DR, Roman RJ. Contribution of 20-HETE to the vasodilator actions of nitric oxide in renal arteries. Am J Physiol. 1998;275:F370–F378. doi: 10.1152/ajprenal.1998.275.3.F370. [DOI] [PubMed] [Google Scholar]
- 4.Oyekan AO, Youseff T, Fulton D, Quilley J, McGiff JC. Renal cytochrome P450 ω-hydroxylase activity are differently modified by nitric oxide and sodium chloride. J Clin Invest. 1999;104:1131–1137. doi: 10.1172/JCI6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang M-H, Wang J, Chang H-H, Zand BA, Jiang M, Nasjletti A, Laniado-Schwartzman M. Regulation of renal CYP4A expression and 20-HETE synthesis by nitric oxide in pregnant rats. Am J Physiol. 2003;285:F295–F302. doi: 10.1152/ajprenal.00065.2003. [DOI] [PubMed] [Google Scholar]
- 6.Alonso-Galicia M, Drummond HA, Reddy KK, Falck JR, Roman RJ. Inhibition of 20-HETE production contributes to the vascular responses to nitric oxide. Hypertension. 1997;29:320–325. doi: 10.1161/01.hyp.29.1.320. [DOI] [PubMed] [Google Scholar]
- 7.Tunctan B, Yaghini FA, Estes A, Malik KU. Inhibition by nitric oxide and cyclooxygenase of cytochrome P450 4A expression and activity contributes to endotoxin-induced hypotension in rats. Nitric Oxide. 2006;14:51–57. doi: 10.1016/j.niox.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Alonso-Galicia M, Falck JR, Reddy KM, Roman RJ. 20-HETE agonists and antagonists in the renal circulation. Am J Physiol. 1999;277:F790–F796. doi: 10.1152/ajprenal.1999.277.5.F790. [DOI] [PubMed] [Google Scholar]
- 9.Yu M, Cambj-Sapunar L, Kehl F, Maier KG, Takeuchi Miyata N, Ishimoto T, Reddy LM, Falck JR, Gebremedhin D, Harder DR, et al. Effects of a 20-HETE antagonist and agonists on cerebral vascular tone. Eur J Pharmacol. 2004;486:297–306. doi: 10.1016/j.ejphar.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 11.Korkmaz B, Ozveren E, Buharalioglu CK, Tunçtan B. Extracellular signal-regulated kinase (ERK1/2) contributes to endotoxin-induced hyporeactivity via nitric oxide and prostacyclin production in rat aorta. Pharmacology. 2006;78:123–128. doi: 10.1159/000095962. [DOI] [PubMed] [Google Scholar]
- 12.Ozveren E, Korkmaz B, Buharalioglu CK, Tunçtan B. Involvement of calcium/calmodulin-dependent protein kinase II to endotoxin-induced vascular hyporeactivity in rat superior mesenteric artery. Pharmacol Res. 2006;54:208–218. doi: 10.1016/j.phrs.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Roman RJ, Lombard JH. Does 20-hydroxyeicosatetraenoic acid contribute to sex differences in cardiovascular risk by increasing oxidative stress. Hypertension. 2007;50:37–38. doi: 10.1161/HYPERTENSIONAHA.107.090803. [DOI] [PubMed] [Google Scholar]
- 14.Tunctan B, Yaghini FA, Estes A, Malik KU. Prostaglandins inhibit cytochrome P450 4A activity and contribute to endotoxin-induced hypotension in rats via nitric oxide production. Arch Pharm Res. doi: 10.1007/s12272-001-1238-x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tunctan B, Korkmaz B, Yildirim H, Tamer L, Atik U, Buharalioğlu CK. Increased production of nitric oxide contributes to renal oxidative stress in endotoxemic rat. Am J Infect Dis. 2005;1:111–115. [Google Scholar]
- 16.Tunctan B, Korkmaz B, Yildirim H, Tamer L, Atik U, Buharalioğlu CK. Reversal of endotoxin-induced hypotension by inhibition of inducible nitric oxide synthase activity is associated with improved oxidative status in rat heart, aorta and mesenteric artery. Turk J Med Sci. 2006;36:71–80. [Google Scholar]
- 17.Tunctan B, Korkmaz B, Dogruer ZN, Tamer L, Atik U, Buharalioglu CK. Inhibition of extracellular signal-regulated kinase (ERK1/2) activity reverses endotoxin-induced hypotension via decreased nitric oxide production in rats. Pharmacol Res. 2007;56:56–64. doi: 10.1016/j.phrs.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Singh H, Cheng J, Deng H, Kemp R, Ishizuka T, Nasjletti A, Schwartzman ML. Vascular cytochrome P450 4A expression and 20-hydroxyeicosatetraenoic acid synthesis contribute to endothelial dysfunction in androgen-induced hypertension. Hypertension. 2007;50:123–129. doi: 10.1161/HYPERTENSIONAHA.107.089599. [DOI] [PubMed] [Google Scholar]
- 19.Wang JS, Singh H, Zhang F, Ishizuka T, Deng H, Kemp R, Wolin MS, Hintze TH, Abraham NG, Nasjletti A, et al. Endothelial dysfunction and hypertension in rats transduced with CYP4A2 adenovirus. Circ Res. 2006;98:962–969. doi: 10.1161/01.RES.0000217283.98806.a6. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, Medhora M, Falck JR, Pritchard KA, Jr, Jacobs ER. Mechanisms of activation of eNOS by 20-HETE and VEGF in bovine pulmonary artery endothelial cells. Am J Physiol. 2006;291:L378–L385. doi: 10.1152/ajplung.00424.2005. [DOI] [PubMed] [Google Scholar]
- 21.Guo AM, Arbab AS, Falck JR, Chen P, Edwards PA, Roman RJ, Scicli AG. Activation of VEGF through ROS mediates 20-HETE–induced endothelial cell proliferation. J Pharmacol Exp Ther. 2007;321:18–27. doi: 10.1124/jpet.106.115360. [DOI] [PubMed] [Google Scholar]
- 22.Karimi G, Fatehi Z, Gholamnejad Z. The role of nitric oxide and protein kinase C in lipopolysaccharide-mediated vascular hyporeactivity. J Pharm Pharm Sci. 2006;9:119–123. [PubMed] [Google Scholar]
- 23.Virdis A, Colucci R, Fornai M, Blandizzi C, Duranti E, Pinto S, Bernardini N, Segnani C, Antonioli L, Taddei S, et al. Cyclooxygenase-2 inhibition improves vascular endothelial dysfunction in a rat model of endotoxic shock: role of inducible nitric-oxide synthase and oxidative stress. J Pharmacol Exp Ther. 2005;312:945–953. doi: 10.1124/jpet.104.077644. [DOI] [PubMed] [Google Scholar]


