Abstract
Decisions concerning treatment changes pervade the management of chronic psychiatric disorders that resist definitive cure, yet empirical evidence for the comparative clinical effectiveness of treatment strategies remains underdeveloped. In this paper we exploit the example of psychosis following substance use to illustrate some new developments in clinical trials design that can provide the most solid evidence base for defining successful strategies. The intent is to explore the strengths and limitations of the methodological approach through a meaningful clinical example, with an emphasis on concepts and issues. Both methodology and clinical science are overviewed.
Keywords: adaptive treatment, treatment strategy, substance induced psychosis, experimental design
Introduction
A patient is brought to the emergency department in a psychotic state, following recent drug use, and is treated with an antipsychotic medication. Within a few days of treatment, the psychosis has cleared, the patient is cooperative, and the clinician must decide whether to continue the antipsychotic, and for how long. The considerations that weigh in this decision include the desire to limit treatment exposure to minimize side effects, and the need to prevent relapse into psychosis. The treatment decision is binary for this individual patient (continue treatment for a fixed course of some specific duration, or stop immediately), but it follows from a strategy that the clinician brings to the management of all such patients. There are three kinds of strategy: 1) always treat for a fixed course, 2) always stop immediately, 3) assess the risk of psychotic relapse, and continue treatment only in patients with high risk.
What kinds of empirical evidence can be used to help clinicians form strategies that lead to better outcomes on average, across the range of patients they will see? In this paper we exploit the example of psychosis following substance use to illustrate some new developments in clinical trials design that can provide the most solid evidence base for defining successful strategies. Similar decision making pervades the management of other chronic psychiatric disorders that resist definitive cure.
Adaptive Treatment Strategy
The third strategy defined above, treatment continuation based on risk, is an example of an “adaptive treatment strategy” (ATS), because it tailors the decision to the specific circumstances of the individual patient, while the other two strategies are fixed and invariant for all patients, regardless of risk.1-3 The ATS requires that patients be stratified according to their risk of psychosis, based on history up to the current time; individuals identified as high risk are advised to continue the antipsychotic. When treatment continuation is not warranted, it is possible to periodically reevaluate the patient’s risk (based on emergence of subsyndromal symptoms and new instances of substance use) and reintroduce the antipsychotic if a chosen “threshold of risk” is reached. In this way the ATS includes a “watchful waiting” stage that extends in time (after medication discontinuation), providing additional potential benefit.
It is tempting to presume that any method for adaptively deciding treatment changes over time would be preferable to either version of “one size fits all” fixed treatment, as in the first two options. But the performance of the ATS depends on two features: first, the ability to accurately assess risk, and second, the efficacy of the reintroduced treatment to control psychosis in patients whose risk rises above the threshold after medication discontinuation. If assessment at intake or during follow-up under- or over-estimates risk, it will cause the ATS to systematically under- or over-treat patients, even if the cut point and threshold are chosen to appropriately balance (true) overall risk and benefit. If the antipsychotic treatments are not effective, then the rationale for watchful waiting disappears. So the choice of strategy demands conclusive evidence, in the specific context of psychosis following substance abuse.
There is some reason to expect that an accurate and reliable risk assessment is available,4,5 and no strong reason to doubt the efficacy of reintroduced treatments, but no direct experimental evidence to show that these two features of the ATS work together to produce better outcomes. So it appears possible to propose a specific ATS meriting rigorous test against other options. We describe how to perform a full-strength experimental test in the next section.
Experimental Tests of ATS
Randomized controlled trials of adaptive strategies for cancer have been conducted since the 1980’s.6-14 These studies are now called sequential, multiple assignment, randomized (SMAR) trials15 because successive courses of treatment are randomly and adaptively assigned at a set of pre-defined decision points (e.g., switch treatment or not), according to the individual subject’s treatment and response history. Psychiatric trials to evaluate adaptive strategies have also been conducted or are underway for treatment of substance abuse,16,17 smoking cessation,18 depression in late life,19 and childhood mania.20 Recent large-scale effectiveness trials for treatment of schizophrenia,21 depression22 and Alzheimer’s disease23 have randomized subjects sequentially according to their treatment and response history. However, the primary analyses for these studies were stage-specific comparisons of medication alternatives, rather than contrasts of whole treatment sequences.
To date, most studies for evaluating whole adaptive strategies have focused on comparing short sequences of decisions about first-line options, second-line options, and so forth. The duration of each treatment stage in such trials is fixed by the length of time thought necessary for an “adequate” test of an individual’s responsiveness to that particular treatment. Evidence from previous short-term clinical trials of those treatments provides an estimate of the actuarial probability of future response given the absence of response after 2, 4, 6 weeks, etc. and these estimates are used to define the duration of each individual test.
The context we consider here (psychosis following substance use) poses a different challenge: devising adaptive decision rules that specify under what circumstances to change treatment (in particular, how to decide that it is appropriate to stop, and, conversely, to restart). Uncertainty surrounding such decisions warrants experimental investigation, particularly when the risk of significant medication side effects is high. Recent studies in substance abuse treatment have explicitly pursued this line of research, by incorporating decision rules that adaptively govern when to change treatment.17,26-28 Their consideration of adaptive timing decisions reflects the widening view that drug dependence is both chronic and relapsing in nature, and that individualized care must address under what circumstances to change the intensity and type of treatment.27
In this article, we consider the methodological and scientific issues arising in the attempt to test strategies for long-term management of patients who present with psychosis following substance use that responds to a short course of an antipsychotic. The intent is to explore the strengths and limitations of the ATS and SMAR approach through a meaningful clinical example, with an emphasis on concepts and issues. We also discuss reasons that this particular clinical problem may be amenable to an approach based on SMAR trials of ATS.
Clinical Background
Findings from a naturalistic longitudinal study of 319 psychiatric emergency admissions recruited from the upper Manhattan section of New York5 motivate and support a risk-based adaptive strategy for deciding how to treat early phase psychosis and co-occurring substance use. Among the 133 patients who were diagnosed with a DSM-IV substance-induced psychosis (SIP) at baseline, 19% experienced psychotic symptoms that persisted during follow-up despite cessation of substance use. Almost a quarter of SIP patients had ongoing substance use, which complicated prospective treatment of their comorbid psychosis.4 In addition, (DSM-IV determined) episodes of SIP can occur after an initial diagnosis of primary psychosis (PP), as observed in the New York Study.5 Thus, a cross-sectional diagnosis of SIP does not rule out the risk of future psychosis, even in the absence of continuing substance abuse. Other clinical studies of SIP provide similar findings,28 undercutting the viability of ‘one size fits all’ approaches to treatment of psychosis and co-occurring substance use.
Psychosis in response to drug use may be a marker of neurobiological vulnerability, a premise that is supported empirically by the New York study4 and other research. In 1996, Boutros and Bowers concluded from a review of the literature that drug abuse can cause PP in patients who might not otherwise develop psychosis independent of substance abuse.29 Boutros et al. further found that increased drug use in the late 1960s was associated with a rapid increase in first admissions to Connecticut hospitals of patients with schizophrenia.30 Prospective follow-up of a Danish cohort of 535 patients treated for a first episode of short-lived psychotic symptoms following cannabis use showed that almost half went on to develop a schizophrenia-spectrum disorder, underscoring the important prognostic value of cannabis-induced SIP.31 Studies of non-clinical samples suggest an interaction between cannabis use and vulnerability to psychosis regarding the risk of PP;32 more recently, Caspi et al.33 have provided evidence for this specific gene-environment interaction.
Example ATS for the Timing of Antipsychotic Medication
We develop a whole ATS for managing the treatment of psychosis following substance use, which potentially includes long-term use of antipsychotic medication. We consider the simplest algorithm for a sequence of timing decisions to illustrate the conceptually distinct approaches to constructing rules for starting and stopping treatment. The ideas apply generally to the context of psychiatric illness that may be emergent or episodic.
A natural way to formulate adaptive decisions that determine under what circumstances to start (or restart) treatment is through the use of “threshold” rules, which are used to track the patient’s risk over time and initiate treatment once it exceeds some a priori risk threshold specified by the clinician.20 This approach has appeal when it provides a reasonable model for the latent neurobiological process, and can also serve as a useful metaphor for the emerging clinical course of illness, even when there is limited etiological understanding. Operationally, the threshold rule is carried out sequentially at a pre-defined set of decision points during watchful waiting, using a data-based summary of history to date to calculate if the selected risk criterion for initiating treatment has been met. The adaptive tailoring of the rule occurs prospectively at each decision point, which in turn determines how long the patient is treated with watchful waiting (which ceases if active treatment is started). By contrast, we conceptualize stopping rules as “ballistic”, to be carried out at a single decision point to specify a particular duration of active treatment. Elapsed time since the start of remission (the decision point) is a natural metric for formulating medication discontinuation, given the absence of further prospective information about the patient’s risk. (We assume that any patient who becomes symptomatic would remain on medication.)
As described in Table 1, the example ATS specifies adaptive rules for three stages of decision making, under the assumption that the patient’s SIP noted at intake has cleared after a few days of treatment with an antipsychotic. The first two stages adaptively begin long-term use of antipsychotic medication; the third determines when to stop such use if initiated.
TABLE 1.
Whole ATS for the Timing of Long-Term Antipsychotic Medication (AP)
| STAGE | DECISION | TYPE OF DECISIONRULE | NUMBER OF DECISION POINTS |
|---|---|---|---|
| 1 | Whether to continue initial AP | Cross-sectional, Threshold | 1 |
| 2 | When (if ever) to reintroduce AP | Sequential, Threshold | Adaptively determined |
| 3 | How long to maintain AP | Cross-sectional, Ballistic | 1 |
Stage 1 is a single decision point that determines whether or not the antipsychotic medication administered at intake should be continued after psychotic symptoms have cleared. Using a cross-sectional risk assessment at this time permits the clinician to extend medication usage beyond emergency treatment, given sufficiently high risk. The intent is to identify the most vulnerable patients with the cross-sectional assessment, thereby providing a decision rule that helps to minimize unnecessary exposure to antipsychotic drugs for less vulnerable patients. Although the decision rule could be criterion-based, later stages of decision making can more easily incorporate the initial risk assessment if it uses a data-based summary. In this case, it operates like a cross-sectional threshold rule. Either type of strategy will stratify patients according to intake history(including response to initial antipsychotic) in order to tailor continuation to individual risk.
Stage 2 is a threshold rule that gives rise to a sequence of decision points for prospectively monitoring the risk of a patient who discontinues the antipsychotic drug after psychotic symptoms have cleared. The intent is to adaptively begin the patient on a long-term course of the antipsychotic medication during follow-up, according to the evolving course of symptoms and substance use, as well as other patient history, including risk of psychosis at intake. In this context, each follow-up visit constitutes a decision point and a potential time to reintroduce the drug. The clinical intuition underlying this stage is that a true signal about psychosis vulnerability emerges with continued follow-up, thereby effecting a better result (i.e., minimal duration of psychosis and treatment risk) on average across patients.
Stage 3 is a single decision point to determine the timing of medication discontinuation for patients who have been placed on an antipsychotic for the long term, either once initial symptoms have cleared (via Stage 1) or during follow-up (via Stage 2). Although the rule could simply specify a fixed duration of treatment for all patients, it is possible to build in some adaptation here, as was done in the decision rule for Stage 1. In particular, it may be sensible to stratify duration of time on medication according to patient characteristics and history at the time psychotic symptoms remit, including response to the (possibly reintroduced) antipsychotic. Lower-risk patients would continue on medication for a shorter period of time, with the intent of making an informed tradeoff between the risk of relapse and the burden of treatment with an antipsychotic.
Because of the novelty of sequential threshold rules, we take up Stage 2 of the ATS in more detail. To be concrete, suppose at each visit during the second decision stage, the clinician re-evaluates the patient’s substance abuse and symptom history to date using the Time Line Follow Back (TLFB)34 and the Scale of Prodromal Symptoms(SOPS).35 A simple strategy would be to require a certain threshold (quantity and severity) of substance use to forestall initiation of long-term antipsychotic treatment even when risk of psychosis exceeded the risk threshold.36 Given a judicious choice of thresholds, the strategy might be clinically described as “Start watchful waiting (WW), continue WW for two months after psychotic symptoms clear, and then if the patient has had a SOPS rating of 4 or more for at least 6 out of the eight preceding weeks on either suspiciousness or delusional thinking items, start the patient on an antipsychotic unless use of non-alcoholic drugs during the past 2 months exceeded 4 times weekly for at least 5 weeks. If the patient does not meet these criteria, continue WW. If the SOPS ever reaches 6 on any positive symptom rating for more than half the days over one month, start the patient on active treatment; if non-alcohol drug use during the same month exceeds 4 times a week, discontinue medication after clearing of the SOPS exacerbation and resume WW.” Additional criteria could be specified to cover alcohol use.37 The strategy could also be elaborated to include the initial risk assessment or supplemental stepped care algorithms to adaptively intensify substance abuse services,38 but the simple version suffices for purposes of illustration.
To translate the clinical description to canonic form, we use 1) a cumulative score and 2) threshold for each part of the dual threshold rule. The score, which summarizes the patient’s history to date, is calculated on a regular basis as a simple summation. For example, at time t, , defines a score S that summarizes risk for the past 2 months, where xt = 1 if the patient is “ill” at week t, or else xt = -1. An increasing S reflects continuing illness, while a decreasing one reflects continuing wellness; treatment is changed the first time S reaches (or exceeds) the chosen risk threshold, say θ. Defining illness as “SOPS rating of 4 or more on suspiciousness or delusional thinking items” and setting θ = 4 specifies the example threshold rule for psychotic risk, described above in words. (Requiring at least 6 “risky” weeks during the past two months implies that S must be at least 6 * 1 + 2 * (-1) = 4 once the 2 symptom-free weeks are taken into account.) The rule for substance use is analogously specified, with its own cumulative score D and threshold ϕ, so that Stage 2 of the strategy is concisely expressed in terms of specific choices for (S, θ, D, ϕ). In this formulation, it is useful to think of the scores S and D as being more or less fixed, and the thresholds θ and ϕ as being the parameters that are “dialed in” by the clinician to create a specific strategy, with higher threshold values keeping patients on watchful waiting for a longer time. The cumulative method of scoring the patient’s history is clinically suitable for the context of emergent or episodic psychiatric illness because it is sensitive to risk heterogeneity (between patients) and fluctuating course (within patient). See Dawson et al.20 for a demonstration of these properties for an ATS of this type for treating childhood mania.
SMAR Design to Test the Example ATS
Randomized studies of adaptive strategies, such as SMAR trials, preserve the fundamental principles of clinical trial methodology, including the principle of clinical equipoise that dictates ethical choices of treatment alternatives. When uncertainty centers on how to decide that it is appropriate to start (or restart) treatment, the threshold parameterization of an ATS provides a natural structure for specifying timing alternatives. The ordered continuum of possible threshold values allows equipoise to be expressed in terms of an upper and lower bound for the range of thresholds thought to be clinically competitive. The first two strategies in the Introduction (always treat for a fixed course, always stop immediately) correspond to the extreme thresholds “start immediately” and “never start”, where treatment in this case is long-term medication with an antipsychotic. The rationale for bracketing equipoise with these extremes derives from presumptions about risk heterogeneity for patients with co-occurring psychosis and substance use. Specifically, we assume that a person’s predisposition to psychosis is not binary or categorical. Rather, there is a continuum of vulnerability to psychosis that is bracketed by patients whose risk factors are weighted substantially toward either PP or SIP. The “start immediately” option represents what might be appropriate for a patient diagnosed with PP, whereas “never start” might be appropriate for a patient diagnosed with SIP. Because risk or response heterogeneity often necessitates adaptive approaches to managing psychiatric disorders, (presumed) population heterogeneity provides a general rationale for structuring treatment alternatives for ATS evaluation.
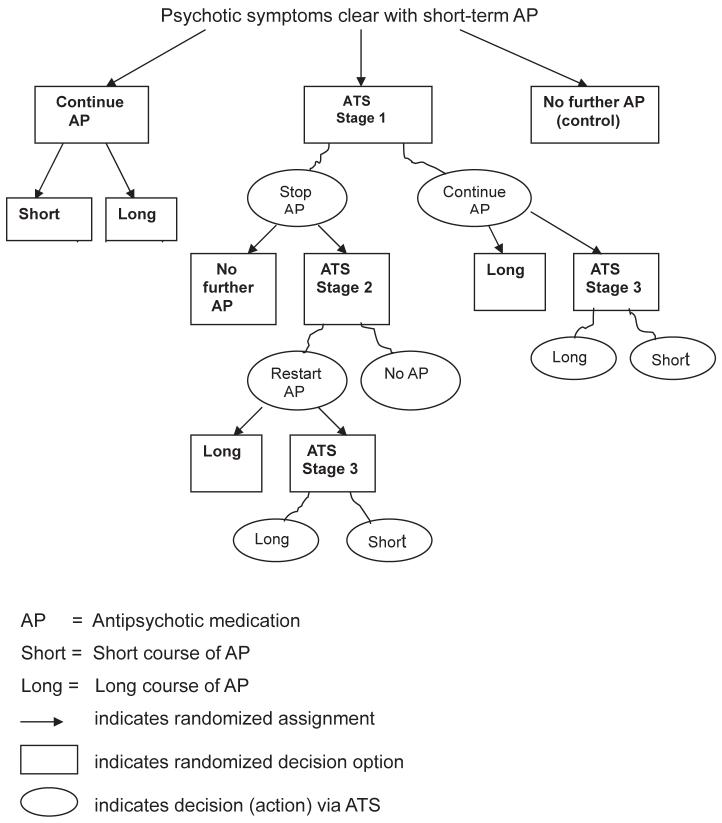
Figure 1 shows a SMAR trial design for the example ATS and alternatives, in which patients are sequentially randomized at decision points. Notice that the number of randomizations any given patient undergoes depends on treatment assignment and outcome because randomization itself is adaptive. Subjects assigned initially to an adaptive check at baseline will never be randomized again if their risk is low enough throughout the study. In contrast, more vulnerable subjects may be randomized up to three times during the trial. Of particular note is that all subjects are randomized prior to a definitive DSM-IV diagnosis of PP, to account for sequential equipoise due to diagnostic uncertainty at intake and during prospective follow-up (see Clinical Background). The SMAR alternatives may be more conventionally evaluated by randomizing subjects at baseline to one of the multistage strategies represented in the decision tree underlying Figure 1.39,40 Either design approach provides an alternative to the classic design that requires eligible subjects to have a diagnosed disorder, which implicitly presumes that the optimal risk threshold for starting treatment is meeting full-criterion diagnosis. As such, the classic design may fail to fully address clinical equipoise when it centers on appropriate timing of medication.
FIGURE 1.
SMAR Trial for Example ATS for Timing of Antipsychotic Medication (AP)
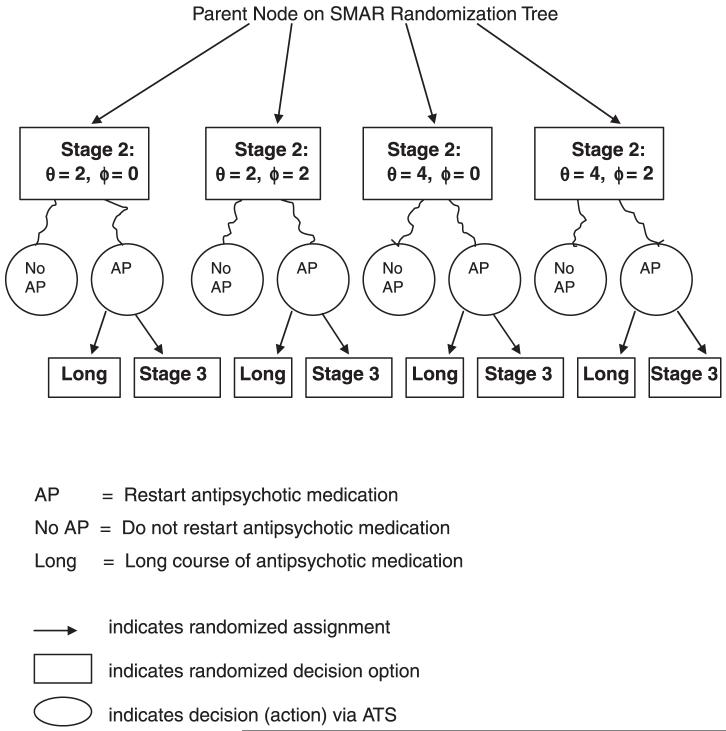
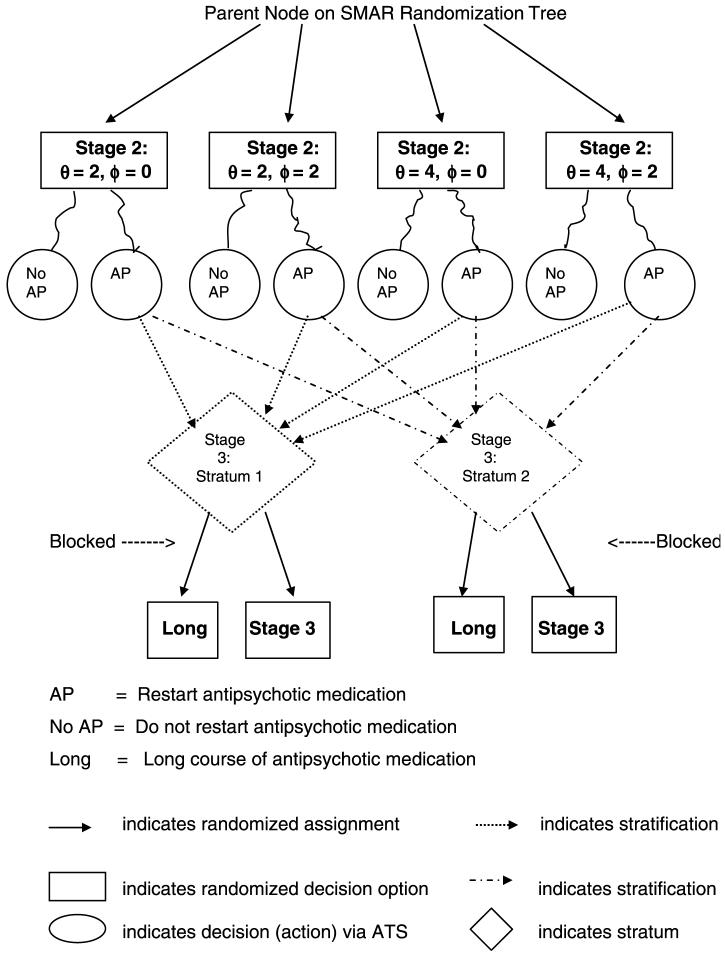
The SMAR alternatives in Figure 1 can be expanded to include other choices for θ or ϕ if there is no clear consensus for intermediate threshold values. Introducing more threshold options splits the sample multiplicatively because SMAR operates like a decision tree, with each stage of randomization nested within previous stages. One way to obviate the potential for successively dwindling sample sizes is to “coarsen” adaptive randomization by redefining how subjects are grouped for the next stage of treatment assignment. For example, rather than having assignment to “stop” options nested within Stage 2 threshold alternatives (for restarting antipsychotic medication), as in Figure 2A, randomization could be nested within strata that cut across those alternatives, as in Figure 2B. In this case, the stratification must preserve the properties of randomization needed for valid causal inference. This implies that the potential confounding effects on future outcomes (after Stage 3) due to differences in prior patient risk are controlled via the stratification, as they would be controlled by SMAR randomization. (Technically, the stratification must act as a statistical surrogate for priori patient history.) A natural choice for coarsening SMAR randomization is the stratification driving the “next” adaptive decision; for the example set up, this would be the strata in Stage 3 of the ATS, which might be based on average SOPS ratings during the past month coupled with known baseline predictors of remission.41-42 Figure 2B indicates that coarsened randomization is blocked on the prior outcome-treatment paths that map into the strata, in order to balance important heterogeneity. The overall effect is to increase statistical power for ATS evaluation.
FIGURE 2A.
A SMAR Randomization for Stages 2 and 3 of Example ATS
FIGURE 2B.
B Coarsened Randomization After Stage 2 of Example ATS
We note that the SMAR design is to be carried out as a single clinical trial to test the whole ATS, despite there being distinct stages of decision making. This requirement derives from mathematical theory, which states that optimization of a sequence of decisions over time requires simultaneous evaluation of the entire sequence of decisions and outcomes.43 In general, this precludes the “myopic” approach that considers each decision stage in a separate study and then sequentially pieces together the stage-specific optimal rules.3,15,20
Handling Non-Compliance and Drop-Out
As in classic experiments, SMAR trials will have subjects who deviate from randomized protocol, or drop out from the study entirely. We distinguish these two issues, and consider them explicitly for the design in the previous section.
Non-compliance with assigned ‘treatment’ may occur once a patient is placed on a long-term course of an antipsychotic. The Intent to Treat Principle (ITT) applies here, but may be more interpretable when treatments are determined adaptively (at least for some of the alternatives). Consider specifically subjects who are placed on active treatment adaptively either at baseline or during follow-up, but fail to remain on medication. In this case, sequential determination of treatment effectively limits non-compliance to a “riskier” subset of the study population. Reducing heterogeneity in this way removes some of the variance from ITT comparisons, which average over randomized assignment, independent of adherence to protocol. This may not only improve efficiency, but also help to clarify the meaning of ITT causal effects. Sequential randomization also serves to experimentally control treatment variation in ITT effects for general SMAR designs, nesting non-compliance within successive decision stages.3 If concerns about non-compliance are paramount, it is even possible to make “non-adherence to current regime” part of the decision algorithm, in which case the ATS is self-regulating with respect to ITT side effects.39 The stepped-care algorithms for substance abuse are examples of tailoring the patient’s intensity of services to adherence.38
Drop-out, in the sense of not adhering to measurement, rather than treatment protocol, may occur during any prospective stage of a SMAR design. Like classical longitudinal studies, clinical investigators who evaluate adaptive strategies need to address the challenge of retaining patients with chronic psychiatric disorders. The example ATS incorporates a rescue algorithm for break-through psychosis as part of the strategy, which precludes the need to “terminate” subjects who require rescue treatment. Even so, recent large scale multi-stage studies, such as STAR*D,22 suggest that incorporating patient preferences and adaptive treatments into the design is not sufficient to overcome the propensity for more severely ill subjects to drop out of experimental monitoring. Two methodological advances for SMAR trials help to address the resulting loss of statistical power due to reduced sample sizes. One is a new way of analyzing the data sequentially so as to capitalize on their nested structure to improve efficiency.44,45 The other draws upon methods for statistical selection and screening, in which the goal is to select the best among a number of competing alternatives. These methods have been well developed for the field of computer simulation for system evaluation, and more recently applied to the design of SMAR cancer trials.10 Converting ATS evaluation from a hypothesis testing problem to one of statistical selection may reduce sample size requirements considerably; see Dawson et al.20 for a detailed worked example.
Discussion
In 1991, Greenhouse argued in favor of trials that randomized subjects to treatment strategies to avoid selection bias in maintenance therapy studies,46 but psychiatric research has just begun to embrace this line of thinking more generally. A recent overview of measurement-based approaches to treating depression highlights the need for innovative study designs to evaluate sequential adaptive algorithms.47 To encourage broader use of clinical trial methodology for testing ATS, we have considered the treatment of patients who present with apparent substance-induced psychosis, as way of illustrating concepts and issues. The (untested) hypotheses underlying the example is that the presumed overt manifestation of the patient’s predisposition to PP allows adaptive approaches to managing long-term antipsychotic treatment, and that an adaptive strategy will provide better overall outcomes than fixed ‘one size fits all’ rules used for extending acute treatment of SIP symptoms.
We consider SMAR designs to be appropriate for effectiveness studies, given that the intent is to provide clinicians with decision rules that will be replicable and feasible in real world practice settings. The example ATS will be more effective than the alternative non-adaptive treatments if 1) the decision rules produce superior outcomes (e.g., reduce duration of psychosis and treatment risk); and 2) it is possible to prospectively monitor patients in the target study population. The first condition simply requires that the ATS be more efficacious than the treatment alternatives, while the second requires that patients who seek treatment for apparent SIP be available for regular follow-up. A pure efficacy analysis of trial data, which would censor a patient’s data at the point of non-adherence to randomized treatment, could help pinpoint whether differences in effectiveness were due to difficulties in following patients over time. If this were the case, non-adaptive approaches that placed all patients on long-term antipsychotic treatment once psychotic symptoms cleared might prove superior, thereby reframing decisional uncertainty in terms of how long to continue the antipsychotic after psychosis clears with medication. Alternatively, the strategy that discontinued the initial antipsychotic immediately after symptoms cleared (i.e., the control option), while providing rescue treatment for recurrent psychosis, could prove to be most effective when taking into account the adverse effects due to long-term use of an antipsychotic. Such a result would largely endorse the current de facto standard, which relies on patients to seek treatment once psychosis, with or without concurrent substance use, recurs. Either scenario shifts interest to evaluation of cross-sectional (baseline) adaptive decision rules, such as Stage 1 in the example ATS that keeps high risk patients on an antipsychotic after psychosis clears.
The use of adaptive threshold rules to formalize individualized timing decisions during watchful waiting applies to the prodrome and relapse phases of psychiatric disorders, in addition to putative conversion from SIP to PP. In particular, the non-adaptive treatment alternatives in the example SMAR design closely resemble the experimental (start antipsychotic medication immediately) and control (no antipsychotic medication) options used in clinical trials to assess the efficacy of antipsychotics in preventing or delaying the progression to PP in “ultrahigh risk” patients.48-49 The inclusion of adaptive strategies in such trials serves two purposes. First, an ATS directly addresses the challenges posed by risk and response heterogeneity, providing an intermediate alternative to options of fixed time duration, through longitudinal individualized risk assessment.47 Second, randomized assignment to adaptive initiation of an antipsychotic should also lessen ethical concerns over falsely identified cases raised by prevention research. We note that patients with co-occurring substance use and psychosis differ in important ways from the “ultra-high risk” population; as characterized here, they are treatment seeking and highly symptomatic, with clear markers of vulnerability.
Our exposition of the ATS and SMAR approach has been rooted in clinical markers of disease, but the ideas are flexible enough to respond to advances in genomics and neuroscience. Not only can scientific evidence be incorporated in the algorithmic details, we maintain that chronic, episodic disorders intrinsically require treatment adaptation over time. Even with the advent of genetic testing for schizophrenia and related psychotic disorders, there would still be a great deal of uncertainty about when onset would occur, if at all. Given the side effect burden of antipsychotic medication, the decision to begin active treatment would likely remain an adaptive one carried out over time, rather than at some pre-determined time after genetic testing. This supposition supports the belief that advances in health information technology will play a complementary role to science in improving patient’s outcomes, by integrating adaptive strategies into routine clinical practice.47 Clinical trial methodology of the type we describe here is needed to develop an evidence base for optimizing psychiatric care via electronic systems for medical records and decision support. ✤
Acknowledgment
We acknowledge the invaluable help of Dr. Carol Caton for her influential participation in discussions that have led to this work.
References
- 1.Lavori PW, Dawson R, Rush JA. Flexible treatment strategies in chronic disease: Clinical and research implications. Biol Psychiatry. 2000;48:605–614. doi: 10.1016/s0006-3223(00)00946-x. [DOI] [PubMed] [Google Scholar]
- 2.Murphy SA, Oslin DW, Rush JA, Zhu J. Methodological challenges in constructing effective treatment sequences for chronic psychiatric disorders. Neuropsychopharmacology. 2007;32:57–262. doi: 10.1038/sj.npp.1301241. [DOI] [PubMed] [Google Scholar]
- 3.Lavori PW, Dawson R. Adaptive treatment strategies in chronic disease. Ann Rev Med. 20072008;59:443–453. doi: 10.1146/annurev.med.59.062606.122232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caton CM, Hasin DS, Shrout PE, Drake RE, Dominguez B, Samet S, Schanzer B. Predictors of psychosis remission in psychotic disorders that co-occur with substance use. Schizophr Bull. 2006;32:618–625. doi: 10.1093/schbul/sbl007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caton CL, Hasin DS, Shrout PE, Drake RE, Dominguez B, First MB, Samet S, Schanzer B. Stability of early-phase primary psychotic disorders with concurrent substance use and substance-induced psychosis. Brit J Psychiatry. doi: 10.1192/bjp.bp.105.015784. [DOI] [PubMed] [Google Scholar]
- 6.Schaid DJ, Ingle JN, Wieand S, Ahmann DL. A design for phase II testing of anticancer agents within a phase III clinical trial. Controlled Clinical Trials. 1998;9:107–118. doi: 10.1016/0197-2456(88)90032-3. [DOI] [PubMed] [Google Scholar]
- 7.Thall PF. A review of phase 2-3 clinical trial designs. Lifetime data analysis. 2008;14:S9–S12. doi: 10.1007/s10985-007-9049-x. [DOI] [PubMed] [Google Scholar]
- 8.Stone RM, Berg DT, George SL, Dodge RK, Paciucci PA, Schulman EJL, Moore JO, Powell BL, Schiffer CA, for the Cancer and Leukemia Group B Granulocyte-macrophage colony-stimulating factor after initial chemotherapy for elderly patients with primary acute myelogenous leukemia. New Engl J Med. 1995;332:1671–1677. doi: 10.1056/NEJM199506223322503. [DOI] [PubMed] [Google Scholar]
- 9.Tummarello D, Mari D, Granziano F, Isidori P, Cetto G, Pasini F, Santo A, Cellerino R. A randomized, controlled, phase III study of cyclophosphamide, doxorubicin and vincristine with etoposide (CAV-E) or teniposide (CAV-T), followed by recombinant interfereon-α maintenance therapy or observation, in small cell lung carcinoma patients with complete responses. Cancer. 1997;80:2222–2229. [PubMed] [Google Scholar]
- 10.Thall PF, Millikan R, Sung H-G. Evaluating multiple treatment courses in clinical trials. Stats in Med. 2000;19:1011–1028. doi: 10.1002/(sici)1097-0258(20000430)19:8<1011::aid-sim414>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 11.Milikan RE, Thall PF, Lee SJ, Jones D, Cannon MW, Kuebler JP, Wade J, III, Logothetis CJ. Randomized multicenter II trial of two multicomponent regimens in androgen independent prostate cancer. Journal of Clinical Oncology. 2003;21:878–883. doi: 10.1200/JCO.2003.04.057. [DOI] [PubMed] [Google Scholar]
- 12.Habermann TM, Weller EA, Morrison VA, Gascoyne RD, Cassileth PA, Cohn JB, Dakvil SR, Woda B, Fisher RI, Peterson BA, Horning SJ. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncology. 2006;24:3121–3127. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- 13.Thall PF, Logothetis CJ, Pagliaro L, Wen S, Brown MA, Williams D, Milikan RE. Adaptive therapy for androgen independent prostate cancer: A randomized selection trial including four regimens. J Natl Cancer Inst. 2007;99:1613–1622. doi: 10.1093/jnci/djm189. [DOI] [PubMed] [Google Scholar]
- 14.Thall PF, Wooten LH, Logothetis CJ, Milikan RE, Tannir NM. Bayesian and frequentist two-stage treatment strategies based on sequential failure times subject to interval censoring. Stat in Med. 2007;26:4687–4702. doi: 10.1002/sim.2894. [DOI] [PubMed] [Google Scholar]
- 15.Murphy S. An experimental design for the development of adaptive treatment strategies. Stat in Med. 2005;24:1455–1481. doi: 10.1002/sim.2022. [DOI] [PubMed] [Google Scholar]
- 16.Breslin FC, Sobell MB, Sobell LC, Cunningham JA, Sdao-Jarvie K, Borson D. Problem drinkers: evaluation of a stepped-care approach. J Substance Abuse. 1999;10:217–232. doi: 10.1016/s0899-3289(99)00008-5. [DOI] [PubMed] [Google Scholar]
- 17.Brooner RK, Kidorf MS, King VL, Stoller KB, Peirce JM, Bigelow GE, Kolodner K. Behavioral contingencies improve counseling attendance in an adaptive treatment model. J Substance Abuse Treatment. 2004;27:223–232. doi: 10.1016/j.jsat.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Prochaska JO, Velicer WF, Fava JL, Rossi JS, Tsoh JY. Evaluating a population-based recruitment approach and a stage-based expert system intervention for smoking cessation. Addict Behav. 2001;26:583–602. doi: 10.1016/s0306-4603(00)00151-9. [DOI] [PubMed] [Google Scholar]
- 19.Unutzer J, Katon W, Williams JW, Callahan CM, Harpole L, Hunkeler EM, Hoffing M, Arean P, Hegel T, Schoenbaum M, Oishi SM, Langston CA. Improving primary care for depression in late life: the design of a multicenter randomized trial. Med Care. 2001;39:785–799. doi: 10.1097/00005650-200108000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Dawson R, Lavori PW, Luby JL, Ryan ND, Geller B. Adaptive strategies for childhood mania. Biol Psychiatry. 2007;61:758–764. doi: 10.1016/j.biopsych.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 21.Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK. Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators: Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1286–1288. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 22.Rush AJ, Fava M, Wisniewski SR, Lavori PW, Trivedi MH, Sackeim HA, Thase ME, Nierenberg AA, Quitkin FM, Kashner TM, Kupfer DJ, Rosenbaum JF, Alpert J, Stewart JW, McGrath PJ, Biggs MM, Shores-Wilson K, Lebowitz BD, Ritz L, Niederehe G. Sequenced treatment alternatives to relieve depression (STAR*D): rationale and design. Control Clinical Trials. 2004;25:119–42. doi: 10.1016/s0197-2456(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 23.Schneider LS, Tariot PN, Lyketsos CG, Dagerman KS, Davis KL, Davis S, Hsiao JK, Jeste DV, Katz IR, Olin JT, Pollock BG, Rabins PV, Rosenheck RA, Small GW, Lebowitz B, Lieberman JA, National Institute of Mental Health Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Alzheimer disease trial methodology. Am J Geriatr Psychiatry. 2001;9:346–360. [PubMed] [Google Scholar]
- 24.McHugo GJ, Drake RE, Brunette MF, Xie H, Essock SM, Green AI. Enhancing validity of co-occurring disorders treatment research. Schizophr Res. 2006;32:655–665. doi: 10.1093/schbul/sbl009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lavori PW, Dawson R. A design for testing clinical strategies: biased individually tailored within-subject randomization. JR Stat Soc Ser A Stat Soc. 2000;163:29–38. [Google Scholar]
- 26.McKay JR, Lynch KG, Shepard DS, Pettinati HM. The effectiveness of telephone-based continuing care for alcohol and cocaine dependence: 24-month outcomes. Arch Gen Psychiatry. 2005;62:199–207. doi: 10.1001/archpsyc.62.2.199. [DOI] [PubMed] [Google Scholar]
- 27.Murphy SA, Lynch KG, Oslin D, McKay JR, TenHave T. Developing adaptive treatment strategies in substance abuse research. Drug Alcohol Depend. 2007;88(S2):S24–S30. doi: 10.1016/j.drugalcdep.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathias S, Lubman DI, Hides L. Substance-induced psychosis: a diagnostic conundrum. J Clin Psych. 2008 doi: 10.4088/jcp.v69n0304. in press; published online ahead of print. [DOI] [PubMed] [Google Scholar]
- 29.Boutros NN, Bowers MB., Jr Chronic substance-induced psychotic disorders: state of the literature. J Neuropsychiatry Clin Neurosci. 1996;8:262–269. doi: 10.1176/jnp.8.3.262. MB. [DOI] [PubMed] [Google Scholar]
- 30.Boutros NN, Bowers MB, Jr, Quinlan D. Chronological association between increases in drug abuse and psychosis in Connecticut state hospital. J Neuropsychiatry Clin Neurosci. 1998;10:48–54. doi: 10.1176/jnp.10.1.48. [DOI] [PubMed] [Google Scholar]
- 31.Arendt M, Rosenberg R, Foldager L, Perto G. Cannabis-induced psychosis and subsequent schizophrenia-spectrum disorders: follow-up study of 535 incident cases. Brit J Pscyhiatry. 2005;187:510–515. doi: 10.1192/bjp.187.6.510. [DOI] [PubMed] [Google Scholar]
- 32.Verdoux H, Gindre C, Sorbara F, Tournier M, Swensen JD. Effects of cannabis and psychosis vulnerability in daily life: an experience sampling test study. Pscychological Med. 2003;33:23–32. doi: 10.1017/s0033291702006384. [DOI] [PubMed] [Google Scholar]
- 33.Caspi A, Moffitt T, Cannon M, McClay J, Murray R, Harrington H, Taylor A, Arseneault L, Williams B, Braithwaite A. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment Interaction. Biol Psychiatry. 2005;57:1117–1127. doi: 10.1016/j.biopsych.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 34.Sobell LC, Sobell MB. Timeline Follow-Back: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen J, editors. Measuring Alcohol Consumption: Psychosocial and Biological Methods. Humana Press; 1992. [Google Scholar]
- 35.Miller TJ, McGlashan TH, Rosen JL, Somjee L, Markovich PJ, Stein K, Woods SW. Prospective diagnosis of the initial prodrom for schizophrenia based on the structured interview for prodromal syndromes: preliminary evidence of interrater reliability and predictive validity. Am J Psychiatry. 2002;159:863–865. doi: 10.1176/appi.ajp.159.5.863. [DOI] [PubMed] [Google Scholar]
- 36.Schanzer BM, First MB, Dominguez B, Hasin DS, Caton CL. Diagnosing psychotic disorders in the emergency department in the context of substance use. Psychiatric Serv. 2006;57:1468–1473. doi: 10.1176/ps.2006.57.10.1468. M.P.H. [DOI] [PubMed] [Google Scholar]
- 37.Hasin D, Trautman K, Miele G, Samet S, Smith M, Endicott J. Psychiatric Research Interview for Substance and Mental Disorders (PRISM): Reliability for substance abusers. Amer J Psychiatry. 1996;153:1195–1201. doi: 10.1176/ajp.153.9.1195. [DOI] [PubMed] [Google Scholar]
- 38.Sobell MB, Sobell LC. Stepped care as a heuristic approach to the treatment of alcohol problems. J Consult Clin Psychol. 2000;68:573–579. 2007; 190:105-111. [PubMed] [Google Scholar]
- 39.Lavori P, Dawson R. Dynamic treatment regimes: practical design considerations. Clinical Trials. 2004;1:9–20. doi: 10.1191/1740774s04cn002oa. [DOI] [PubMed] [Google Scholar]
- 40.Dawson R, Lavori PW. Comparison of designs for adaptive treatment strategies: baseline vs. adaptive randomization. J Stat Planning & Inference. 2003;117:365–385. [Google Scholar]
- 41.Yung AR, Philips LJ, Yuen HP, Francey SM, McFarlane CA, Hallgren M, McGorry PD. Psychosis prediction: 12-month follow up of a high-risk ‘prodromal’ group. Schizophr Res. 2003;60:21–32. doi: 10.1016/s0920-9964(02)00167-6. [DOI] [PubMed] [Google Scholar]
- 42.Yung AR, Philips LJ, McGorry PD, McFarlane CA, Francey S, Harrigan S, Patton GC, Jackson HJ. Prediction of psychosis: a step towards indicated prevention of schizophrenia. Brit J Psychiatry. 1998;172:14–20. [PubMed] [Google Scholar]
- 43.Bellman R. Dynamic Programming. Princeton University Press; Princeton, New Jersey: 1957. [Google Scholar]
- 44.Lavori PW, Dawson R. Improving the efficiency of estimation in randomized trials of adaptive treatment strategies. Clinicial Trials. 2007;4:297–308. doi: 10.1177/1740774507081327. [DOI] [PubMed] [Google Scholar]
- 45.Dawson R, Lavori PW. Sequential causal inference: application to randomized trials of adaptive treatment strategies. Stats in Med. 2008;27:1626–1645. doi: 10.1002/sim.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greenhouse JB, Stangl D, Kupfer DJ, Prien RF. Methodologic issue in maintenance therapy clinical trials. Arch Gen Psychiatry. 1991;48:313–318. doi: 10.1001/archpsyc.1991.01810280029004. [DOI] [PubMed] [Google Scholar]
- 47.Trivedi MH, Daly EJ. Measurement-based care for refractory depression: A clinical decision support model for clinical research and practice. Drug & Alcohol Dependence. 2007;88S:S61–S71. doi: 10.1016/j.drugalcdep.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McGorry PD, Yung AR, Philips LJ, Yuen HP, Francey S, Cosgrave EM, Germano D, Vravin J, McDonald T, Blair A, Adlard S, Jackson H. Randomized controlled trial of interventions designed to reduce the risk of progression to first-episode psychosis in a clinical sample with subthreshold symptoms. Arch Gen Psychiatry. 2002;59:921–928. doi: 10.1001/archpsyc.59.10.921. [DOI] [PubMed] [Google Scholar]
- 49.McGlashan TH, Zipursky RB, Perkins D, Addington J, Miller T, Woods SW, Hawkins KA, Hoffman RE, Preda A, Epstein I, Addington D, Lindborg S, Trzaskoma Q, Tohen M, Breier A. Randomized, Double-blind trial of Olanzapine versus placebo in patients prodromally symptomatic for psychosis. Am J Psychiatry. 2006;163:790–799. doi: 10.1176/ajp.2006.163.5.790. [DOI] [PubMed] [Google Scholar]