Abstract
Background
The incidence of Pseudomonas aeruginosa bacteremia has not been defined in a population-based investigation.
Methods
Retrospective, population-based incidence study using resources of Rochester Epidemiology Project of Olmsted County, Minnesota. We identified all Olmsted County residents with P. aeruginosa bacteremia between 1/1/1997 and 12/31/2006 by microbiology records in the only two laboratories in the county. Medical records were reviewed to confirm diagnosis, residency status, and clinical characteristics.
Results
Age-adjusted incidence per 100,000 person-years for total P. aeruginosa bacteremia was 10.8 (95% confidence interval [CI], 7.5-14.0) in males and 3.7 (95% CI, 2.2-5.2) in females; and for monomicrobial P. aeruginosa bacteremia was 8.4 (95% CI, 5.5-11.2) in males and 2.5 (95% CI, 1.3-3.8) in females. There was no significant change in incidence of total P. aeruginosa bacteremia over the past decade (p=.418). Incidence increased exponentially with age; with greater magnitude of increase in males compared to females for total and monomicrobial P. aeruginosa bacteremia (p=.007 and p=.015, respectively). In patients with monomicrobial P. aeruginosa bacteremia, median age was 69 years; and 78.4% of cases were either nosocomial or health care-associated. Most patients had multiple comorbid conditions. The urinary tract was the most common primary source of infection. The 28-day all-cause mortality of monomicrobial P. aeruginosa bacteremia was 25.5%. In vitro susceptibility to ciprofloxacin was 95.3%.
Conclusions
To our knowledge, this is the first population-based incidence study of P. aeruginosa bacteremia. The incidence of P. aeruginosa bacteremia has remained stable over the past decade. Fluoroquinolone susceptibility is high among local P. aeruginosa bacteremia isolates.
Keywords: bacteremia, epidemiology, mortality, antibiotic susceptibility, Pseudomonas aeruginosa
INTRODUCTION
The incidence of Pseudomonas aeruginosa bacteremia has never been defined in a population-based investigation.1 Data that address P. aeruginosa bacteremia incidence are, for the most part, derived from cross-sectional studies that have been performed at large tertiary care centers where referral bias is a major limitation. P. aeruginosa is the third most common gram-negative pathogen causing bloodstream infections. Estimates from a cross-sectional study performed in tertiary care centers in North and Latin America published by the SENTRY Antimicrobial Surveillance Program showed that P. aeruginosa contributed to 10.6% of gram-negative nosocomial and community-acquired bloodstream infections in 1997.2
Increasing resistance of P. aeruginosa to fluoroquinolones and other antimicrobial agents has greatly impacted management decisions in patients with this infection. Oral therapy is no longer a treatment option in many patients and, in others, there may be no safe and active parenterally administered antibiotic available for use. It is estimated that resistance to ciprofloxacin in P. aeruginosa blood isolates in intensive care units in this country have increased from 9% to 31.7% between 1995 and 2002.3 With the increasing use of newer fluoroquinolones, resistance is expected to continue to increase. In one investigation, exposure to levofloxacin was associated with increased risk of isolation of fluoroquinolone-resistant P. aeruginosa.4
The aims of this study are to establish the incidence, certain clinical characteristics, short- and long-term outcomes, and in vitro antibiotic susceptibility patterns of P. aeruginosa bacteremia in patients from Olmsted County, Minnesota. To our knowledge, our work is the first incidence investigation of P. aeruginosa bacteremia and fluoroquinolone-resistant P. aeruginosa bacteremia in a population-based setting.
METHODS
Setting
Olmsted County is located in southeastern Minnesota. It has a population of 124,277 according to the 2000 census.5 With the exception of a lower prevalence of injection drug use, a higher prevalence of middle-class individuals, and a higher proportion being employed in the health-care industry, the population characteristics of Olmsted County residents are similar to those of US non-Hispanic whites.6-7 The Rochester Epidemiology Project (REP) is a unique medical records-linkage system that encompasses care delivered to residents of Rochester and Olmsted County, Minnesota. The microbiology laboratories at Mayo Medical Center and Olmsted Medical Center are the only two laboratories in Olmsted County. These two medical centers are geographically isolated from other urban centers. The closest competing medical centers are in Minneapolis, Minnesota (139 km to the north), LaCrosse, Wisconsin (114 km to the east), Iowa City and Des Moines, Iowa (317 and 333 km to the south, respectively), and Sioux Falls, South Dakota (376 km to the west). Although best known as a tertiary referral center, Mayo Clinic has always provided primary, secondary, and tertiary care to local residents. Because the Mayo and Olmsted Medical Centers offer care in every medical and surgical specialty and subspecialty, local residents are able to obtain health care within the community, rather than seeking health care at a distant geographic location.6,8
Case Ascertainment
A population-based retrospective cohort with P. aeruginosa bacteremia from 1/1/1997 through 12/31/2006 was identified using the microbiology databases at the Mayo Medical Center Rochester, and Olmsted Medical Center. We used complete enumeration of Olmsted County, Minnesota population. All patients with positive blood cultures for Pseudomonas species during the study period were considered for inclusion, regardless of age, gender, or whether they were hospitalized or in the ambulatory care setting at the time of bacteremia. Among 5,268 episodes of gram-negative bacteremia identified in both clinical microbiology laboratories during the study period, 742 (14.1%) and 656 (12.5%) were due to Pseudomonas species and P. aeruginosa, respectively. Patients with an initial episode of P. aeruginosa bacteremia were included for analysis; patients without valid research authorization (n=10), or lived outside Olmsted County (n=574), and those with recurrent P. aeruginosa bacteremia (n=3) were excluded. Medical records were reviewed by the primary investigator (M.N.A.) to confirm the diagnosis, determine patient residency status, and obtain baseline clinical features, outcome, and isolate in vitro susceptibility data. Patients were followed from the time of the initial episode of P. aeruginosa bacteremia until the latest health care encounter; long-term follow-up was available through the REP.
Case definition
P. aeruginosa bacteremia was defined as growth of P. aeruginosa in a blood culture. Monomicrobial P. aeruginosa bacteremia was defined as growth of P. aeruginosa as the only isolate in a blood culture and polymicrobial P. aeruginosa bacteremia as the growth of P. aeruginosa and other organisms in a blood culture, excluding coagulase-negative staphylococci and Propionibacterium spp. The term total P. aeruginosa bacteremia was used to describe cases of both monomicrobial and polymicrobial P. aeruginosa bacteremia combined. Recurrent P. aeruginosa bacteremia was defined as P. aeruginosa bacteremia occurring 90 days after the initial episode of P. aeruginosa bacteremia. Cases of P. aeruginosa bacteremia were classified into community-acquired, health care-associated, or nosocomial.9 Blood cultures were identified using standard microbiology techniques according to the Clinical and Laboratory Standards Institute (CLSI). Both laboratories are certified by the College of American Pathologists. CLSI methods were employed to evaluate in vitro antibiotic susceptibility results of P. aeruginosa isolates.
Statistical Analysis
Chi-square or Fisher’s exact test was used to test for associations between categorical variables and Student’s t-test was used to test for differences in a continuous variable between levels of a categorical variable. The incidence rate, expressed as the number of new cases per 100,000 person-years, was calculated assuming that the entire population of Olmsted County was at risk of P. aeruginosa bacteremia. The 2000 Olmsted County census figures were used with a projected population growth rate after 2000 of 1.9% per year as the denominator. Analysis was restricted to the initial episode of P. aeruginosa bacteremia during the study period and incidence rates were directly adjusted to the US 2000 white population.5 Ninety-five percent confidence intervals (CI) for incidence rates were estimated assuming that the rates follow a Poisson distribution.
Poisson regression was used to examine incidence trends in overall P. aeruginosa bacteremia and in monomicrobial and polymicrobial P. aeruginosa bacteremia using the SAS procedure GENMOD (version 8, SAS Institute Inc, Cary, NC). Counts for calendar years from 1997 to 2006, age, and gender were used as the unit of observation. Rate ratios (RR) and 95% CI of P. aeruginosa bacteremia rates in different age groups (grouped as <18 [reference], 19-59, 60-79, and ≥80 years) were estimated. Comparisons of incidence trends across age groups were performed by including the 2-way interaction term of gender with age after adjustment for all main effects. For Kaplan-Meier analyses, the log rank test was used to detect differences in survival rates between groups using JMP (version 6.0, SAS Institute Inc, Cary, NC). The level of significance for all statistical testing was defined as p<0.05 (2-sided) except when testing for interactions, where p<0.10 were accepted.
To examine the potential effect of referral bias on in vitro susceptibility results to fluoroquinolones, we compared ciprofloxacin and levofloxacin susceptibility results for isolates from Olmsted County patients with P. aeruginosa bacteremia to blood culture isolates from referral patients. We matched 69 Olmsted County residents with P. aeruginosa bacteremia to 69 patients with first episodes of P. aeruginosa bacteremia who lived outside Olmsted County, but were referred to the Mayo Clinic for care. Patients were matched for the exact year of onset of P. aeruginosa bacteremia, gender, and closest age (82.6% of patients were matched within 5 years of age) at onset of bacteremia.
RESULTS
Among 742 (62.8% male) cases of Pseudomonas spp. bacteremia identified by both microbiology laboratories during the study period, 51 (69% male) and 18 (56% male) unique Olmsted County residents had monomicrobial and polymicrobial P. aeruginosa bacteremia, respectively. Patients with monomicrobial and polymicrobial P. aeruginosa bacteremia had a median age of 69 and 78 years, respectively.
Temporal trends by age and gender
Figure 1 shows age and gender-adjusted incidence rates of P. aeruginosa bacteremia from 1997-2006 for total, monomicrobial, and polymicrobial P. aeruginosa bacteremia. There was no significant change in the incidence of total P. aeruginosa bacteremia between 1997 and 2006 (p=0.418). Although not statistically significant, there was a slight upward trend in the incidence of total P. aeruginosa bacteremia between the years of 2001 and 2006.
Figure 1.
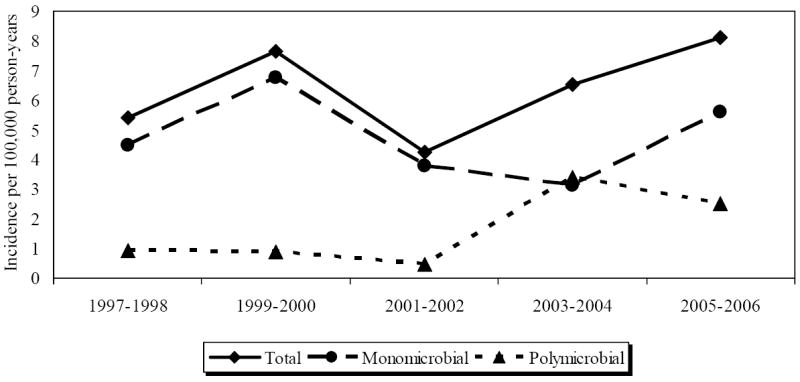
Age- and gender-adjusted incidence of Pseudomonas aeruginosa bacteremia by calendar year
Figure 2 shows that the incidence rate of total P. aeruginosa bacteremia increased exponentially across age for both males and females. A similar trend was seen in both monomicrobial and polymicrobial P. aeruginosa bacteremia. The magnitude of the increase in incidence across age was greater in males than females for both total and monomicrobial P. aeruginosa bacteremia (p=0.007 and p=0.015, respectively for age-by-gender interaction). Compared with the reference age (<18 age group), the rate ratios for the 19-59, 60-79, and ≥80 age groups were 0.66 (95% CI 0.18-2.44), 14.20 (95% CI 4.85-41.55), and 59.22 (95% CI 19.80-177.12), respectively, for males; and 0.96 (95% CI 0.29-3.18), 2.87 (95% CI 0.77-10.70), and 10.93 (95% CI 3.20- 37.33), respectively, for females.
Figure 2.
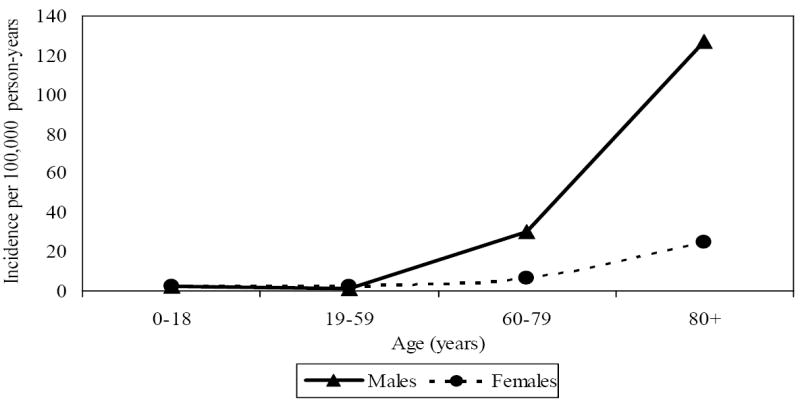
Incidence of total Pseudomonas aeruginosa bacteremia by age group and gender: 1997-2006
The age-adjusted incidence rate per 100,000 person-years for total P. aeruginosa bacteremia was 10.8 (95% CI 7.5-14.0) for males and 3.7 (95% CI 2.2-5.2) for females; and for monomicrobial P. aeruginosa bacteremia was 8.4 (95% CI 5.5-11.2) for males and 2.5 (95% CI 1.3-3.8) for females. The age-adjusted incidence of polymicrobial P. aeruginosa bacteremia was much less at 2.4 (95% CI 0.9-3.9) for males and 1.2 (95% CI 0.4-2.0) for females.
Clinical characteristics of monomicrobial and polymicrobial P. aeruginosa bacteremia
The clinical characteristics of patients with monomicrobial and polymicrobial P. aeruginosa bacteremia are shown in Table 1. Patients with polymicrobial P. aeruginosa bacteremia were more likely to have community-acquired infection (p=0.022) and an abdominal or biliary source of P. aeruginosa bacteremia (p=0.004) as compared to patients with monomicrobial P. aeruginosa bacteremia. Over 78% of monomicrobial P. aeruginosa bacteremia cases were either nosocomial or health care-associated. Furthermore, there was an association between age and classification of monomicrobial P. aeruginosa bacteremia. Sixty percent (15/25) of patients ≥ 70 years old had nosocomial or health care-associated P. aeruginosa bacteremia compared to 96.2% (25/26) of patients < 70 years old (p=0.007).
Table 1.
Clinical characteristics of patients with monomicrobial and polymicrobial P. aeruginosa bacteremia+
| Characteristic | Monomicrobial N=51 | Polymicrobial N=18 |
|---|---|---|
| Age | 69.0 (49.0-81.0) | 78.0 (64.8-82.3) |
| Male gender | 35 (68.6) | 10 (55.6) |
| Non-white race | 2 (3.9) | 2 (11.1) |
| Diabetes mellitus | 12 (23.5) | 1 (5.6) |
| End stage renal disease | 5 (9.8) | 0 (0) |
| Malignancy | 23 (45.1) | 6 (33.3) |
| Hematologic | 10 (19.6) | 3 (16.7) |
| Solid tumor | 13 (25.5) | 3 (16.7) |
| Immunocompromised | 20 (39.2) | 5 (27.8) |
| Chemotherapy | 11 (21.6) | 4 (22.2) |
| Corticosteroids | 9 (17.6) | 1 (5.6) |
| Neutropenia | 8 (15.7) | 3 (16.7) |
| Other immunosuppressive medications | 4 (7.8) | 1 (5.6) |
| Transplant recipients | 3 (5.9) | 0 (0) |
| Recent surgical procedure | 18 (35.3) | 1 (5.6) |
| Central venous catheter | 21 (41.2) | 1 (5.6) |
| Foley catheter | 11 (21.6) | 1 (5.6) |
| Prior antibiotic therapy | 21 (41.2) | 3 (16.7) |
| Classification: | ||
| Nosocomial | 11 (21.6) | 1 (5.6) |
| Health care-associated | 29 (56.9) | 8 (44.4) |
| Community-acquired* | 11 (21.6) | 9 (50.0) |
| ICU admission | 12 (23.5) | 7 (38.9) |
| Hypotension | 19 (37.3) | 4 (22.2) |
| Fever/hypothermia | 32 (62.7) | 13 (72.2) |
| Leukocytosis | 27 (52.9) | 9 (52.9) |
| Leukopenia | 12 (23.5) | 5 (29.4) |
| Pitt bacteremia score ≥ 4 | 11 (21.6) | 4 (22.2) |
| Source: | ||
| Urinary tract | 16 (31.4) | 4 (22.2) |
| Abdominal/biliary** | 1 (2.0) | 5 (27.8) |
| Respiratory tract | 11 (21.6) | 4 (22.2) |
| Catheter-related | 5 (9.8) | 0 (0) |
| Skin and soft tissue | 3 (5.9) | 0 (0) |
| Primary bacteremia++ | 15 (29.4) | 5 (27.8) |
| 28-day all-cause mortality | 13 (25.5) | 4 (22.2) |
Continuous data are expressed as median (interquartile range), whereas categorical data are the observed number (%) for each level.
Primary bacteremia is defined as bacteremia with no clearly established site of active infection.
p=0.022,
p=0.0038 (only p values <0.05 are shown).
Approximately, 30% of patients with monomicrobial P. aeruginosa bacteremia had primary bacteremia, defined as bacteremia with no clearly established site of active infection. An ad hoc analysis showed that patients with primary monomicrobial P. aeruginosa bacteremia were more likely to be neutropenic (5/15 [33.3%]) as compared to those who had a known source of bacteremia (3/36 [8.3%], p=0.039). Patients with primary monomicrobial P. aeruginosa bacteremia were also more likely to have a central venous catheter (10/15 [66.7%]) as compared to those with a known source (11/36 [30.6%], p=0.028). This suggests that gastrointestinal translocation of P. aeruginosa and line-associated infections might play an important role in the pathogenesis of primary P. aeruginosa bacteremia.
Among the 18 patients with polymicrobial P. aeruginosa bacteremia, 6 (33.3%) had aerobic gram-positive organisms in the same blood culture, 5 (27.8%) had other aerobic gram-negative organisms, 5 (27.8%) had anaerobic organisms, and the remaining 2 (11.1%) had more than two organisms in a blood culture.
Mortality
Monomicrobial P. aeruginosa bacteremia has poor prognosis with a 28-day and 1-year all-cause mortality of 25.5% and 47.5%, respectively. Patients with a Pitt bacteremia score10 ≥ 4 had both a higher 28-day and 1-year all-cause mortality than patients with Pitt bacteremia score < 4 (p=0.045, and p=0.002, respectively) as in Figure 3. The 28-day all-cause mortality for community-acquired, health care-associated, and nosocomial monomicrobial P. aeruginosa bacteremia was 9.1%, 27.6%, and 36.6%, respectively. Although nosocomial P. aeruginosa bacteremia had a considerable higher 28-day all-cause mortality rate than community-acquired P. aeruginosa bacteremia, due to the limited sample size, the difference in mortality across the three groups was not statistically significant (p=0.315). Likewise, although not statistically significant (p=0.368) patients < 70 years of age had a higher 28-day all-cause mortality rate (30.8%) in comparison to patients ≥ 70 years old (20.0%). However, the 1-year all-cause mortality was comparable across age groups; 46.2% in patients < 70 years, and 49.1% in patients ≥ 70 years (p=0.916). It is conceivable that the relatively high 28-day all-cause mortality rate in patients < 70 years old in comparison to patients ≥ 70 could be due to the higher prevalence of immunocompromise in patients < 70 years (16/26 [61.5%] vs. 4/25 [16.0%], p=0.001). Additionally, patients < 70 years old were less likely to have a urinary source of P. aeruginosa bacteremia compared to patients ≥ 70 years old (3/26 [11.5%] vs. 13/25 [52.0%], p=0.002) and were less likely to have community-acquired P. aeruginosa bacteremia compared to patients ≥ 70 years.
Figure 3.
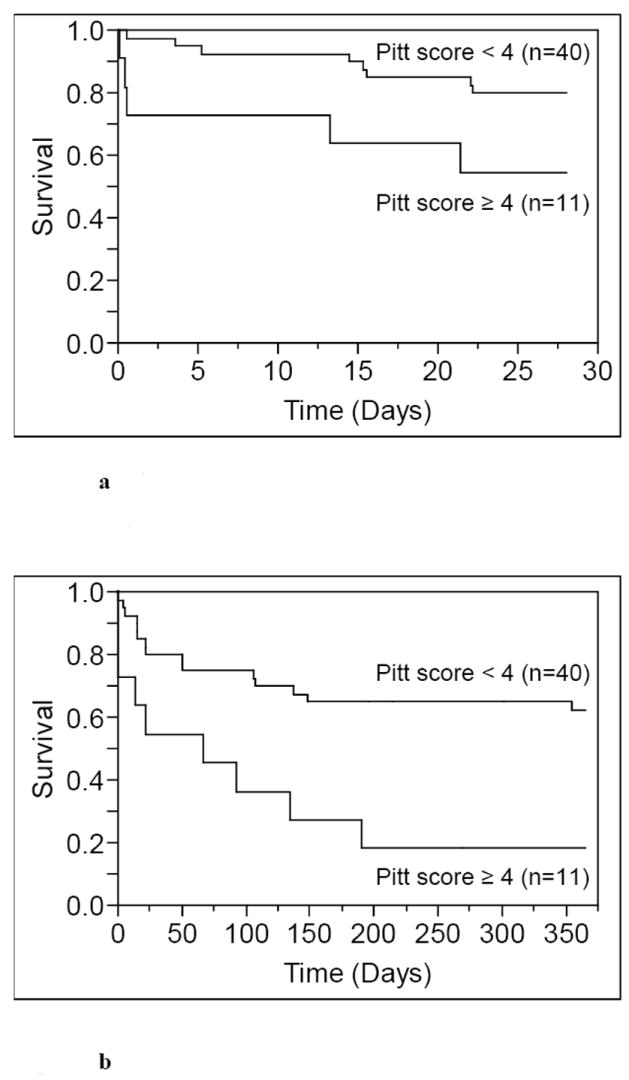
a Kaplan-Meier 28-day survival curves of patients with monomicrobial P. aeruginosa bacteremia by Pitt bacteremia score (p=0.045).
b Kaplan-Meier 1-year survival curves of patients with monomicrobial P. aeruginosa bacteremia by Pitt bacteremia score (p=0.002).
In vitro susceptibility of P. aeruginosa bacteremia
In vitro susceptibilities to ciprofloxacin and levofloxacin among P. aeruginosa isolates were 95.3% and 93.5%, respectively (Table 2). Only 2 isolates of 64 tested (3.1%) were resistant, and 1 isolate (1.6%) had intermediate susceptibility to ciprofloxacin. Likewise, only 2 of 62 isolates tested (3.2%) were resistant and another 2 (3.2%) had intermediate susceptibility to levofloxacin. Even when the 3 isolates from recurrent cases of P. aeruginosa bacteremia were included in the analysis, 62 of 67 isolates tested (92.5%) were susceptible to ciprofloxacin and 59 of 64 isolates (92.2%) were susceptible to levofloxacin.
Table 2.
Antibiotics in vitro Susceptibility of P. aeruginosa+
| Antibiotic | Number of susceptible isolates/number of isolates tested | Susceptibility % |
|---|---|---|
| Levofloxacin | 58/62 | 93.5 |
| Ciprofloxacin | 61/64 | 95.3 |
| Gentamicin | 62/64 | 96.9 |
| Amikacin | 63/64 | 98.4 |
| Ceftazidime | 63/64 | 98.4 |
| Cefepime | 63/63 | 100 |
| Piperacillin-tazobactam | 64/64 | 100 |
| Imipenem | 64/64 | 100 |
| Meropenem | 62/62 | 100 |
Including both monomicrobial and polymicrobial P. aeruginosa bacteremia isolates.
In vitro susceptibility results for both ciprofloxacin and levofloxacin were significantly higher in isolates from patients of Olmsted County as compared to those from non-Olmsted County residents (Table 3).
Table 3.
The effect of referral bias on reporting of P. aeruginosa bacteremia isolates in vitro susceptibility to fluoroquinolone antibiotics
| Characteristic | Olmsted County residents N=69 | Non-Olmsted County residents N=69 | p-value |
|---|---|---|---|
| Year of onset of bacteremia | 1997-2006 | 1997-2006 | - |
| Age+ | 72 (55-82) | 71 (56-79) | - |
| Male gender++ | 45 (65.2) | 45 (65.2) | - |
| Ciprofloxacin susceptibility* | 61/64 (95.3) | 53/64 (82.8) | 0.023 |
| Levofloxacin susceptibility* | 58/62 (93.5) | 50/62 (80.6) | 0.032 |
Data are given as median age at onset of bacteremia in years (interquartile range)
Data are given as the observed number (%).
Data are given as number of susceptible isolates/number of isolates tested (%)
DISCUSSION
Incidence
Based on an age- and gender-adjusted incidence of 6.4 per 100,000 person-years (95% CI, 4.9-8.0), we demonstrated that P. aeruginosa bacteremia (monomicrobial and polymicrobial) is a relatively rare syndrome. A previous study in Olmsted County from 2003 to 2005 demonstrated an age- and gender-adjusted incidence of 188.9, 93.2, 80.9, 47.7, and 32.0 per 100,000 person-years for all cases, gram-positive, gram-negative, Escherichia coli, and Staphylococcus aureus bloodstream infections, respectively.11 The incidence of P. aeruginosa bacteremia was higher in males than in females, particularly after the age of 50 years. In contrast, the incidence of E. coli bacteremia was higher in females in all age groups.11
Clinical characteristics
Although this was a population-based study, the majority of patients with P. aeruginosa bacteremia had multiple comorbidities and is comparable to findings in other studies of P. aeruginosa bacteremia that were based on hospital or ICU cohorts.12-15 This is probably because most patients, especially with monomicrobial P. aeruginosa bacteremia, acquired the infection in a hospital or health care setting. Many patients with P. aeruginosa bacteremia were acutely ill; 21.7% of patients had a Pitt bacteremia score ≥ 4, and 27.5% required ICU admission.
Mortality
The all-cause 28-day mortality of 25.5% in patients with monomicrobial P. aeruginosa bacteremia in the current study was less than that reported in previous investigations of P. aeruginosa bacteremia.12,16-19 The 30-day mortality of P. aeruginosa bacteremia was as high as 39% in one recent investigation.13 We believe that the lower mortality demonstrated in our population-based study is likely due, in part, to the exclusion of referral patients who characteristically have more complications with worse outcomes.
The prolonged follow-up (median, 581 days) described in the current investigation is a unique advantage of our work. Because advanced age and multiple comorbidities were commonplace among our cohort, a one-year all-cause mortality rate of 47.5% in patients with monomicrobial P. aeruginosa bacteremia is conceivable.
In vitro antibiotic susceptibility testing
Antibiotic susceptibility results of local P. aeruginosa blood isolates were somewhat unanticipated; 95.3% of isolates were susceptible to ciprofloxacin. This was considerably higher than that described in recent cross sectional studies from tertiary care hospitals in North America and Europe where in vitro susceptibility results to ciprofloxacin ranged from 64.4-70.8% among all P. aeruginosa isolates, and from 68.3-74.9% among P. aeruginosa blood culture isolates.20-24 This is consistent with previous work suggested that blood isolates of P. aeruginosa were more susceptible to fluoroquinolones than were isolates from other sites of infection.25
The higher in vitro susceptibility rates of P. aeruginosa bacteremia isolates in Olmsted County residents compared to non-Olmsted County residents suggests that referral bias likely influenced susceptibility results reported at our institution and possibly those from other tertiary care centers. Data from the United Kingdom and Ireland that were collected from a wide geographic area by 25 clinical laboratories indicate that the low rate of ciprofloxacin resistance described in blood culture isolates from patients in Olmsted County is reflective of that (7.4%) seen among strains from other non-referral populations.26 In addition, susceptibility patterns can differ from one geographic area to another.
There are few limitations in our study. First, since the population of Olmsted County is fairly small, the number of patients with P. aeruginosa bacteremia during the study period was also small. This limited the ability to perform a multivariate model to determine risk factors for mortality. Nonetheless, we had enough statistical power to examine age and gender effects on incidence rates. Second, the population of Olmsted County consists mainly of middle class whites; therefore, the results of the study may be generalized only to communities with similar population characteristics.
CONCLUSIONS
This is the first population-based study that defines the incidence and long-term outcome of P. aeruginosa bacteremia. The incidence of P. aeruginosa bacteremia increased exponentially with age, and was higher in males than in females, especially after the age of 50 years. Most cases of monomicrobial P. aeruginosa bacteremia were either nosocomially-acquired or health care-associated, especially in patients under the age of 70 years. The relatively low all-cause mortality and in vitro resistance to fluoroquinolones among blood culture isolates in our population as compared to previously reported investigations from tertiary care centers is likely due to referral bias that can impact data generated from the latter institutions.
Acknowledgments
Funding. The study receieved funding from the small grants program at the the Mayo Clinic, Rochester, MN. The funding source had no role in study design.
Footnotes
Potential conflicts of interest. MNA, JWW, BDL, JEE, and LMB: No conflict.
MNA have full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
A poster of this study was presented at the Infectious Diseases Society of America 45th annual meeting on October 5th, 2007 in San Diego, CA.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pier GB, Ramphal R. Pseudomonas aeruginosa. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and Practice of Infectious Diseases. 6. Vol. 2. Philadelphia, PA: Elsavier Churchill Livingstone; 2005. pp. 2587–2615. [Google Scholar]
- 2.Diekema DJ, Pfaller MA, Jones RN, et al. Survey of bloodstream infections due to gram-negative bacilli: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, and Latin America for the SENTRY Antimicrobial Surveillance Program, 1997. Clin Infect Dis. 1999;29(3):595–607. doi: 10.1086/598640. [DOI] [PubMed] [Google Scholar]
- 3.Friedland I, Gallagher G, King T, Woods GL. Antimicrobial susceptibility patterns in Pseudomonas aeruginosa: data from a multicenter Intensive Care Unit Surveillance Study (ISS) in the United States. J Chemother. 2004;16(5):437–41. doi: 10.1179/joc.2004.16.5.437. [DOI] [PubMed] [Google Scholar]
- 4.Kaye KS, Kanafani ZA, Dodds AE, Engemann JJ, Weber SG, Carmeli Y. Differential effects of levofloxacin and ciprofloxacin on the risk for isolation of quinolone-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2006;50(6):2192–6. doi: 10.1128/AAC.00060-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.US Census Bureau. Olmsted County QuickFacts. http://quickfacts.census.gov/qfd/states/27/27109.html.
- 6.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 7.Steckelberg JM, Melton LJ, 3rd, Ilstrup DM, Rouse MS, Wilson WR. Influence of referral bias on the apparent clinical spectrum of infective endocarditis. Am J Med. 1990;88(6):582–8. doi: 10.1016/0002-9343(90)90521-e. [DOI] [PubMed] [Google Scholar]
- 8.Tleyjeh IM, Steckelberg JM, Murad HS, et al. Temporal trends in infective endocarditis: a population-based study in Olmsted County, Minnesota. Jama. 2005;293(24):3022–8. doi: 10.1001/jama.293.24.3022. [DOI] [PubMed] [Google Scholar]
- 9.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16(3):128–40. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 10.Paterson DL, Ko WC, Von Gottberg A, et al. International prospective study of Klebsiella pneumoniae bacteremia: implications of extended-spectrum beta-lactamase production in nosocomial Infections. Ann Intern Med. 2004;140(1):26–32. doi: 10.7326/0003-4819-140-1-200401060-00008. [DOI] [PubMed] [Google Scholar]
- 11.Uslan DZ, Crane SJ, Steckelberg JM, et al. Age- and sex-associated trends in bloodstream infection: a population-based study in Olmsted County, Minnesota. Arch Intern Med. 2007;167(8):834–9. doi: 10.1001/archinte.167.8.834. [DOI] [PubMed] [Google Scholar]
- 12.Chamot E, Boffi El, Amari E, Rohner P, Van Delden C. Effectiveness of combination antimicrobial therapy for Pseudomonas aeruginosa bacteremia. Antimicrob Agents Chemother. 2003;47(9):2756–64. doi: 10.1128/AAC.47.9.2756-2764.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang CI, Kim SH, Kim HB, et al. Pseudomonas aeruginosa bacteremia: risk factors for mortality and influence of delayed receipt of effective antimicrobial therapy on clinical outcome. Clin Infect Dis. 2003;37(6):745–51. doi: 10.1086/377200. [DOI] [PubMed] [Google Scholar]
- 14.Vidal F, Mensa J, Almela M, et al. Epidemiology and outcome of Pseudomonas aeruginosa bacteremia, with special emphasis on the influence of antibiotic treatment. Analysis of 189 episodes. Arch Intern Med. 1996;156(18):2121–6. [PubMed] [Google Scholar]
- 15.Aliaga L, Mediavilla JD, Llosa J, Miranda C, Rosa-Fraile M. Clinical significance of polymicrobial versus monomicrobial bacteremia involving Pseudomonas aeruginosa. Eur J Clin Microbiol Infect Dis. 2000;19(11):871–4. doi: 10.1007/s100960000392. [DOI] [PubMed] [Google Scholar]
- 16.Hilf M, Yu VL, Sharp J, Zuravleff JJ, Korvick JA, Muder RR. Antibiotic therapy for Pseudomonas aeruginosa bacteremia: outcome correlations in a prospective study of 200 patients. Am J Med. 1989;87(5):540–6. doi: 10.1016/s0002-9343(89)80611-4. [DOI] [PubMed] [Google Scholar]
- 17.Osmon S, Ward S, Fraser VJ, Kollef MH. Hospital mortality for patients with bacteremia due to Staphylococcus aureus or Pseudomonas aeruginosa. Chest. 2004;125(2):607–16. doi: 10.1378/chest.125.2.607. [DOI] [PubMed] [Google Scholar]
- 18.Scheetz MH, Bolon MK, Scarsi KK, Fotis MA, Postelnick MJ. Lack of effect of fluoroquinolone resistance on mortality in subjects with Pseudomonas aeruginosa bacteraemia. J Infect. 2006;52(2):105–10. doi: 10.1016/j.jinf.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Blot S, Vandewoude K, Hoste E, Colardyn F. Reappraisal of attributable mortality in critically ill patients with nosocomial bacteraemia involving Pseudomonas aeruginosa. J Hosp Infect. 2003;53(1):18–24. doi: 10.1053/jhin.2002.1329. [DOI] [PubMed] [Google Scholar]
- 20.Gales AC, Jones RN, Turnidge J, Rennie R, Ramphal R. Characterization of Pseudomonas aeruginosa isolates: occurrence rates, antimicrobial susceptibility patterns, and molecular typing in the global SENTRY Antimicrobial Surveillance Program, 1997-1999. Clin Infect Dis. 2001;32(Suppl 2):S146–55. doi: 10.1086/320186. [DOI] [PubMed] [Google Scholar]
- 21.Fluit AC, Jones ME, Schmitz FJ, Acar J, Gupta R, Verhoef J. Antimicrobial susceptibility and frequency of occurrence of clinical blood isolates in Europe from the SENTRY antimicrobial surveillance program, 1997 and 1998. Clin Infect Dis. 2000;30(3):454–60. doi: 10.1086/313710. [DOI] [PubMed] [Google Scholar]
- 22.Unal S, Masterton R, Goossens H. Bacteraemia in Europe--antimicrobial susceptibility data from the MYSTIC surveillance programme. Int J Antimicrob Agents. 2004;23(2):155–63. doi: 10.1016/j.ijantimicag.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Decousser JW, Pina P, Picot F, et al. Frequency of isolation and antimicrobial susceptibility of bacterial pathogens isolated from patients with bloodstream infections: a French prospective national survey. J Antimicrob Chemother. 2003;51(5):1213–22. doi: 10.1093/jac/dkg201. [DOI] [PubMed] [Google Scholar]
- 24.Koprnova J, Beno P, Korcova J, et al. Bacteremia due to Pseudomonas aeruginosa: results from a 3-year national study in the Slovak Republic. J Chemother. 2005;17(5):470–6. doi: 10.1179/joc.2005.17.5.470. [DOI] [PubMed] [Google Scholar]
- 25.Baddour LM, Hicks DV, Tayidi MM, et al. Risk factor assessment for the acquisition of fluoroquinolone-resistant isolates of Pseudomonas aeruginosa in a community-based hospital. Microb Drug Resist. 1995;1(3):219–22. doi: 10.1089/mdr.1995.1.219. [DOI] [PubMed] [Google Scholar]
- 26.Reynolds R, Potz N, Colman M, Williams A, Livermore D, MacGowan A. Antimicrobial susceptibility of the pathogens of bacteraemia in the UK and Ireland 2001-2002: the BSAC Bacteraemia Resistance Surveillance Programme. J Antimicrob Chemother. 2004;53(6):1018–32. doi: 10.1093/jac/dkh232. [DOI] [PubMed] [Google Scholar]


