Abstract
Object
A hollow fiber catheter was developed to improve the distribution of drugs administered via direct infusion into the central nervous system (CNS). It is a porous catheter that significantly increases the surface area of brain tissue into which a drug is infused.
Methods
Dye was infused into the mouse brain through convection-enhanced delivery (CED) using a 28-gauge needle compared with a 3-mm-long hollow fiber catheter. To determine whether a hollow fiber catheter could increase the distribution of gene therapy vectors, a recombinant adenovirus expressing the firefly luciferase reporter was injected into the mouse striatum. Gene expression was monitored using in vivo bioluminescent imaging. To assess the distribution of gene transfer, an adenovirus expressing green fluorescent protein was injected into the striatum using a hollow fiber catheter or a needle.
Results
Hollow fiber catheter—mediated infusion increased the volume of brain tissue labeled with dye by 2.7 times relative to needle-mediated infusion. In vivo imaging revealed that catheter-mediated infusion of adenovirus resulted in gene expression that was 10 times greater than that mediated by a needle. The catheter appreciably increased the area of brain transduced with adenovirus relative to a needle, affecting a significant portion of the injected hemisphere.
Conclusions
The miniature hollow fiber catheter used in this study significantly increased the distribution of dye and adenoviral-mediated gene transfer in the mouse brain compared with the levels reached using a 28-gauge needle. Compared with standard single-port clinical catheters, the hollow fiber catheter has the advantage of millions of nanoscale pores to increase surface area and bulk flow in the CNS. Extending the scale of the hollow fiber catheter for the large mammalian brain shows promise in increasing the distribution and efficacy of gene therapy and drug therapy using CED.
Keywords: adenovirus, catheter, convection-enhanced delivery, gene therapy, glioma, in vivo imaging, mouse
Direct infusion of drugs into brain parenchyma using CED allows dosing of large areas of tissue and concentrating the infusate in situ, thereby circumventing the delivery obstacles posed by the blood—brain barrier and dilution of the infusate in the bloodstream.6,10,13,17,22 Convection-enhanced delivery is a technique that relies on bulk flow to establish a pressure gradient over time, resulting in continuous diffusion and widespread distribution of infusate in the brain.3,16 The extent of drug distribution achieved using CED depends on many factors, including interstitial pressure, type of tissue infused (that is, tumor, gray matter, or white matter), molecular weight of the infusate, infusion volume and rate, and diameter and type of infusion catheter(s) used.7,16,19
Data from studies in the cat and nonhuman primate brain have shown that CED allows uniform distribution of infusate, covering distances of 1 to 2.9 cm from the site of catheter placement.3,12 Hadaczek and colleagues6 have demonstrated that CED-mediated infusion of adeno-associated virus in a 35.5-μl volume covered nearly 75% of the putamen in rhesus monkeys. The distribution of infused liposomes or viral vectors can be increased further when mannitol is coadministered to increase the size of the interstitial space and to promote diffusion.17,18
Despite its proven efficacy in drug delivery in the CNS, CED is not without its limitations. Whereas the interstitial pressure in normal brain tissue is relatively low (1–2 mm Hg), that in brain tumor tissue can be more than 25 times greater, which may account for the uneven distribution and leakage of drug into the subarachnoid space that has been observed in brain tumor clinical trials.7,15,27 This phenomenon is not surprising considering that most catheters used for infusion have a single lumen from which infusate is delivered and that the infusate will usually follow the path of lowest interstitial pressure. Gliomas are composed of necrotic areas and regions of infiltrative growth into normal brain, therefore the interstitial pressure varies greatly, creating counterproductive pressure gradients in peritumoral tissue. Multiport catheters have been developed to improve infusate distribution, but data from gel models have shown that only the proximal ports deliver infusate, rendering the remaining ports useless for delivery.23 New catheters capable of minimizing reflux along the outside of the catheters and allowing for more homogeneous drug delivery would be beneficial in the treatment of brain tumor via CED.
Convection-enhanced delivery at low flow rates (that is, 0.5 μl/minute) in normal brain results in relatively homogeneous distribution, whereas higher flow rates (that is, 5 μl/minute) will cause reflux of the infusate along the catheter track and away from the target tissue.19 Attempting to infuse large volumes of drug over a short time period creates a deforming force on tissue, eventually narrowing the interstitial space and promoting a shear plane or tissue tearing;18,19,27 therefore, a long administration time is required to deliver even 1 ml of infusate, because higher flow rates negate the desired distribution of drug via CED. Novel methods to increase the flow rate and decrease the total infusion time would simplify and shorten clinical treatment using CED.
In this study we evaluated a new catheter designed to increase drug distribution using CED. We reasoned that the use of a porous hollow fiber catheter instead of a single-lumen infusion device would decrease deforming forces on the tissue surrounding the catheter and consequently increase bulk flow and total distribution of the infusate.20 We used a hollow fiber catheter made of polysulfone with pore diameters of 0.45 μm, thereby providing multiple pathways for infusate to travel around each cell in the brain, which can be anywhere from 10 to 100 μm in diameter. As an initial test we used a 3-mm hollow fiber catheter and compared the distribution of dye injected into agarose gel and mouse brain relative to the distribution achieved with a 28-gauge needle, which is commonly used to deliver viral and nonviral vectors into the brain via CED.17,21,27 In addition, we used recombinant adenoviral vectors expressing the firefly luciferase and GFP reporter genes to compare the distribution achieved using a hollow fiber catheter and a needle. Results of in vitro and in vivo studies with dye and viral vectors revealed that a hollow fiber catheter significantly increases the distribution of infusate compared with a single-lumen needle. These findings indicate that porous catheters may improve the distribution of CED-mediated drug delivery in the CNS, compared with single-lumen catheters.
Materials and Methods
Hollow Fiber Catheter Construction
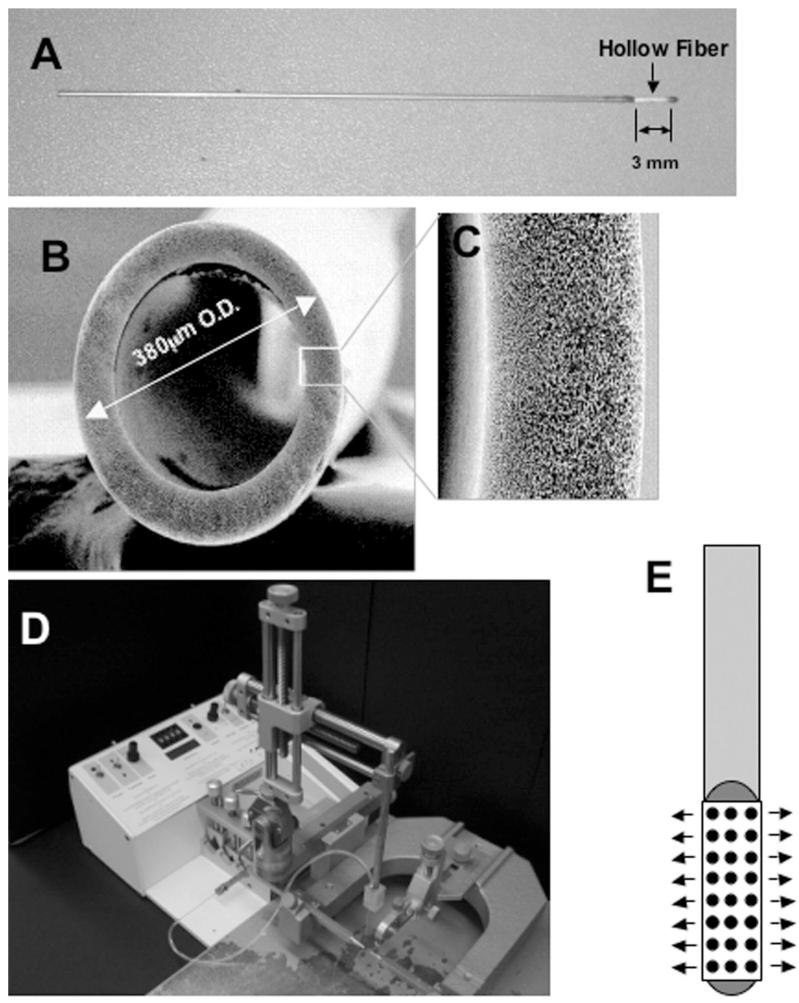
Hollow fiber catheters (Twin Star Medical, Inc.; Fig. 1) consisted of a single 380-μm-diameter hollow fiber made of polysulfone with a sealed distal tip. The proximal hollow fiber was attached to tubing that allows handling of the hollow fiber and its attachment to an infusion pump. The exposed hollow fiber was 3 mm in length. The wall of the hollow fiber was composed of a porous material with multiple interconnected passageways. The nominal pore size was 0.45 μm.
Fig. 1.
A: Photograph illustrating a 3-mm hollow fiber catheter attached to the end of a stainless-steel cylinder body. The outer diameter of the hollow fiber catheter (380 μm) is similar to that of a conventional 28-gauge needle (355 μm). B: Magnified cross-sectional image featuring the hollow fiber catheter. Original magnification × 200. C: Magnified image depicting the area of the hollow fiber catheter outlined by the white box in panel B. Original magnification × 1000. D: Photograph showing the system used for stereotactic CED into the mouse brain. The needle or catheter was mounted directly onto the arm of the frame. The catheter(s) were connected to tubing attached to a syringe, which delivered infusate at a flow rate controlled by the micropump. E: Schematic depicting the hollow fiber catheter shown in panel A. Infusate is delivered along the shaft of the entire hollow fiber as depicted by the arrows.
Mice and Experimental Groups
Thirty-six B6CBA mice were used for the in vivo experiments in this study. The University of Minnesota Research Animal Resources bred the mice, which were kept in a specific pathogen—free housing facility at the University of Minnesota. All experiments involving animals were conducted according to an approved Institutional Animal Care and Use Committee protocol, and every effort was made to minimize pain and discomfort. The housing area was maintained on a 12-hour light/12-hour dark cycle at 22 ± 2°C throughout the study.
The tissue processing for each assay conducted on brain tissue is described in detail in the corresponding sections of the proceeding text. For the dye studies, nine mice were used (three mice/group), and these animals were killed and perfused 1 hour after injection of the dye. For the quantification of the brain tissue area covered with dye, another six mice were used and perfused identically (three mice/group). Seven mice were treated with firefly luciferase—encoding virus: three via the hollow fiber catheter, three via the needle, and one untreated (control). In vivo imaging was conducted 24, 48, and 72 hours after gene delivery. In eight mice (four animals/group), the brains were tested for gene expression 48 hours later. For the GFP transduction experiment, six mice were used (three animals/group), and the brains were harvested 48 hours after gene delivery for histological analysis.
Anesthesia, Surgery, and Infusate Delivery
Before surgery, animals were deeply anesthetized with a ketamine/xylazine cocktail solution (53.7 mg/ml ketamine and 9.26 mg/ml xylazine) delivered at 1 ml/kg via an intraperitoneal injection. Anesthetized mice were promptly secured in a stereotactic frame (David Kopf Instruments). A skin incision was made along the dorsal midline of the skull to expose the sagittal crest. A bur hole was made using a 0.5-mm dental drill (Medidenta, Inc.) at the appropriate position on the skull. For striatal injections, we used the following coordinates: 0.5 mm anterior, 2.2 mm mediolateral, and 3.0 mm deep from the bregma. A 28-gauge Hamilton syringe needle or 3-mm hollow fiber catheter was used for each injection into the striatum by connecting to sterilized Tygon tubing (Cole-Parmer Instrument Company), which was attached to a CMA/100 programmable microinjector (Carnegie Medicin AB).
For CED of adenovirus, all mice were given a 200-μl injection of 25% mannitol solution (American Regent, Inc.) into the lateral tail vein 1 hour before surgery to potentiate vector diffusion.17,18 Virus (8.19 × 108 pfu) was diluted in sterile saline for a final volume of 60 μl at a final concentration of 1.31 × 107 pfu/μl. The virus solution was drawn into the Tygon tubing through a Hamilton syringe so that no dead space was apparent; the tubing was then connected to a 28-gauge needle or 3-mm hollow fiber catheter and flushed until solution dripped from the needle tip. The needle or catheter was attached to the arm of the stereotactic frame with tape and slowly inserted into the brain to target the striatum through the bur hole that had been made with a dental drill. Two microliters of virus was unilaterally infused into the right striatum over 20 minutes (0.1 μl/minute) using a CMA/100 programmable microinjector (Carnegie Medicin AB). The needle or catheter was slowly withdrawn over 2 minutes, after injection of the vector. After withdrawing the needle or catheter, bone wax was inserted into the bur hole. The skin was then closed with 6-0 Ethicon sutures (Ethicon, Inc.). After surgery, ketoprofen (5 mg/kg subcutaneously, MP Biomedical) was administered for postoperative analgesia. Mice were monitored until they regained consciousness and were then returned to their housing area.
For in vivo administration of dye, 2 g of Evans blue powder (Fisher Scientific) was solubilized in 100 ml of sterilized saline solution. Before use, the solution was filtered through a 0.45-μm microfilter (Millipore). The surgical procedure for dye injection was identical to that used for adenoviral injection, with the single difference that mannitol was not administered prior to infusion. The flow rate was 0.1 μl/minute. Bolus dye injections were performed using the same coordinates, although the injection was given within 10 seconds, and the needle was left in the brain for 20 minutes after the injection. The needle was withdrawn over 30 seconds.
Recombinant Adenoviral Vectors
The RAdLUC and RAdGFP used in this study are first-generation replication-defective recombinant adenovirus type 5 vectors expressing the transgene under the transcriptional control of the human cytomegalovirus intermediate early promoter within the E1 region. Both the RAdLUC and RAdGFP vector seed stock were the generous gift of Dr. Harvey Herschman and were constructed as described previously.14 The vectors were scaled up in our laboratory by infecting the human embryonic kidney 293 cell line with 3 IU/cell of vector seed stock. Cells were harvested 72 hours later and purified by three-step CsCl gradients, as previously described in detail.26 The vectors were titered in triplicate by end point dilution, a cytopathic effect assay. The titer determined was 8.19 × 1010 pfu/ml. The vector preparation was screened for the presence of replication-competent adenovirus5,26 and lipopolysaccharide contamination (Cambrex).4,26 The virus preparations used were free from replication-competent adenovirus and lipopolysaccharide contamination. The expression of firefly luciferase encoded within RAdLUC was verified by infecting the vector onto COS-7 cells with a multiplicity of infection of 100 pfu/cell and by measuring bioluminescence activity 72 hours later using the Dual Luciferase Reporter Assay System (Promega).
Dye Infusion Into Agarose Gel and Distribution Calculations
Evans blue dye was infused via a hollow fiber catheter and 28-gauge needle into a 4% agarose gel by using a microinjection pump. The total volume was 2 μl and the flow rate was 0.1 μl/minute. Gel was firmly shaped before injection. The data shown (Fig. 2A) are representative results of three separate experiments. The volume of dye distribution was calculated according to the equation for the volume of an ellipsoid: V = 4/3πabc, where a, b, and c are the lengths of the three semi-axes.
Fig. 2.
A: Image showing the distribution of 2 μl of dye infused into an agarose gel over 20 minutes using a 28-gauge needle or a 3-mm hollow fiber. B: Images of tissue sections depicting the distribution of 2 μl of dye infused into the mouse brain via stereotactic targeting to the striatum using a bolus injection (10 seconds) or CED (20-minute injection) with a 28-gauge needle or a hollow fiber catheter. C: Bar graph demonstrating the volume of dye distribution in murine brain infused with 2 μl of dye over 20 minutes using a hollow fiber catheter or 28-gauge needle (three mice/group, five sections per animal). Use of the hollow fiber catheter significantly increased the volume of brain labeled with dye. *p ≤ 0.05, t-test.
Perfusion and Tissue Processing
One hour after delivery of the Evans blue dye into the striatum, mice were deeply anesthetized with the ketamine/xylazine cocktail and then given freshly prepared Z-fix solution (formaldehyde and ionized zinc in buffer solution; Anatech, Ltd.) through cardiac perfusion. Dye-injected mouse brain was frozen with optimal cutting temperature embedding compound (Sakura) in a liquid N2 chamber. The brain-embedded cube was cut coronally using a JUNG CM3000 cryostat machine (IMEB, Inc.) to a 10-μm thickness and was mounted onto slides. Frozen sections on the slides were dehydrated by dipping in 50, 70, 80, 90, and 100% ethanol and then 100% xylene for 1 minute sequentially. Dried sections were mounted with DPX mounting media (Fluka Chemie) for histological analysis and topped with a coverslip.
Mice injected with RAdGFP via CED were perfused identically 48 hours after gene delivery. After perfusion, brains were incubated in the Z-fix solution overnight for additional fixing time, then transferred into a 30% sucrose solution for 4 days. For RAdGFP-injected brains, 30-μm-thick coronal sections were cut through the striatum using a microtome.
Measurement of Evans Blue and GFP Distribution in the Murine Brain
Each section from dye-injected brain was photographed using a SPOT black and white camera (model 310, Diagnostic Instruments, Inc.), which was attached to a Nikon Eclipse E600 microscope (Nikon Instruments, Inc.) with a magnification of 1. Pictures were analyzed by measuring the dye-distributed area using ImageJ software (freeware, developed by Wayne Rasband). Once the software was calibrated and an area of interest was highlighted, the program calculated the exact size of the measured area. We collected and measured one 0.1-mm-thick section from every five sections obtained from the dye-injected mouse brain. The volume of dye distribution (mm3) was calculated as the sum of distributed areas in each section in mm2 × 5 × 0.01 mm.
Green fluorescent protein expression was visualized with a Zeiss Atto Arc HBO 110W upright fluorescence microscope (Carl Zeiss, Inc.) in combination with nuclear marker DAPI mountant (Vector Laboratories). Full-size coronal sections were photographed using a Leica microscope (Leica Microsystems, Inc.) and SPOT RT color camera (Diagnostic Instruments, Inc.).
Firefly Luciferase Assays
Each brain was homogenized mechanically in 1× tissue lysis buffer (Promega). Firefly luciferase assays were performed on mouse brain lysates using a luciferase assay kit according to the manufacturer’s instructions (Promega). The background luciferase activity was determined from brain tissue from untreated mice (noninjected).
Statistical Analyses
All statistics analyses were conducted using StatView, a statistical software program (SAS Institute, Inc.). An unpaired Student t-test was used to compare values from the needle and hollow fiber catheter groups, and a probability level less than 0.05 was considered significant.
Results
Hollow Fiber Increased Dye Distribution In Vitro and In Vivo
A 3-mm hollow fiber catheter was compared with a 28-gauge needle to model a single-lumen catheter of similar size and scale. As a preliminary experiment, the distribution efficiency of the hollow fiber was compared with that of a 28-gauge needle by infusing dye into a 4% agarose gel. A hollow fiber catheter was inserted into the gel several centimeters away from the needle, and 2 μl of Evans blue dye was infused through each catheter at a flow rate of 0.1 μl/minute to deliver a final volume of 2 μl. Compared with the infusion volume achieved with the 28-gauge needle using the same infusion parameters, the hollow fiber catheter—infused volume was calculated to be 3.7 times greater (Fig. 2A). The volume of distribution achieved using the catheter was 189.97 mm3, as opposed to 50.94 mm3 with the conventional needle.
To determine whether the hollow fiber catheter could improve the distribution of small molecules in vivo, dye was injected into the mouse brain via CED. When 2 μl of Evans blue dye was infused via CED into the right striatum over 20 minutes by using a hollow fiber catheter or a 28-gauge needle, a noticeable increase in dye distribution was observed in coronal sections (flow rate of 0.1 μl/minute; Fig. 2B). This experiment was repeated: three mice were treated using a catheter and three with a needle (flow rate of 0.1 μl/minute). Within 1 hour of dye infusion, the mice were killed and the brains were sectioned to determine dye distribution. An automatic picture area measurement program (ImageJ) was used to measure the area of dye distribution in each section. The area of dye distribution was measured in every fifth 10-μm-thick section covering the entire labeled area of brain, allowing the total volume of dye distribution to be determined. The distribution of Evans blue dye in the brain was 2.7 times larger when the catheter was used relative to conventional needle use (Fig. 2C); this difference was statistically significant (p < 0.05).
To confirm that convection was being achieved during the aforementioned 20-minute infusions, a bolus injection was given. A bolus injection of 2 μl of dye resulted in re-flux, with dye leaking onto the cortical surface (not shown); very little dye was delivered into the parenchyma, with most of it appearing around the needle track (Fig. 2B). This result indicates that CED was achieved during the 20-minute infusion into the mouse brain.
Hollow Fiber Increased Adenoviral-Mediated Gene Transfer In Vivo
Experiments were conducted to establish how the hollow fiber catheter performed as a gene therapy infusion catheter. Delivery of the gene therapy vector was evaluated by injecting the adenoviral vector RAdLUC into the mouse striatum. Adult mice were injected with 1.4 × 107 particles of RAdLUC in 2 μl of saline, which was delivered over 20 minutes using a hollow fiber catheter or needle (three animals/group, flow rate of 0.1 μl/minute). Luciferase expression was then measured at 24, 48, and 72 hours after injection using in vivo bioluminescent imaging. Mice that received RAdLUC through a catheter displayed a more than 10-fold increase in measured luciferase expression compared with mice treated with an identical dose of virus using a needle; this difference was significant and remained constant from 24 to 72 hours after injection of the virus (Fig. 3B).
Fig. 3.
A: Photographs depicting adult mice 24 hours after injection with RAdLUC using a hollow fiber catheter or a conventional needle (three mice/ group, flow rate of 0.1 μl/minute). Luciferase expression was measured 24, 48, and 72 hours after injection using in vivo bioluminescent imaging. B: Graph demonstrating firefly luciferase expression in animals featured in panel A. *p ≤ 0.05, Student t-test. C: Bar graph revealing luciferase expression 48 hours after injection with RAdLUC using the same parameters mentioned in panel A. Only the murine brains were homogenized and assayed for luciferase activity 48 hours after gene delivery. *p ≤ 0.05, Student t-test. RLU = relative light units.
To corroborate the in vivo imaging data, this experiment was repeated using an identical dose of virus and identical infusion parameters (four animals/group). The mice were killed 48 hours after injection of RAdLUC. The brains were removed, homogenized, and immediately assayed for luciferase expression using an in vitro activity assay. Using this technique for measuring luciferase expression, we noted that the hollow fiber catheter increased gene transfer fourfold relative to needle transfer. Although the variability in luciferase expression was high (Fig. 3C), this difference was statistically significant (p < 0.05). In summary, results of both the in vivo imaging study and in vitro assay revealed a significant increase in total luciferase expression when a hollow fiber catheter was used compared with a conventional needle.
Hollow Fiber Increased Distribution of Adenoviral Vector In Vivo
Firefly luciferase is a useful reporter gene to determine total gene transfer and expression but does not address the distribution of gene transfer into the brain parenchyma. To determine the distribution of transduced cells using a hollow fiber catheter or a needle, the adenoviral vector RAdGFP was injected into the mouse striatum. The infusion parameters were identical to those used for RAdLUC: 1.4 × 107 particles of RAdGFP in 2 μl of saline delivered over 20 minutes using a hollow fiber catheter or needle (three animals/group, flow rate of 0.1 μl/minute). Two days after the administration of RAdGFP, all mice were killed and the brains were analyzed using fluorescence microscopy to visualize GFP expression. Both the hollow fiber catheter and the conventional needle facilitated transduction of a large portion of the striatum. However, brains infused using a catheter displayed a much larger area of transduced cells compared with the area revealed following needle use, including cells in the cortex and near the lateral ventricles (Fig. 4A). The GFP-positive cells colocalized with DAPI (stains nuclei), ruling out nonspecific autofluorescence as potentially yielding a false-positive signal for transduced cells (Fig. 4B). The same trend was evident in all mice; catheter-mediated infusion resulted in greater distribution of gene transfer. To quantify the area of gene delivery, measurements of GFP-positive areas were made rostral to caudal relative to the injection site, covering the majority of transduced tissue. Hollow fiber catheter delivery was associated with a significant increase in the GFP-transduced area (Fig. 4C).
Fig. 4.
A: Fluorescence microscopy images demonstrating the distribution of GFP-transduced cells in representative adult mice injected with RAdGFP delivered using a hollow fiber catheter or needle (three animals/group, flow rate of 0.1 μl/minute). Note the increase in gene transfer in representative sections when the catheter was used. Original magnification × 2. B: Fluorescence microscopy images demonstrating GFP expression and DAPI staining of cell nuclei. The GFP-positive cells colocalized with DAPI, indicating that GFP expression was not due to autofluorescence caused by necrosis. Bar = 100 μm. C: Bar graph revealing the area of GFP-transduced brain tissue in four sections from three animals at the indicated distance (mm) from the bregma. Use of the hollow fiber catheter led to a significant increase in the labeled area throughout a large region of brain. *p ≤ 0.05, Student t-test.
Discussion
The objective of CED is to utilize the interstitial pathways between cells as highways for drug delivery by establishing a pressure gradient and using convection to “push” the drug away from the catheter. This delivery method permits optimal distribution of infusate when a small-diameter catheter is used and when the flow rate is very low (that is, ≥ 0.5 μl/minute).18,19 The problem with conventional CED is that the process can require hours to days to deliver therapeutic doses. Increasing the flow rate modestly to 5.0 μl/minute causes reflux along the catheter in normal brain tissue,19 and in patients with brain tumor high flow rates can result in the infusate leaving the cerebrum entirely.7,27 This low infusion rate required for CED is due to impedance mismatch, which is a concept first used in electrical engineering. Impedance mismatch refers to a condition in which one system is unable to accommodate input from another system. In electrical engineering, a signal could be reflected back along a sending cable if the receiving cable was not of sufficient conductance. The primary limitation in the delivery of agents to brain tissue is the low capacity of convective fluid flow within the brain.
As derived from the Darcy law, flow through a porous medium is the product of the cross-sectional area and pore velocity. The cross-sectional area available for flow in brain parenchyma is the extracellular space, which is known to be quite limited. The velocity of fluid movement within the brain is the energy-consuming, rate-limiting step of moving a drug through the tissue.
Rosenberg and associates24 determined the velocity of fluid movement in white matter to be 10 μm/minute toward the ventricle of a normal brain. Bauman and colleagues2 calculated the velocity of nanoscale flow in isotropic tissue phantoms to be 10 μm/second. There are few options available to increase flow within the tissue. Increasing the extra-cellular space is one method. Administration of hypertonic mannitol, which decreases free water content in the brain, thereby shrinking cells and widening interstitial pathways, has been shown to dramatically increase the distribution of genetic vectors injected into the CNS.17,18 Unfortunately, there is no method of increasing interstitial fluid velocity. Increasing the driving pressure will not increase velocity but serve only to deform the tissues. The deformation of tissue accelerates impedance mismatch due to compaction of tissue, and further decreases porosity and hydraulic conductance. If the free fluid accumulates at a rate faster than it can dissipate, it will either flow backward along the shaft of the needle or tear into the tissue by shear force.
The problem of impedance mismatch is ameliorated with hollow fiber catheters because impedance mismatch is reduced, not by decreasing the impedance within the tissue but by increasing impedance in the catheter. Hollow fiber catheter walls contain millions of nanoscale pores. Essentially, the porosity of the hollow fiber catheter approximates the porosity of brain tissue, so impedance mismatch is avoided. This concept has been called “pore connectedness.”29 Increased impedance to flow through the hollow fiber catheter wall is compensated for by an increased surface area and a reduced potential for generation of the tissue-deforming force. The surface area of a 3-mm hollow fiber is more than 25 times the surface area of the open tip of a 28-gauge needle. Given that force is a product of pressure and area, the open tip of a 28-gauge needle will generate 874,000 times more deforming force than the pores of the hollow fiber catheter (Fig. 5). Of course, a significant limitation in drug delivery studies with rodents is scaling up to human-sized catheters. Another advantage of the hollow fiber catheter is the uniform drug delivery along the length of the catheter. Transmural outflow is reasonably homogeneous from the proximal to distal end, compared with that in multiple-pore catheters, because of the high transmural resistance and low intraluminal resistance to flow.23 Linear homogeneity allows the use of long catheters up to several centimeters in length, which would be a clinically relevant catheter. With increased surface area of the hollow fiber, relatively large volumetric flow is possible at nominal flow velocities.
Fig. 5.

Schematic demonstrating tissue—pore interaction for the conventional catheter and hollow fiber catheter. The tissue deforming force is a product of infusion pressure and cross-sectional area of the tissue—pore interface. A 1-mm pore of a conventional CED catheter generates five million times more tissue deforming force than the nanoscale pores of hollow fiber catheters.
In the current study, we postulated that a long, cylindrical catheter containing multiple pores for infusion improves the distribution of infusate via CED for the following reasons: 1) nanoscale pores over a large surface area reduce the deforming force locally by increasing the area of tissue in contact with the infusion point(s); 2) the infusate is “pushed” out over a 360° radius as opposed to a single infusion point; and 3) the drug is infused around each cell body, ranging from 10 to 100 μm in the brain, through small 0.45-μm pores along the catheter shaft. In summary, the advantage of a large surface area for delivery of an agent is that maximal interstitial flow is achieved while interstitial fluid velocity is controlled and pore connectedness is maintained. The end result is that more cells come into contact with the active agent, delivered by homogeneous, interstitial, convective flow.
To preliminarily determine whether the hollow fiber catheter could provide any meaningful benefit compared with a single-lumen catheter, a miniature 3-mm hollow fiber catheter was constructed and tested against a 28-gauge needle, which served as a model of a single-lumen catheter of similar size and scale. A pilot study that was conducted by infusing dye into an agarose gel revealed that hollow fiber catheter delivery doubled the area of dye diffusion compared with the 28-gauge needle (Fig. 2A). Similar results were observed in the mouse striatum, where hollow fiber catheter infusion increased the volume of brain labeled with dye 2.7 times relative to needle-infused volume (Fig. 2C).
The Evans blue dye that was used in this study has a molecular weight of 960 D, which is similar to many commonly used chemotherapeutic drugs such as paclitaxel (854 D), 5-FU (130 D), carboplatin (371 D), and carmustine (240 D). This finding indicates that the hollow fiber catheter may be useful in increasing the distribution of chemotherapy in the brain via CED. A next step toward investigating this hypothesis will be the use of the hollow fiber catheter for CED in an animal model of glioblastoma multiforme. Morrison and colleagues19 have established a mathematical model predicting that total brain size will not significantly alter the distribution of infusate in the brain via CED; therefore, it is likely that the outcome of this study can be applied to humans.
The results obtained using a hollow fiber catheter to infuse firefly luciferase—encoding adenovirus were not expected. We initially hypothesized that the hollow fiber catheter would increase the distribution of adenovirus relative to a needle, but not increase the total level of gene expression when an identical dose of virus was administered. After the injection of 1.4 × 107 pfu of adenovirus into the mouse striatum under identical infusion parameters, the total luciferase expression measured by in vivo imaging was more than 10 times greater when the hollow fiber catheter was used compared with the 28-gauge needle. One possible explanation for this increase in expression is that the copies of vector per cell may be higher when a conventional needle is used, but more total cells are transduced with the hollow fiber catheter. The distribution of GFP-encoding adenovirus injected into the striatum confirmed that the hollow fiber catheter increased the distribution of GFP-transduced cells (Fig. 4). Perhaps the transcription and translation of luciferase DNA/RNA has a maximum threshold on a per cell basis, and therefore increasing vector copies per cell beyond that threshold cannot proportionally increase gene expression. Instead, increasing the total number of cellstransduced may be a more efficient way of generating peak total gene expression. This phenomenon has been observed in in vitro transduction experiments with adenoviral vectors, in which coinfection of two vectors has reduced the expression of each individual transgene compared with cells infected with a single vector alone.1
In a separate experiment, the firefly luciferase—encoding adenovirus was injected under conditions identical to those in the aforementioned study, but the brains were removed, homogenized, and assayed in vitro for total luciferase activity. In this experiment, the hollow fiber catheter increased gene expression fourfold, which represents a significantly smaller difference relative to what was measured using in vivo imaging (compare Fig. 3B and C). It is important to note that luciferase bioluminescent imaging relies on visible light penetrating tissue and reaching the camera and that tissue depth will therefore affect the light measured because tissue absorbs visible light. The hollow fiber catheter was associated with more widespread transduction relative to when a needle was used; there were more transduced cells distal from the injection site (Fig. 4A and C). Therefore, the emitted light may have less tissue to pass through to reach the camera compared with cells transduced more focally when a needle was used. This hypothesis would explain why the in vivo imaging showed a more than 10-fold increase, and the whole-brain activity assay showed a fourfold increase in total gene expression when the hollow fiber catheter was used rather than a needle. Nevertheless, despite the discrepancies between in vivo and in vitro assays, data from these studies revealed that the hollow fiber catheter increases the distribution and amount of gene transfer relative to a single-lumen catheter in the mouse brain.
Achieving global distribution of gene therapy vectors in brain tissue remains a formidable obstacle to clinical efficacy in human patients. There are several diseases affecting the brain that are caused by the absence of an enzyme, such as mucopolysaccharidosis, Canavan disease, and Niemann—Pick disease. To achieve clinical improvements for these diseases, it will be necessary to transduce cells throughout the brain rather than just small areas because the missing enzyme is required globally.9 Likewise, gene therapy approaches that rely on RNA interference to destabilize endogenous RNA molecules that cause pathogenesis, such as spinocerebellar ataxia and Huntington disease, require that large areas of the brain are transduced to show behavioral correction in animal models.8,28 Gliomas are yet another example of a disease requiring widespread transduction by gene therapy, as the invasive migratory cells are commonly found several centimeters away from the bulk tumor mass.11,25 Convection-enhanced delivery has opened the possibility of achieving widespread distribution of genetic vectors and drugs in the human brain, but reflux of the infusate, long infusion times, and tissue deformation have limited its efficacy. The hollow fiber catheter is a simple, scalable tool that shows promise in improving the efficacy of drug delivery via CED.
Conclusions
A hollow fiber catheter was compared with a 28-gauge needle, a model of a single-lumen catheter of similar size, for CED. Results of both the in vitro gel model and in vivo mouse model indicated that the hollow fiber catheter significantly increased the distribution of Evans blue dye compared with a needle. When a recombinant adenoviral vector was used to compare the hollow fiber catheter and a needle, luciferase in vivo imaging showed that the former device increased gene expression by 10 times. The whole-brain luciferase activity measured in vitro was four times greater when a hollow fiber catheter was used for infusion into the mouse striatum. These results were consistent with those obtained using a GFP-encoding virus, which revealed a significant increase in the distribution of GFP-transduced cells when a hollow fiber catheter was used. Taken together, these results demonstrate that the hollow fiber catheter may be useful in improving drug distribution via CED in human patients.
Acknowledgments
The work conducted at the University of Minnesota was supported by a grant from the Biomedical Engineering Institute (J.R.O.). The work performed at the Gene Therapeutics Research Institute was supported by Grant Nos. UO1 NS052465-01, 1R01 NS44556.01, RO3 TW006283-01 (M.G.C.), 1RO1 NS 42893.01, U54 NS045309-01, and 1R21 NS047298-01 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health; the Bram and Elaine Goldsmith Chair in Gene Therapeutics (P.R.L.); the Medallion Group Chair in Gene Therapeutics (M.G.C.); The Linda Tallen and David Paul Kane Annual Fellowship (M.G.C.); and the Board of Governors at Cedars-Sinai Medical Center (P.R.L.).
Abbreviations used in this paper
- CED
convection-enhanced delivery
- CNS
central nervous system
- DAPI
4,6′-diamino-2-phenylindole-dihydrochloride
- GFP
green fluorescent protein
- pfu
plaque-forming units
- RAdGFP
recombinant adenovirus encoding GFP
- RAdLUC
RAd encoding firefly luciferase
References
- 1.Ali S, King GD, Curtin JF, Candolfi M, Xiong W, Liu C, et al. Combined immunostimulation and conditional cytotoxic gene therapy provide long-term survival in a large glioma model. Cancer Res. 2005;65:7194–7204. doi: 10.1158/0008-5472.CAN-04-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauman MA, Gillies GT, Raghavan R, Brady ML, Pedain C. Physical characterization of neurocatheter performance in brain phantom gelatin with nanoscale porosity: steady-state and oscillatory flows. Nanotechnology. 2004;15:92–97. [Google Scholar]
- 3.Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci U S A. 1994;91:2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cotten M, Baker A, Saltik M, Wagner E, Buschle M. Lipopolysaccharide is a frequent contaminant of plasmid DNA preparations and can be toxic to primary human cells in the presence of adenovirus. Gene Ther. 1994;1:239–246. [PubMed] [Google Scholar]
- 5.Dion LD, Fang J, Garver RI., Jr Supernatant rescue assay vs. polymerase chain reaction for detection of wild type adenovirus-contaminating recombinant adenovirus stocks. J Virol Methods. 1996;56:99–107. doi: 10.1016/0166-0934(95)01973-1. [DOI] [PubMed] [Google Scholar]
- 6.Hadaczek P, Kohutnicka M, Krauze MT, Bringas J, Pivirotto P, Cunningham J, et al. Convection-enhanced delivery of adeno-associated virus type 2 (AAV2) into the striatum and transport of AAV2 within monkey brain. Hum Gene Ther. 2006;17:291–302. doi: 10.1089/hum.2006.17.291. [DOI] [PubMed] [Google Scholar]
- 7.Hall WA, Sherr GT. Convection-enhanced delivery of targeted toxins for malignant glioma. Expert Opin Drug Deliv. 2006;3:371–377. doi: 10.1517/17425247.3.3.371. [DOI] [PubMed] [Google Scholar]
- 8.Harper SQ, Staber PD, He X, Eliason SL, Martins IH, Mao Q, et al. RNA interference improves motor and neuropathological abnormalities in a Huntington’s disease mouse model. Proc Natl Acad Sci U S A. 2005;102:5820–5825. doi: 10.1073/pnas.0501507102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodges BL, Cheng SH. Cell and gene-based therapies for the lysosomal storage diseases. Curr Gene Ther. 2006;6:227–241. doi: 10.2174/156652306776359522. [DOI] [PubMed] [Google Scholar]
- 10.Kawakami K, Kawakami M, Kioi M, Husain SR, Puri RK. Distribution kinetics of targeted cytotoxin in glioma by bolus or convection-enhanced delivery in a murine model. J Neurosurg. 2004;101:1004–1011. doi: 10.3171/jns.2004.101.6.1004. [DOI] [PubMed] [Google Scholar]
- 11.Kuratsu J, Itoyama Y, Uemura S, Ushio Y.Regrowth patterns of glioma—cases of glioma regrew away from the original tumor Gan No Rinsho 1989. (Jpn)351255–1260. [PubMed] [Google Scholar]
- 12.Laske DW, Morrison PF, Lieberman DM, Corthesy ME, Reynolds JC, Stewart-Henney PA, et al. Chronic interstitial infusion of protein to primate brain: determination of drug distribution and clearance with single-photon emission computerized tomography imaging. J Neurosurg. 1997;87:586–594. doi: 10.3171/jns.1997.87.4.0586. [DOI] [PubMed] [Google Scholar]
- 13.Laske DW, Youle RJ, Oldfield EH. Tumor regression with regional distribution of the targeted toxin TF-CRM107 in patients with malignant brain tumors. Nat Med. 1997;3:1362–1368. doi: 10.1038/nm1297-1362. [DOI] [PubMed] [Google Scholar]
- 14.Liang Q, Dmitriev I, Kashentseva E, Curiel DT, Herschman HR. Noninvasive of adenovirus tumor retargeting in living subjects bya soluble adenovirus receptor-epidermal growth factor (sCAR-EGF) fusion protein. Mol Imaging Biol. 2004;6:385–394. doi: 10.1016/j.mibio.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Lidar Z, Mardor Y, Jonas T, Pfeffer R, Faibel M, Nass D, et al. Convection-enhanced delivery of paclitaxel for the treatment of recurrent malignant glioma: a phase I/II clinical study. J Neurosurg. 2004;100:472–479. doi: 10.3171/jns.2004.100.3.0472. [DOI] [PubMed] [Google Scholar]
- 16.Lieberman DM, Laske DW, Morrison PF, Bankiewicz KS, Oldfield EH. Convection-enhanced distribution of large molecules in gray matter during interstitial drug infusion. J Neurosurg. 1995;82:1021–1029. doi: 10.3171/jns.1995.82.6.1021. [DOI] [PubMed] [Google Scholar]
- 17.Mamot C, Nguyen JB, Pourdehnad M, Hadaczek P, Saito R, Bringas JR, et al. Extensive distribution of liposomes in rodent brains and brain tumors following convection-enhanced delivery. J Neurooncol. 2004;68:1–9. doi: 10.1023/b:neon.0000024743.56415.4b. [DOI] [PubMed] [Google Scholar]
- 18.Mastakov MY, Baer K, Xu R, Fitzsimons H, During MJ. Combined injection of rAAV with mannitol enhances gene expression in the rat brain. Mol Ther. 2001;3:225–232. doi: 10.1006/mthe.2001.0246. [DOI] [PubMed] [Google Scholar]
- 19.Morrison PF, Chen MY, Chadwick RS, Lonser RR, Oldfield EH. Focal delivery during direct infusion to brain: role of flow rate, catheter diameter, and tissue mechanics. Am J Physiol. 1999;277:R1218–R1229. doi: 10.1152/ajpregu.1999.277.4.R1218. [DOI] [PubMed] [Google Scholar]
- 20.Neeves KB, Lo CT, Foley CP, Saltzman WM, Olbricht WL. Fabrication and characterization of microfluidic probes for convection enhanced drug delivery. J Control Release. 2006;111:252–262. doi: 10.1016/j.jconrel.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 21.Ohlfest JR, Demorest ZL, Motooka Y, Vengco I, Oh S, Chen E, et al. Combinatorial antiangiogenic gene therapy by nonviral gene transfer using the sleeping beauty transposon causes tumor regression and improves survival in mice bearing intracranial human glioblastoma. Mol Ther. 2005;12:778–788. doi: 10.1016/j.ymthe.2005.07.689. [DOI] [PubMed] [Google Scholar]
- 22.Patel SJ, Shapiro WR, Laske DW, Jensen RL, Asher AL, Wessels BW, et al. Safety and feasibility of convection-enhanced delivery of Cotara for the treatment of malignant glioma: initial experience in 51 patients. Neurosurgery. 2005;56:1243–1253. doi: 10.1227/01.neu.0000159649.71890.30. [DOI] [PubMed] [Google Scholar]
- 23.Raghavan R, Brady ML, Rodriguez-Ponce MI, Hartlep A, Pedain C, Sampson JH. Convection-enhanced delivery of therapeutics for brain disease, and its optimization. Neurosurg Focus. 2006;20(4):E12. doi: 10.3171/foc.2006.20.4.7. [DOI] [PubMed] [Google Scholar]
- 24.Rosenberg GA, Kyner WT, Estrada E. Bulk flow of brain interstitial fluid under normal and hyperosmolar conditions. Am J Physiol. 1980;238:F42–F49. doi: 10.1152/ajprenal.1980.238.1.F42. [DOI] [PubMed] [Google Scholar]
- 25.Silbergeld DL, Chicoine MR. Isolation and characterization of human malignant glioma cells from histologically normal brain. J Neurosurg. 1997;86:525–531. doi: 10.3171/jns.1997.86.3.0525. [DOI] [PubMed] [Google Scholar]
- 26.Southgate T, Kingston P, Castro MG. Gene transfer into neural cells in vivo using adenoviral vectors. Current Protocols in Neuroscience. 2000;(13 Suppl):4.23.21–24.23.40. doi: 10.1002/0471142301.ns0423s13. [DOI] [PubMed] [Google Scholar]
- 27.Vogelbaum MA. Convection enhanced delivery for the treatment of malignant gliomas: symposium review. J Neurooncol. 2005;73:57–69. doi: 10.1007/s11060-004-2243-8. [DOI] [PubMed] [Google Scholar]
- 28.Xia H, Mao Q, Eliason SL, Harper SQ, Martins IH, Orr HT, et al. RNAi suppresses polyglutamine-induced neurodegeneration in a model of spinocerebellar ataxia. Nat Med. 2004;10:816–820. doi: 10.1038/nm1076. [DOI] [PubMed] [Google Scholar]
- 29.Yuan F, Krol A, Tong S. Available space and extracellular transport of macromolecules: effects of pore size and connectedness. Ann Biomed Eng. 2001;29:1150–1158. doi: 10.1114/1.1424915. [DOI] [PubMed] [Google Scholar]