Abstract
A gradient reversed-phase high performance liquid chromatographic method was developed for determining NSC 737664 (2-[(2R)-2-methylpyrrolidin-2-yl]-1H-benzimidazole-4-carboxamide; ABT-888) in human plasma and urine. Chromatographic separation used a mobile phase composed of 0.1% formic acid in water and 0.1% formic acid in acetonitrile, and a C18 column (150 mm × 4.6 mm, 5µ). Quantitation was performed using UV detection at 300 nm. Chromatographic peak identity was confirmed using positive-ion electrospray ionization mass spectrometry. The method was shown to be specific, accurate and reproducible, and thereby appropriate for monitoring plasma and urine levels of the agent in support of a phase 0 clinical study.
Keywords: ABT-888, PARP inhibitor, phase 0 study
INTRODUCTION
Understanding DNA repair mechanisms and how to inhibit them have become general objectives in cancer therapy (1). Of particular interest is the inhibition of poly(ADP-ribose) polymerase (PARP) (2–4), a key enzyme implicated in DNA repair. The first PARP inhibitor to enter clinical trials was Pfizer’s AG-014699 (5,6).
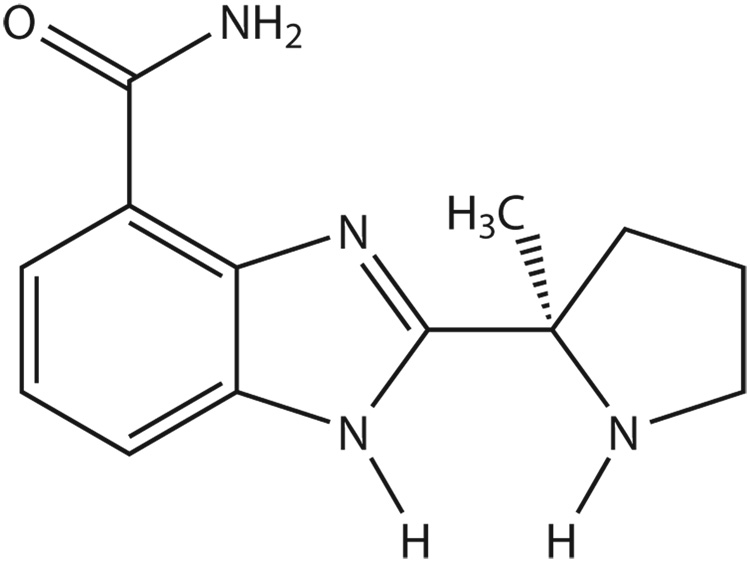
Optically pure 2-[(2R)-2-methylpyrrolidin-2-yl]-1H-benzimidabole-4-carboxamide (NSC 737664, also referred to as “ABT-888”) (see Figure 1) has been developed by Abbott Laboratories (7) and has been shown to be an orally bioavailable inhibitor of PARP (8,9).
Figure 1.
Chemical structure of NSC 737664 (ABT-888).
This compound is of high interest to the Developmental Therapeutics Program of the National Cancer Institute (NCI), and was recently entered into a phase 0 clinical trial (10,11). One objective of the study was to monitor in real-time (i.e. provide the results of sample analysis generally within 48 hours of receiving the samples) plasma and urine concentrations in patients following oral administration of NSC 737664. An earlier report by Donawho, et.al. (8), focused on the pre-clinical evaluation of NSC 737664 in several animal models, and included a brief description of analytical methodology used to assay the compound in plasma and brain homogenate. Although this provided a basis for analyzing NSC 737664, we found it necessary to modify the methodology in order to meet the anticipated urgency created by a clinical setting. Thus, our efforts focused on developing analytical methodology suitable for the determination of NSC 737664 in plasma and urine in real-time support of a phase 0 clinical trial.
EXPERIMENTAL
Chemicals
2-[(2R)-2-Methylpyrrolidin-2-yl]-1H-benzimidazole-4-carboxamide (NSC 737664; ABT-888), and 2-(N-propylpiperidine-4-yl)-1H-benzimidazole-4-carboxamide (NSC 733606; ABT-472; used as the internal standard) were supplied by the Division of Cancer Treatment and Diagnosis of the National Cancer Institute under a Collaborative Research Agreement with Abbott Laboratories. All solvents and chemicals (methanol, acetonitrile, water, formic acid, and ammonium formate) were HPLC or reagent grade and were obtained from various sources and used as received.
Sample preparation
Plasma samples (100 µL) were prepared for high performance liquid chromatographic (HPLC) analysis by adding 300 µL of a 3.5 µM solution of the internal standard (IS; NSC 733606) in acetonitrile to precipitate plasma proteins. The resulting mixture was vigorously mixed for 15 seconds, and then centrifuged at 9,000 × g for 20 minutes. The supernatant was removed, placed in a separate vial, and taken to dryness in a vacuum centrifuge. The resulting residue was re-dissolved in 120 µL of water, and 100 µL were injected on column.
Urine samples (1000 µL) were prepared for high performance liquid chromatographic (HPLC) analysis by first adding 20 µL of a 500 µM solution of the internal standard in acetonitrile followed by vigorous mixing. The resulting solution was applied to a Varian Bond Elut® C18 solid phase extraction cartridge (Varian, Inc., Harbor City, CA) that had been preconditioned with methanol followed by water. The sample was eluted with a solution of formic acid in methanol (1:200, v/v) and the eluent was collected in a 15 mL glass culture tube. The sample was then applied to a Varian Bond Elut® PRS (strong cation exchange) solid phase extraction cartridge (Varian, Inc., Harbor City, CA) that had been preconditioned with methanol. The sample was eluted with a 0.4 M solution of ammonium formate in methanol, and the eluent was collected in a 15 mL glass culture tube. The sample was then taken to dryness under vacuum in a vacuum centrifuge, and the residue was re-dissolved in 220 µL of water, and 200 µL were injected on column.
Sample analysis
The chromatographic system consisted of an Agilent (Agilent Technologies, Santa Clara, CA) 1100 Series autosampler, 1100 Series quaternary pump, and 1100 Series ultraviolet (UV) diode array detector controlled through a Windows NT-based ChemStation. Reversed-phase chromatography was conducted at ambient temperature with a flow rate of 0.7 mL/minute using a 150 mm × 4.6 mm I.D. Symmetry Shield (5µ , C18) column (Waters Corporation, Milford, MA). A mobile phase composed of (A) a solution of 0.1% formic acid in water and (B) a solution of 0.1% formic acid in a 40/60 mixture of acetonitrile/water was used for gradient elution with the following gradient profile: 0–3 min, 100% A; 3–11 min, 100% A to 100% B; 11–16 min, 100% B; 16–19 min, 100% B to 100% A; 19–28 min, 100% A. The column effluent was monitored at a wavelength of 300 nm for UV absorption. Following detection by UV absorption, the effluent was then subjected to analysis by scanning positive-ion electrospray ionization mass spectrometry using an Agilent ion-trap mass spectrometer (model G2445A). Ions representing the (M+H)+ species of NSC 737664 and NSC 733606 (the internal standard) were monitored at m/z 245 and m/z 287, respectively, to verify chromatographic peak identity. Under these conditions, the retention times of NSC 737664 and the internal standard were 11.3 minutes and 9.0 minutes, respectively. Chromatograms were integrated for peak area.
Quantitation
A series of plasma and urine standards were prepared for analysis and run together with pharmacokinetic plasma specimens on a daily basis. Ratios of the UV chromatographic peak area for NSC 737664 to that of the internal standard (IS) were calculated. Standard curves were constructed by plotting the peak area ratios against the added analyte concentration in the plasma standards. Linear least squares regression was performed using a weighting factor of 1/y2 without inclusion of the origin, to determine the slope, y-intercept, and correlation coefficient of the best fit line. Analyte concentrations in unknown samples were calculated using results of the regression analysis. Each unknown sample was initially assayed in duplicate, with additional analyses performed if the replicate determinations deviated from the average by more than 10%. Specimens with concentrations exceeding the upper limit of the standard curve were assayed upon appropriate dilution with blank plasma or blank urine.
Assay validation
Accuracy and repeatability of the assay were assessed by analyzing the back-calculated sample concentrations and regression parameters from a series of calibration curves of NSC 737664 in plasma or urine that were prepared and analyzed on separate days. The relative standard deviation (RSD) of the mean predicted concentration for the independently assayed standards provided the measure of repeatability. The lower limit of quantitation was defined as the minimum concentration amenable to analysis with an inter-day RSD not exceeding 20%. Accuracy of the assay was assessed by expressing the mean predicted analyte concentration as a percentage of its known concentration in the standard solutions.
Phase 0 study design and drug administration
This clinical trial was conducted under an NCI-sponsored Investigational New Drug (IND) study with the approval from the Institutional Ethics Committee and the NCI Institutional Review Board (IRB). Protocol design and conduct [9] followed all applicable regulations, guidances, and local policies. NSC 737664 (ABT-888) was supplied by the Division of Cancer Treatment and Diagnosis under a Collaborative Research Agreement with Abbott Laboratories. Criteria for participant eligibility has been described elsewhere (12). A single dose of NSC 737664 was administered by mouth on day 1 only. Serial sampling of blood was performed at pre-selected time points for the first 24 hours after dosing. Urine was collected in 8-hour aliquots for the first 24 hours after dosing. Additionally, blood and urine samples were acquired prior to dosing.
Blood was collected into potassium EDTA tubes and immediately chilled in an ice bath. Samples were centrifuged at 3,000 RPM for 15 minutes in a refrigerated centrifuge (4 °C), the plasma was separated, flash frozen, and stored at −70 °C until assayed. Urine was simply aliquoted into tubes, flash frozen, and stored at −70 °C until assayed.
RESULTS AND DISCUSSION
Specificity of the method
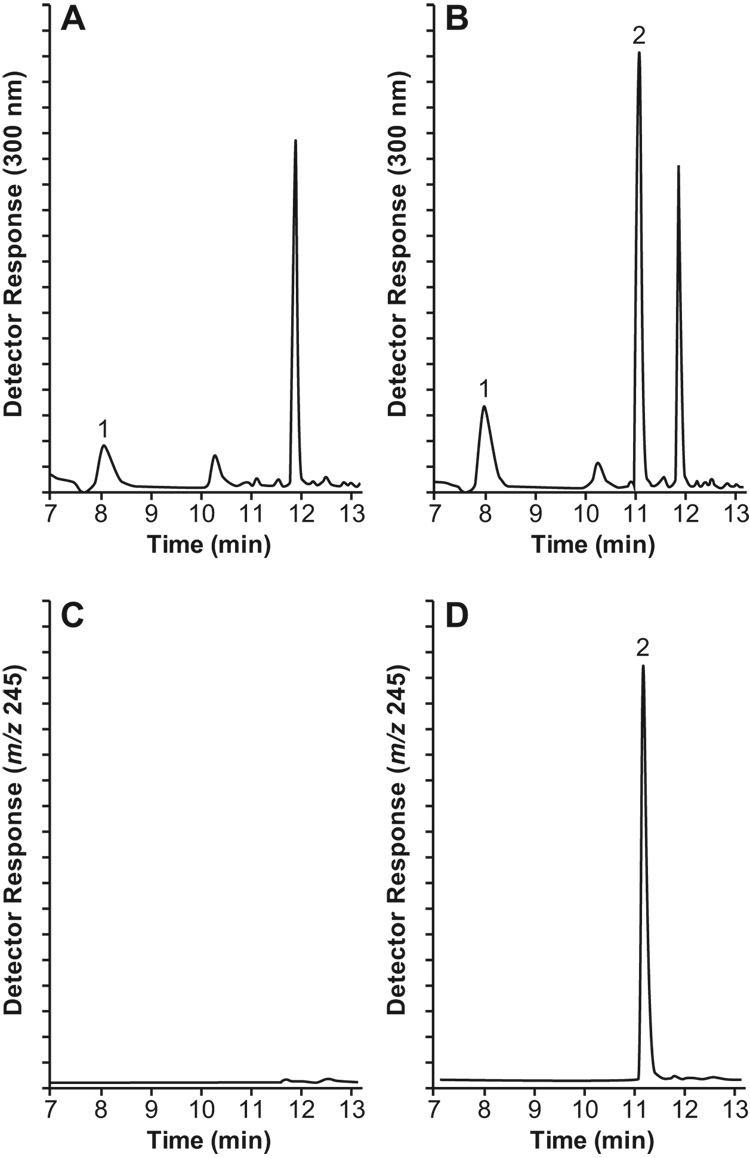
The identity of the chromatographic peak, presumed by UV absorption at 300 nm to be that of eluting NSC 737664, was confirmed by scanning positive-ion electrospray mass spectrometry. While mass spectrometric detection would without doubt provide a higher degree of specificity, detection by UV absorption demonstrated adequate specificity (see Figure 2) and higher degree of repeatability (see Table 1). A small, co-eluting peak of endogenous origin was sometimes observed in the UV chromatograms of human plasma samples. When observed in a plasma blank, the peak was integrated for area (generally found to be < 10% of the area determined for the 2.5 µM standard) and then subtracted from peak areas of samples within the run.
Figure 2.
Time-selected detector response vs. time profiles reconstructed from data acquired during liquid chromatographic separation: (A) UV absorbance at 300 nm of an extract of human plasma to which only the internal standard had been added, and (B) of an extract of human plasma spiked with NSC 737664 at a concentration of 2.5 µM; (C) extracted ion detection (m/z 245) of an extract of human plasma to which only the internal standard had been added, and (D) of an extract of human plasma spiked at a concentration of 2.5 µM. Peak Assignments: 1, internal standard; 2, NSC 737664.
Table 1.
Accuracy and Repeatability for Assaying NSC 737664 in Human Plasma by HPLC with UV Detection and Verification of Peak Identity by MS
| UV Detection | MS Detection | |||||
|---|---|---|---|---|---|---|
| Concentration Added (μM) | Accuracy (%) | Repeatability (%) | S/N | Accuracy (%) | Repeatability (%) | S/N |
| 5.0 | 104.2 | 11.6 | 96.8 | 10.2 | ||
| 2.5 | 98.0 | 9.2 | 96.0 | 10.6 | ||
| 1.0 | 95.0 | 10.8 | 105.0 | 10.0 | ||
| 0.50 | 110.0 | 12.3 | 130.0 | 21.9 | ||
| 0.25 | 104.0 | 11.8 | 136.0 | 31.8 | ||
| 0.10 | 94.0 | 18.4 | 10 | 97.0 | 29.7 | 18 |
Accuracy and repeatability of the assay were assessed from 12 different standard curves of NSC 737664 in human plasma separately prepared and analyzed over a 44-week period.
Linearity of calibration and inter-day reproducibility
The chromatographic peak area of NSC 737664 was found to be directly proportional to the added concentration of NSC 737664 in human plasma from about 0.10 to 5.0 µM. Mean values (± SD) of the linear regression parameters for 12 standard curves of NSC 737664 in human plasma, independently prepared and assayed over a 44-week period were: slope, 0.1890 ± 0.0313 liter/µmole; y-intercept, 0.0084 ± 0.0072; correlation coefficient, 0.972 ± 0.025. Coefficients of variation of the mean predicted NSC 737664 concentrations ranged from 9.2 to 18.4%.
The chromatographic peak area of NSC 737664 was also found to be directly proportional to the added concentration of NSC 737664 in human urine from about 1.00 to 25.0 µM. Coefficients of variation of the mean predicted NSC 737664 concentrations ranged from 7.8 to 12.4% for 9 standard curves of NSC 737664 in human urine, independently prepared and analyzed over an 8-week period.
Accuracy and repeatability
Back-calculated sample concentrations were analyzed from 12 different calibration curves of NSC 737664 in human plasma independently prepared and analyzed over a 44-week period. Accuracy of the assay was assessed by expressing the mean predicted analyte concentration as a percentage of its known concentration in the standard solution, whereas repeatability reflects inter-day variation. As shown in Table 1, the repeatability for inter-day quantitation of NSC 737664 in human plasma with UV detection was <20% for all concentrations included in the standard curve. Similarly, the repeatability for inter-day quantitation of NSC 737664 in human urine was <20% for all concentrations included in the standard curve (Table 2).
Table 2.
Accuracy and Repeatability for Assaying NSC 737664 in Human Urine by HPLC with UV Detection.
| NSC 737664 | ||
|---|---|---|
| Concentration Added (μM) | Accuracy (%) | Repeatability (%) |
| 25.0 | 108.5 | 12.4 |
| 10.0 | 101.4 | 7.8 |
| 5.0 | 99.5 | 7.9 |
| 2.50 | 95.7 | 10.6 |
| 1.00 | 105.3 | 8.8 |
Accuracy and repeatability of the assay were assessed from 9 different standard curves of NSC 737664 in human urine separately prepared and analyzed over an 8-week period.
Analyte stability
A human plasma standard of NSC 737664 (5.0 µM) was incubated for 72 hours at 37°C. At selected times, three aliquots of the plasma mixture were removed and analyzed for remaining NSC 737664. After 72 hours’ incubation at 37°C, the concentration of NSC 737664 had declined to about 0.6 µM, indicating that about 12% of the NSC 737664 remained. In a separate experiment, another sample (5.0 µM human plasma standard of NSC 737664) was prepared, stored at −70°C and, at selected times, similarly sampled and analyzed for remaining NSC 737664. No significant change in the concentration of NSC 737664 in the human plasma sample was noted after 1 month of storage at −70°C.
Lower limit of quantitation
Using UV detection for quantitation, the lowest point of the matrix standard curve which is both repeatable (18.4%, n = 14) and accurate (94.0%, n = 14) is the 0.10 µM human plasma sample standard (see Table 1). The 0.10 µM standard possesses a signal-to-noise ratio of about 10. NSC 737664 is easily detectable at 0.05 µM but is no longer accurate or repeatable. Thus, the lower limit of detection (LOD) of NSC 737664 is about 0.05 µM, and the lower limit of quantitation (LOQ) in human plasma is about 0.10 µM.
Absolute recovery
Four pairs of standard curves were prepared and analyzed. Each pair of standard curves consisted of a set of six standard samples of NSC 737664 in matrix (human plasma) and in non-matrix. Comparing absolute detector responses for the internal standard in matrix and non-matrix shows an extraction efficiency of 95.8% for the internal standard. For NSC 737664, the matrix standard curves gave an average slope of 39.18 ± 2.39, and the non-matrix standard curves gave an average slope of 46.82 ± 1.12. The ratio of the slopes therefore provides the measure of absolute recovery (83.7%) for NSC 737664 from human plasma. Similarly, the absolute recovery of NSC 737664 from human urine was determined (75.6%).
Disposition of NSC 737664
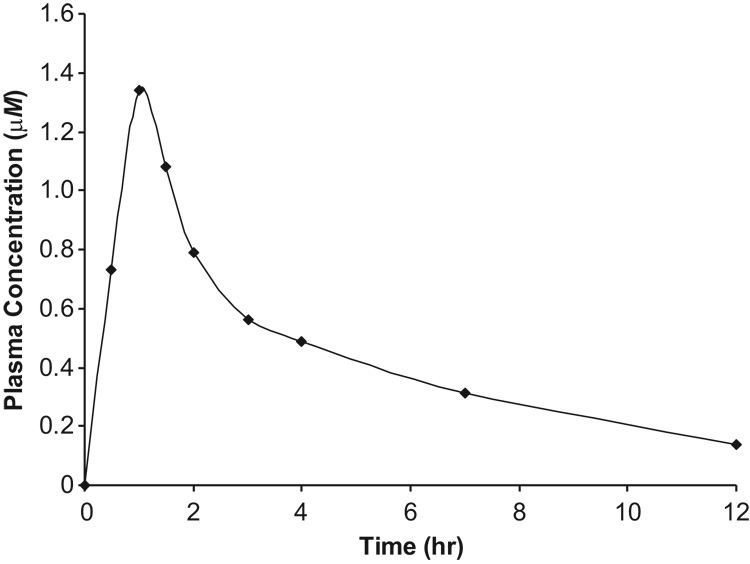
Following a single oral dose of 50 mg, NSC 737664 was rapidly and highly absorbed into the central compartment. A plasma drug concentration of 0.73 µM was observed at 30 minutes post-dosing, and a maximum of 1.34 µM was observed at 60 minutes post-dosing (Figure 3). NSC 737664 was detected in the 24-hr sample, but was below the lower limit of quantitation of the assay. The last quantifiable time point was 12 hours, at which time the plasma drug concentration had declined to 0.14 µM.
Figure 3.
Plasma Concentration vs. Time Profile of NSC 737664 (ABT-888) in a Participant of a Phase 0 Clinical Study Following a Single, Oral Dose of 50 mg.
Urine was collected in three 8-hour aliquots. The first aliquot (0–8 hours) represented a collection of 1175 mL of urine, which assayed to 110.5 µM of unchanged NSC 737664. The second and third aliquots (8–16 and 16–24 hours) represented collections of 800 mL of urine (39.1 µM) and 700 mL of urine (23.2 µM), respectively. Thus, the first, second and third aliquots of urine contained 31.7, 7.6, and 4.0 mg of NSC 737664, respectively, indicating that 43.3 mg (87 %) of the initial drug dose had been excreted unchanged into the urine within the first 24 hours post-dosing.
CONCLUSIONS
A specific assay for determining NSC 737664 in human plasma has been developed. The method involves preliminary isolation of the compound from plasma by protein-precipitation. Following separation using liquid chromatography and detection by UV, the lowest concentration of NSC 737664 that could be quantified with acceptable reproducibility (RSD <20%) in 100 µL of plasma was 0.10 µM. The assay has been shown to be specific, accurate and reproducible, thereby rendering the procedure appropriate for monitoring plasma levels of the agent in support of a phase 0 clinical study.
A participant in a phase 0 clinical study of NSC 737664 was provided a single oral dose of 50 mg. Drug plasma concentrations and urinary excretion were monitored. NSC 737664 was seen to be rapidly and highly absorbed, as evidenced by a plasma level of 0.73 µM only 30 minutes post-dosing. Drug plasma concentrations were quantifiable for the first 12 hours post-dosing, although NSC 737664 could still be detected at 24 hours. Assaying the participant’s urine indicated that about 87% of the drug was excreted unchanged within 24 hours post-dosing.
ACKNOWLEDGEMENTS
This work was facilitated by consultations and technical assistance by Raymond Klecker and Lawrence W. Anderson of the FDA clinical pharmacology laboratory, as part of the NCI-FDA Interagency Oncology Task Force. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. NO1-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This research was supported [in part] by the Developmental Therapeutics Program in the Division of Cancer Treatment and Diagnosis of the National Cancer Institute.
REFERENCES
- 1.Curtin N. Therapeutic potential of drugs to modulate DNA repair in cancer. Expert Opin. Ther. Targets. 2007;11(6):783–799. doi: 10.1517/14728222.11.6.783. Review. [DOI] [PubMed] [Google Scholar]
- 2.De Murcia G, Menissier De Murcia J. Poly(ADP-ribose) polymerase: a molecular nick-sensor. Trends Biochem. Sci. 1994;19:172–176. doi: 10.1016/0968-0004(94)90280-1. [DOI] [PubMed] [Google Scholar]
- 3.Schreiber V, Dantzer F, Ame JC, De Murcia G. Poly(ADP-ribose): novel functions for an old molecule. Nat. Rev. Mol. Cell Biol. 2006;7:517–528. doi: 10.1038/nrm1963. [DOI] [PubMed] [Google Scholar]
- 4.Ratnam K, Low JA. Current development of clinical inhibitors of poly(ADP-ribose) polymerase in oncology. Clin. Cancer Res. 2007;13(5):1383–1388. doi: 10.1158/1078-0432.CCR-06-2260. Review. [DOI] [PubMed] [Google Scholar]
- 5.Plummer R, Middleton M, Wilson R, Jones C, Evans J, Robson L, Steinfeldt H, Kaufman R, Reich S, Calvert AH. First in human phase I trial of the PARP inhibitor AG-014699 with temozolomide (TMZ) in patients (pts) with advanced solid tumors. J. Clin. Oncol. 2005;23(16S):3065. [Google Scholar]
- 6.Thomas HD, Calabrese CR, Batey MA, Canan S, Hostomsky Z, Kyle S, Maegley KA, Newell DR, Skalitzky D, Wang LZ, Webber SE, Curtin NJ. Preclinical selection of a novel poly(ADP-ribose) polymerase inhibitor for clinical trial. Mol. Cancer Ther. 2007;6(3):945–956. doi: 10.1158/1535-7163.MCT-06-0552. [DOI] [PubMed] [Google Scholar]
- 7.Wernet W, Penning TD, Giranda VL. Combination therapy with PARP inhibitors. 20070265324. United States Patent Application. 2007 January 17;
- 8.Donawho CK, Luo Y, Luo Y, Penning TD, Bauch JL, Bouska JJ, Bontcheva-Diaz VD, Cox BF, De Weese TL, Dillehay LE, Ferguson DC, Ghoreishi-Haack NS, Grimm DR, Guan R, Han EK, Holley-Shanks RR, Hristiv B, Idler KB, Jarvis K, Johnson EF, Kleinberg LR, Klinghofer V, Lasko LM, Liu X, Marsh KC, McGonigal TP, Meulbroek JA, Olson AM, Palma JP, Rodriguez LE, Shi Y, Stavropoulos JA, Tsurutani AC, Zhu GD, Rosenberg SH, Giranda VL, Frost DJ. ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin. Cancer Res. 2007;13(9):2728–2737. doi: 10.1158/1078-0432.CCR-06-3039. [DOI] [PubMed] [Google Scholar]
- 9.Albert JM, Cao C, Kim KW, Willey CD, Geng L, Xiao D, Wang H, Sandler A, Johnson DH, Colevas AD, Low J, Rothenberg ML, Lu B. Inhibition of poly(ADP-ribose) polymerase enhances cell death and improves tumor growth delay in irradiated lung cancer models. Clin. Cancer Res. 2007;13(10):3033–3042. doi: 10.1158/1078-0432.CCR-06-2872. [DOI] [PubMed] [Google Scholar]
- 10.Kummar S, Kinders R, Gutierrez M, Rubenstein L, Parchment RE, Phillips LR, Low J, Murgo AJ, Tomaszewski JE, Doroshow JH. Inhibition of poly(ADP-ribose) polymerase (PARP) by ABT-888 in patients with advanced malignancies: results of a phase 0 trial. J. Clin. Oncol. 2007;25(18S):3518. [Google Scholar]
- 11.Kinders R, Parchment RE, Ji J, Kummar S, Murgo AJ, Gutierrez M, Collins J, Rubinstein L, Pickeral O, Steinberg SM, Yang S, Hollingshead M, Chen A, Helman L, Wiltrout R, Simpson M, Tomaszewski JE, Doroshow JH. Phase 0 clinical trials in cancer drug development: from FDA guidance to clinical practice. Mol. Interv. 2007;7(6):325–334. doi: 10.1124/mi.7.6.9. [DOI] [PubMed] [Google Scholar]
- 12.Kummar S, Kinders R, Gutierrez ME, Rubenstein L, Parchment RE, Phillips LR, Ji J, Monks A, Low JA, Chen A, Murgo AJ, Collins J, Steinberg SM, Eliopoulos H, Giranda VL, Gordon G, Helman L, Wiltrout R, Tomaszewski JE, Doroshow JH. Phase 0 clinical trial of the poly(ADP-ribose)polymerase (PARP) inhibitor ABT-888 in patients with advanced malignancies. J. Clin. Oncol. doi: 10.1200/JCO.2008.19.7681. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]