Abstract
Statement of Clinical Relevance
Therapies that can overcome the resistance of malignant brain tumors would be a major clinical advance. Here, we investigate the role of cAMP Phosphodiesterase-4 in stimulating brain tumor growth and the therapeutic utility of cAMP Phosphodiesterase-4 inhibition in the treatment of malignant brain tumors. Cyclic AMP Phosphodiesterase-4 was widely expressed in human brain tumors of glial and neuronal lineage, and forced expression of PDE4A1 accelerated intracranial glioblastoma and medulloblastoma xenograft growth. Moreover, targeted inhibition of PDE4, in combination with standard radiation and chemotherapy, induced a unique regression of established intracranial glioblastoma xenografts. These findings identify PDE4 as a novel molecular target for brain tumor therapy and indicate that PDE4 inhibition should be evaluated in clinical trials for malignant brain tumors.
Purpose
As favorable outcomes from malignant brain tumors remain limited by poor survival and treatment-related toxicity, novel approaches to cure are essential. Previously, we identified the cyclic AMP phosphodiesterase-4 (PDE4) inhibitor Rolipram as a potent anti-tumor agent. Here, we investigate the role of PDE4 in brain tumors and examine the utility of PDE4 as a therapeutic target.
Experimental Design
Immunohistochemistry was used to evaluate the expression pattern of a subfamily of PDE4, PDE4A, in multiple brain tumor types. To evaluate the effect of PDE4A on growth, a brain-specific isoform, PDE4A1 was overexpressed in xenografts of Daoy medulloblastoma and U87 glioblastoma cells. To determine therapeutic potential of PDE4 inhibition, Rolipram, temozolomide, and radiation were tested alone and in combination on mice bearing intracranial U87 xenografts.
Results
We found that PDE4A is expressed in medulloblastoma, glioblastoma, oligodendroglioma, ependymoma and meningioma. Moreover, when PDE4A1 was overexpressed in Daoy medulloblastoma and U87 glioblastoma cells, in vivo doubling times were significantly shorter for PDE4A1 overexpressing xenografts compared to controls. In long-term survival and bioluminescence studies, Rolipram in combination with first-line therapy for malignant gliomas (temozolomide and conformal radiation therapy) enhanced the survival of mice bearing intracranial xenografts of U87 glioblastoma cells. Bioluminescence imaging indicated that while temozolomide and radiation therapy arrested intracranial tumor growth, the addition of Rolipram to this regimen resulted in tumor regression.
Conclusion
This study shows that PDE4 is widely expressed in brain tumors and promotes their growth, and that inhibition with Rolipram overcomes tumor resistance and mediates tumor regression.
Keywords: Brain Tumor, Cyclic AMP, Phosphodiesterase, Rolipram, Bioluminescence, PDE4A
Introduction
Despite thirty years of clinical trials, favorable outcomes from malignant brain tumors continue to be limited by poor survival and excessive treatment–related toxicity (1). Targeted therapies based on tumor biology hold enormous promise, but have yet to be proven clinically effective (2). Thus, it is essential to continue our efforts to identify novel regulators of brain tumor growth that can be targeted for effective therapies.
Recent work from our laboratory suggested that cyclic AMP phosphodiesterase-4 (PDE4) might constitute a novel target for the treatment of brain tumors (3). Many human tumor cells originating in the CNS, lung, and breast overexpress phosphodiesterases, and a survey of 60 different human tumor cell lines indicated that the majority of this PDE activity was due to PDE4 (4). The PDE4 family of cyclic AMP phosphodiesterases regulates cAMP levels through their hydrolytic activity (5). There are four PDE4 subfamily genes (A–D), from which at least 20 different functional isoforms are generated. These allow for tissue-specific intracellular compartmentalization of cAMP signaling (5). Through distinct combinations of localization motifs, regulatory sites and protein-protein interacting domains this array of PDE4 molecules performs numerous and often exquisitely specific functions (6). For instance, using interfering RNA, Lynch et al. was able to demonstrate that only PDE4D5 regulated PKA-dependent heterotrimeric G protein switching by β2 – Adrenergic receptors (7). Similarly, dominant negative constructs allowed McCahill and colleagues to determine that PDE4D3 and PDE4C2, but not PDE4A4 or PDE4B1, were specifically required to regulate the basal activity of AKAP450-tethered protein kinase type II (8). Finally, targeted deletion of the PDE4 genes has also indicated that PDE4 isoforms perform non-redundant functions such as the specific requirement for PDE4B in LPS induction of TNF-αsecretion in macrophages (9).
The importance of PDE4 to tumor biology was first suggested by in vitro studies describing the anti-tumor activity of the specific PDE4 inhibitor Rolipram. Rolipram exhibited significant anti-growth effects when tested in vitro against several breast and lung carcinoma cell lines (10, 11), inhibiting growth by up to 60% in mammary carcinoma and melanoma cells (10). Furthermore, in vivo work in our lab demonstrated that Rolipram could slow the intracranial growth of glioblastoma and medulloblastoma xenografts (3).
Several studies have examined the mechanism of Rolipram’s anti-tumor effects. We found that Rolipram inhibits growth by elevating intracellular cAMP levels (3), which was consistent with the findings of McEwan et al. in studies of Rolipram treatment of colon carcinoma cells (12). Additionally, Chen et al. determined that Rolipram inhibits proliferation in A172 glioblastoma cells through the induction of the cell cycle inhibitors p27 and p21 (13). Despite these findings, though, it remains unclear how cAMP elevation regulates growth.
In this study, we provide the first direct in vivo evidence that PDE4 is a regulator of brain tumor growth and an important therapeutic target. We demonstrate that PDE4A expression is common in human brain tumors. Further, we show that the expression level of a brain-specific isoform of PDE4, PDE4A1, is correlated with the rate of intracranial xenograft growth. PDE4A1 is a unique “super-short” form of PDE4 (14). It possesses a truncated amino terminus that lacks the regulatory and protein-protein interacting domains that characterize the longer forms of PDE4, but which confers localization to the Golgi and Golgi-derived vesicles (15–17). Houslay and co-workers have suggested that precise localization of phosphodiesterase action, such as PDE4A1 to the region of the Golgi, is essential for the compartmentalization and appropriate transduction of cAMP dependent processes (18). The present studies establish a previously unknown role for PDE4A1 as a positive regulator of intracranial brain tumor growth and thus implicate mediators in the region of the Golgi in this activity.
The importance of PDE4 as a therapeutic target is evidenced by the effect of Rolipram on intracranial tumor growth and survival over a 5-month period when administered in combination with temozolomide and conformal radiation therapy (a current first-line treatment for glioblastoma). In this regard, we demonstrate that PDE4 inhibition can promote regression of malignant brain tumors when administered as an adjunct to established therapies.
Materials and Methods
All animals were used in accordance with an established Animal Studies Protocol approved by the Washington University School of Medicine Animal Studies Committee.
Chemicals and other reagents
All chemicals were obtained from Sigma (St. Louis, MO) unless otherwise indicated. All tissue culture reagents and media were obtained from Invitrogen (Carlsbad, CA) unless otherwise indicated. A construct containing mCherry cDNA was the kind gift of Dr. Roger Y. Tsien, University of California (San Diego, CA). Murine PDE4A1 (accession number AJ297396) was provided by Dr James Cherry, Boston University (Boston, MA). The human PDE4A-specific antibody was generously provided by Dr. Miles Houslay, University of Glasgow, Glasgow, Scotland)
Tumor cell lines
The following experiments utilize the well-characterized U87 glioblastoma and Daoy medulloblastoma cell lines as models for human brain tumors. In previous in vitro and intracranial xenograft experiments with these cell lines we have characterized the role of CXCR4 and cAMP in the regulation of brain tumor growth, and the anti-tumor effects of CXCR4 antagonists and cAMP elevating drugs (3, 19). These studies form the basis for the present work in which mCherry- and mCherry-PDE4A1-expressing U87 and Daoy cells were generated via lentiviral infection of previously generated eGFP-firefly luciferase expressing cell lines as described (19–21). Viral particles were produced by the Viral Vectors Core Facility of The Hope Center for Neurological Diseases at Washington University School of Medicine. Expression vectors used in viral production contained either a transgene for mCherry fluorescent protein under a CMV promoter alone, or the CMV promoter-mCherry cassette together with a murine PDE4A1 (mPDE4A1) transgene under a ubiquitin promoter. mCherry-expressing cells were sorted and collected by high-speed fluorescence-activated cell sorting (MoFlo™ High Performance Cell Sorter, Dako, Glostrup, Denmark) (Supplemental Figure 1A). Cell lines are referred to as U87-luc, mCherry-U87-luc, PDE4A1-U87-luc, mCherry-Daoy-luc and PDE4A1-Daoy-luc.
Generation of intracranial xenografts
Intracranial xenografts were generated as previously described (3). Homozygous NCR nude mice (Taconic Farms, Germantown, NY) were anesthetized (IP ketamine (87 mg/kg)/xylazine (13 mg/kg), Phoenix Pharmaceuticals, St. Joseph, MO), the cranium was exposed and a small hole was made 2 mm lateral and posterior to the bregma with a size 34 inverted cone burr (Dremel, Racine, WI). Mice were positioned in a stereotactic frame (Stoelting, Wood Dale, IL) and 50,000 cells in 5 μl of PBS were injected through a 27-gauge needle over 1 min at 3 mm below the dura mater. The incision was closed with Vetbond (3M, St. Paul, MN).
Bioluminescence imaging
NCR nude mice bearing intracranial xenografts of U87-luc, mCherry-U87-luc, PDE4A1-U87-luc, mCherry-Daoy-luc or PDE4A1-Daoy-luc, were injected with 150 μg/g D-luciferin (Biosynth, Naperville, IL) as described (3). After anesthesia using 2.5% isoflurane, mice were imaged with a charge-coupled device (CCD) camera-based bioluminescence imaging system (IVIS 50; Xenogen Corp., Alameda, CA; exposure time 1–30 seconds, binning 8, field of view 12, f/stop 1, open filter). Signals were displayed as photons/sec/cm2/sr (22). Regions of interest (ROI) were defined manually and images processed using Living Image and IgorPro Software (Version 2.50) as described (3). Raw data were expressed as total photon flux (photons/sec) (22).
Treatment of mice bearing intracranial xenografts
NCR nude mice bearing intracranial xenografts of U87-luc glioblastoma cells were imaged at least twice after implantation of cells to identify those with growing tumors. As previously described, two weeks after tumor cell implantation, cohorts of mice were divided into control and treatment groups (7–10 mice/group) (3, 19).
Systemic Therapies
Rolipram and temozolomide (Pharmacy, St. Louis Children’s Hospital) treatments were administered via the drinking water. Drug concentrations in the water were adjusted based on measured daily water consumption to deliver 5 mg/kg/day of Rolipram continuously or 21 mg/kg/day × 5 days/month of temozolomide.
Irradiation
Mice were treated with conformal radiotherapy as previously described (23). Subject animals were anesthetized (IP ketamine (87 mg/kg)/xylazine (13 mg/kg)) and positioned within a custom head immobilization device affixed to the microRT© animal couch. The microRT© couch was positioned within the microRT© device using a 3-axis translational couch positioning system. To create the radiotherapy plan, conformal radiotherapy fields were designed using the treatment planning system based on a CT scan of the anesthetized and immobilized mouse using a Phillips Brilliance CT scanner (Phillips Medical Systems, Bothell, WA). Treatment fields were hand-optimized to minimize exposure to the mucous membranes of the oral cavity, oropharynx, nasal structures, esophagus, trachea, eyes, and ears. Final plan consisted of two fields per fraction using right and left lateral beams. For each fraction, each animal was treated per the radiotherapy plan using a clinical I-192 HDR afterloading system (Nucletron, Columbia, Maryland) collimated via the microRT© collimator system. Dose rate was ~90 cGy/min. Each animal was treated with six fractions of 5 Gy, prescribed to midplane, for a total of 30 Gy. Fractions were delivered every other day (i.e., M, W, F) for two consecutive weeks. Animals were observed to tolerate this regimen well.
Immunohistochemistry
In accordance with an Institutional Review Board approved protocol for human research, human brain tumor tissue was retrieved from the pathology files at Washington University School of Medicine. Formalin-fixed, paraffin-embedded tissue was processed and analyzed as previously described (24). Murine-specific PDE4A antibody was applied at a concentration of 0.33 μg/ml (Fabgennix, Frisco, TX), detected using biotin-conjugated secondary antibodies augmented by streptavidin-horseradish peroxidase, and visualized using 3,3′-diaminobenzidine (DAKO, Carpinteria, CA). Human-specific PDE4A was diluted 1:400 and detected as above. Application of isotype-specific IgG to serial sections, in the absence of PDE4A-directed antibody, served as controls for the specificity of antibody staining.
cAMP measurement
Cyclic AMP was measured by competitive immunoassay using a Correlated-EIA™ Enzyme Immunoassay Kit (Assay Designs, Ann Arbor, MI) according to the manufacturer’s instructions (3). Briefly, mCherry-U87-luc, PDE4A1-U87-luc, mCherry-Daoy-luc and PDE4A1-Daoy-luc cells growing in fetal bovine serum-supplemented (10%) minimal Eagle’s alpha-media (Gibco-BRL) were lysed in 0.1M HCl and particulate matter was removed by centrifugation at >600 × g for 10 min. The supernatants were dried down and resuspended in cAMP assay buffer (Assay Designs). Alternatively, mCherry-expressing intracranial xenograft tissue (mCherry-U87-luc, PDE4A1-U87-luc, mCherry-Daoy-luc and PDE4A1-Daoy-luc) was removed under direct fluorescence microscopy and frozen in liquid nitrogen. Frozen tissue was weighed and homogenized with 10 volumes of 10% ice-cold trichloroacetic acid then centrifuged for 10 min at 1500 × g to remove precipitate. The supernatant was washed three times with 8 volumes of water-saturated ether. The aqueous phase was dried down and resuspended in cAMP assay buffer. Absorbance at 405 nm was measured, and cAMP concentrations were calculated based on a standard curve. Protein concentrations were measured by colorimetric assay (Bio-Rad, Hercules, CA) according to the manufacturer’s directions. Cyclic AMP values were normalized to protein for each sample individually.
Western Blot Analysis
Tissue culture extracts were prepared by lysing cells with RIPA buffer (50 mM Tris pH 8.0, 150 mM NaCl, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate and 1% Triton-X-100) supplemented with phosphatase inhibitor cocktail set II (Calbiochem, La Jolla, CA) and a protease inhibitor cocktail (Roche, Indianapolis, IN). Tumor lysates were prepared by sonication in lysis buffer (100 μl/mg tissue). The proteins were resolved with 4–12% or 10% Bis-Tris gels (Invitrogen) and transferred onto Hybond ECL nitrocellulose membrane (Amersham, Piscataway, NJ) according to standard protocols. Membranes were incubated with antibodies directed against murine PDE4A (1:1000, Abcam), human PDE4A (1:400, Houslay) and actin (1:3000, Sigma) overnight at 4°C. This was followed by incubation with horseradish peroxidase-conjugated secondary antibody (1:20,000, Bio-Rad). Peroxidase activity was detected using the enhanced chemiluminescence Supersignal West Pico system (Pierce, Rockford, IL). Quantitation of western blots was performed by densitometry using ImageJ software from the NIH.
Statistical Analysis
Data were analyzed using GraphPad Prism version 4.00 (GraphPad Software, San Diego, CA) or Stata10 (Stata Corporation, College Station, TX). Kaplan–Meier survival curves were analyzed using pairwise log-rank tests. Given the repeated measurements of mice over time, statistical differences in growth curves were analyzed using the generalized estimating equation (GEE) regression analysis. For cAMP comparisons, significance was determined by two-tailed t-test. Statistical significance was assumed for p < 0.05.
Results
Cyclic AMP Phosphodiesterase-4 is widely expressed in human brain tumors
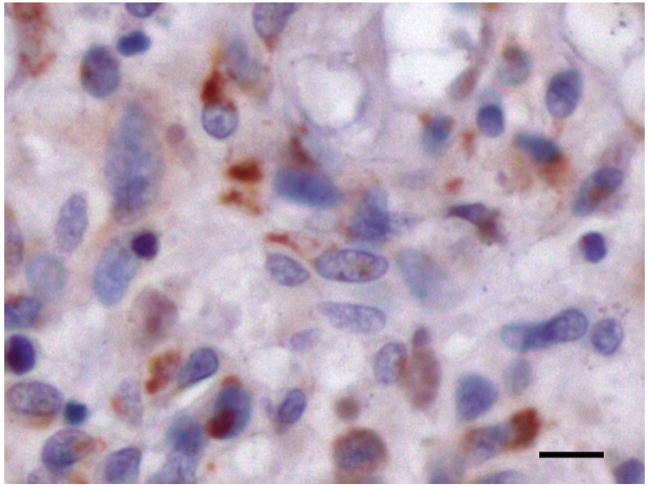
The anti-tumor effect of Rolipram suggested that PDE4 was important in brain tumor biology. Since members of the PDE4A subfamily are distributed throughout the brain (25), we examined the expression pattern of PDE4A in 37 tumors from pediatric and adult patients (Table 1). Among these were four types of parenchymal brain tumors (astrocytomas, medulloblastomas, oligodendrogliomas and ependymomas) as well as meningiomas (non-parenchymal, intracranial tumors) of various grades and histological subtypes. PDE4A was expressed in all tumor types examined (Supplemental Figure 2). Staining in tumor cells was most commonly punctate and frequently perinuclear (Figure 1). All Astrocytomas (15) and medulloblastomas (8) exhibited PDE4A expression with no correlation between grade or histological subtype, respectively. In more limited sample sizes, the majority of ependymomas (4/5), and meningiomas (5/6) and all oligodendrogliomas (3/3) demonstrated PDE4A expression. In most cases, PDE4A expression was found solely in tumor cells. In several specimens however, PDE4A was also evident in endothelial cells. Leukocytes within the lumens of tumor-associated blood vessels were consistently stained (data not shown). Thus, PDE4A expression is widespread in brain tumors.
Table 1.
PDE4A expression in human brain tumors
| Patient | Diagnosis | Age | Sex | PDE4A |
|---|---|---|---|---|
| 1 | Medulloblastoma - c* | 13 | M | 2a |
| 2 | Medulloblastoma - c | 10 | M | 2 |
| 3 | Medulloblastoma - d | 8 | F | 1 |
| 4 | Medulloblastoma - d | 7 | M | 1 |
| 5 | Medulloblastoma - d | 19 | M | 2 |
| 6 | Medulloblastoma - d | 2 | F | 2 |
| 7 | Medulloblastoma - d | 35 | M | 1 |
| 8 | Medulloblastoma - a | 5 | M | 3 |
|
| ||||
| 9 | Astrocytoma – I** | 7 | M | 3 |
| 10 | Astrocytoma – I | 8 | F | 1 |
| 11 | Astrocytoma – I | 12 | F | 3 |
| 12 | Astrocytoma – I | 8 | F | 1 |
| 13 | Astrocytoma – II | 47 | M | 3 |
| 14 | Astrocytoma – II | 57 | M | 2 |
| 15 | Astrocytoma – III | 38 | M | 2 |
| 16 | Astrocytoma – III | 24 | M | 3 |
| 17 | Astrocytoma – III | 47 | F | 3 |
| 18 | Astrocytoma – III | 21 | F | 1 |
| 19 | Astrocytoma – III | 73 | F | 1 |
| 20 | Astrocytoma – IV | 62 | F | 1 |
| 21 | Astrocytoma – IV | 50 | F | 3 |
| 22 | Astrocytoma - IV | 57 | F | 2 |
| 23 | Astrocytoma - IV | 60 | F | 4 |
|
| ||||
| 24 | Oligodendroglioma – II | 44 | F | 1 |
| 25 | Oligodendroglioma – II | 50 | F | 1 |
| 26 | Oligodendroglioma – III | 44 | F | 2 |
|
| ||||
| 27 | Meningioma – I | 65 | F | 1 |
| 28 | Meningioma – I | 70 | F | 3 |
| 29 | Meningioma – II | 72 | F | 2 |
| 30 | Meningioma – III | 29 | F | 3 |
| 31 | Meningioma – III | 70 | F | 3 |
| 32 | Meningioma – III | 61 | M | 0 |
|
| ||||
| 33 | Ependymoma – II | 18 | M | 1 |
| 34 | Ependymoma – II | 68 | F | 2 |
| 35 | Ependymoma – III | 7 | M | 2 |
| 36 | Ependymoma – III | 39 | M | 0 |
| 37 | Ependymoma – III | 6 | F | 2 |
Histological subtypes of medulloblastoma, c = classic, d = desmoplastic, a = anaplastic.
Histological grading of brain tumors based on the World Health Organization classification, grades I-IV.
PDE4A expression grading: 1 = 1–25%, 2 = 26 – 50%, 3 = 51–75%, 4 = 76–100% of tumor cells are positive for PDE4A expression.
Figure 1. PDE4A is expressed in a punctate pattern in brain tumors.
A representative section of an ependymoma stained for PDE4A (brown) is shown. Scale bar = 10 μM.
Cyclic AMP Phosphodiesterase-4A1 stimulates brain tumor growth in vivo
To determine whether the high level of PDE4A expression in human brain tumors was indicative of a growth advantage, we examined the effect of increased PDE4A expression on brain tumor growth. We overexpressed a murine homolog of a brain-specific isoform of PDE4, mPDE4A1 (26), via lentiviral infection of U87 glioblastoma and Daoy medulloblastoma cells that had previously been engineered to express eGFP and firefly luciferase (Supplemental Figure 1A). As expected, increased mPDE4A1 expression (Supplemental Figure 1B) was correlated with lower levels of cAMP as compared to mCherry control cells (Supplemental Figure 1C).
To better understand how PDE4A1 might regulate tumor growth in vivo, mCherry-expressing (control) or mPDE4A1-overexpressing brain tumor cells were stereotactically implanted into the brains of nude mice and intracranial growth was measured by weekly bioluminescence imaging as previously described (3). In both U87 and Daoy cell xenografts, overexpression of mPDE4A1 was associated with significantly accelerated intracranial growth (Figure 2A). Curve fitting of the relation between bioluminescence ratios and time [exponential growth: Y=start*exp(k*X) where k = rate constant, and tumor doubling time = 0.69/k] revealed that expression of mPDE4A1 in Daoy cells was associated with a decrease in doubling time from 1.68 weeks to 1.09 weeks. Similarly, overexpression of mPDE4A1 in U87 cells produced a decrease in tumor doubling time from 0.94 to 0.39 weeks.
Figure 2. Overexpression of PDE4A1 stimulates intracranial tumor growth.
mCherry-Daoy-luc, or mCherry-U87-luc, or PDE4A1-Daoy-luc, or PDE4A1-U87-luc cells were stereotactically implanted into the cortex of nude mice. (A) Tumor growth was measured by weekly bioluminescence imaging. Shown are bioluminescence ratios (total photon flux each week/photon flux from week 1). P-values for differences between control and PDE4A1-overexpressing curves were determined by generalized estimating equation regression analysis. (B) Human and murine PDE4A expression was evaluated by western blot analysis of lysates derived from isolated xenograft tumor tissue. Asterisk indicates human PDE4A1. (C) Cyclic AMP levels were determined by ELISA from diethyl ether extracts of control and PDE4A1-overexpressing Daoy and U87 mCherry-positive tumor tissue isolated under direct fluorescence microscopy. Shown are the means and SEM of values normalized to mCherry controls for both U87 and Daoy tumor tissue samples (n = 13). ** = p<0.01 as determined by two-tailed t-test.
To evaluate the relationship between PDE4A1 expression and intracranial tumor growth, mCherry-positive tumor tissue was recovered from control and mPDE4A1 overexpressing xenografts under direct fluorescence microscopy (Supplemental Figure 3). Western blot analysis of mCherry-U87-Luc and mCherry-Daoy-Luc tumor tissue with the human-specific PDE4A antibody revealed multiple human PDE4A (hPDE4A) isoforms including one with an apparent molecular weight of 78 kD consistent with hPDE4A1 (14) (Figure 2B). Western blot analysis of mCherry control and mPDE4A1 overexpressing Daoy and U87 xenografts with the murine-specific PDE4A antibody confirmed continued xenograft expression of mPDE4A1. Consistent with in vitro studies (Supplemental Figure 1), overexpression of mPDE4A1 in vivo was evident as a prominent band at 68 kD (Figure 2B). In addition, a second band of 83 kD was evident. This latter band is similar to one previously reported for mPDE4A1 expression in COS-7 cells (27).
In order to verify the functionality of the overexpressed PDE4A1, cAMP levels were examined in mCherry-positive tumor tissue. Cyclic AMP was extracted from xenograft tumor tissue and measured by ELISA as previously described (3). Increased mPDE4A1 expression was associated with decreased levels of cAMP in the tumor tissue (Figure 2C). These data are consistent with a role for PDE4 in the stimulation of intracranial brain tumor growth.
Rolipram enhances the survival of mice bearing intracranial xenografts
The growth-promoting activity of PDE4A1 suggests that targeted inhibition of PDE4 with the specific inhibitor Rolipram could be an important addition to current brain tumor therapies. We therefore performed long-term survival and coupled bioluminescence studies in mice bearing intracranial xenografts of U87-luc glioblastoma cells to determine the effects of Rolipram treatment in combination with standard chemoradiation.
Therapy began two weeks after tumor cell implantation. This time was sufficient to establish steadily increasing bioluminescence (a measure of tumor growth) in the xenografts. Mice were assigned to one of eight treatment groups: control, Rolipram, temozolomide, radiation therapy, Rolipram and radiation therapy, Rolipram and temozolomide, radiation therapy and temozolomide (a current first-line therapy), and Rolipram, radiation therapy and temozolomide. Mice underwent bioluminescence imaging weekly and were examined for signs of advancing disease such as weakness, weight loss, and seizures. Mice observed to be suffering from their disease were euthanized.
All treatments were associated with improved survival as compared to controls (Figure 3). Pairwise log-rank tests indicated significant differences in survival between control (no treatment) and the following regimens: temozolomide (p=0.0016), radiation (p=0.0005), radiation and temozolomide (p<0.0001), Rolipram and temozolomide (p<0.0001), Rolipram and radiation (p=0.0006), and radiation, temozolomide and Rolipram (p<0.0001). In terms of overall survival, however, the treatments could be divided into two groups: minimally effective (Figure 3A) and highly effective therapies (Figure 3B). The minimally effective therapies included temozolomide, radiation therapy, Rolipram, and Rolipram and radiation therapy; these mice had an overall survival of 42% or less. The highly effective therapies included Rolipram and temozolomide, radiation therapy and temozolomide, and Rolipram, radiation therapy and temozolomide. Survival increased significantly among mice receiving one of these three therapies. Interestingly, the combination of Rolipram and temozolomide (70% survival) was similar in activity to radiation and temozolomide (a current first-line therapy, 70% survival), while the addition of Rolipram to radiation therapy and temozolomide provided the greatest effect on survival with only a single death over the 150-day experiment (90% survival).
Figure 3. Inhibition of PDE4 with Rolipram enhances survival in mice bearing intracranial U87 xenografts.
Nude mice bearing intracranial U87-luc xenografts were assigned to eight treatment groups (7–10 mice/group) and left untreated (control) or treated with Rolipram, temozolomide, radiation therapy or combinations thereof as described in Experimental Procedures. Survival was monitored over 150 days and data are presented as a Kaplan-Meier curve for (A) minimally effective treatments and (B) highly effective treatments.
Bioluminescence imaging distinguishes treatment effects in vivo
To gain insight into how these treatments improved survival, we examined the bioluminescence imaging data for evidence of anti-tumor growth effects. All mice, whether alive or dead, were included in the analysis. For those mice that had died, their last recorded total photon flux was included in generating the mean photon flux for the group. Bioluminescence data for evaluating treatment efficacy was analyzed by determining the midpoint of maximal growth and rate of maximal growth in control (untreated) mice and comparing it to the other treatment groups.
Several informative patterns of intracranial growth emerged. Tumors in control mice grew in an uninterrupted, exponential fashion and exhibited a midpoint of maximal growth at 6.5 weeks and maximal exponential growth of 7.8 × 109 photons/week (Figure 4). Because all control mice died by week 10, the plateau represents the mean of the final measures of all mice in this group.
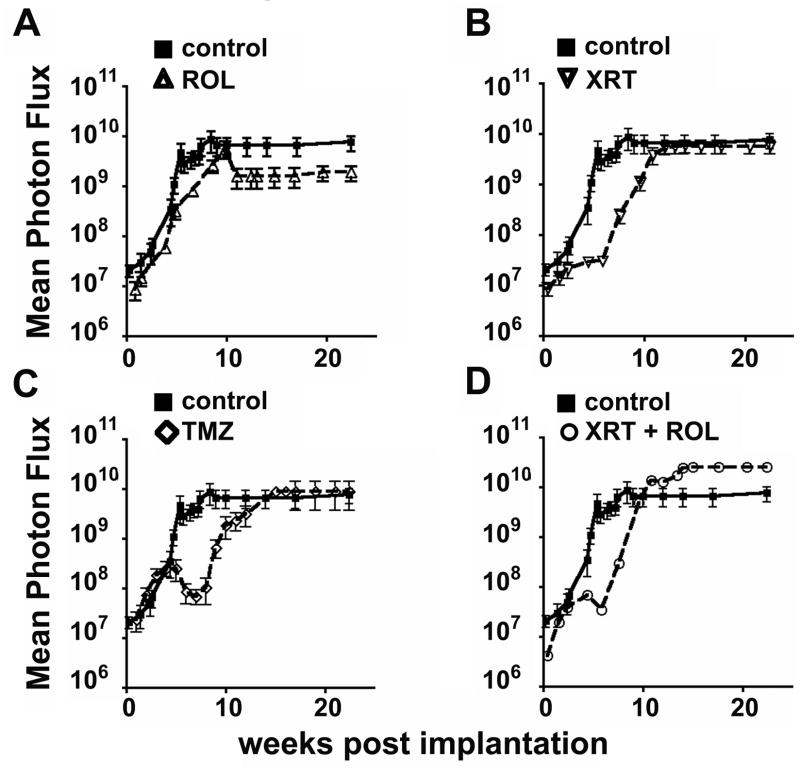
Figure 4. Bioluminescence imaging reveals modes of anti-tumor effects.
Intracranial tumor growth was measured by serial bioluminescence imaging. Shown is mean photon flux over time for the minimally effective therapies (see Figure 3) as compared to control. (A) Rolipram, (B) Radiation, (C) Temozolomide, (D) Radiation and Rolipram.
Interestingly, though radiation and Rolipram had similar effects on overall survival, the two treatments had markedly different effects on growth. Compared to control, Rolipram treatment did not appreciably alter the midpoint of maximal growth, which was 6.7 weeks in the latter group (Figure 4A); however, Rolipram alone decreased the rate of maximal tumor growth to 1.8 × 109 photons/week.
In contrast, radiation therapy induced a transient arrest in growth, delaying the midpoint of maximal growth to 10.5 weeks (Figure 4B). However, after recovery from radiation, the rate of exponential growth in irradiated tumors was similar to that of control mice, with a slope of 5.7 × 109 photons/week. The combination of radiation and Rolipram exhibited similar anti-tumor effects as radiation alone (Figure 4D).
Tumors in temozolomide-treated mice demonstrated a multi-phasic growth response with an initial regression timed to the completion of the second course of therapy (Figure 4C). Exponential growth resumed after 8 weeks, however, with the midpoint at 12.3 weeks and a maximal slope of 8.9 × 109 photons/week.
In contrast to the minimally effective therapies (Figure 4), the highly effective therapies produced more favorable results on intracranial growth. With each of these treatments, no tumor growth beyond an initial phase of 2–3 weeks was observed (Figure 5). Consistent with the survival data, radiation and temozolomide (Figure 5A) versus Rolipram and temozolomide (Figure 5B) were indistinguishable with regard to their inhibition of intracranial growth. Of note, bioluminescence signal persisted in mice treated with either of these regimens, indicating that while these tumors were not growing, they remained viable. The addition of Rolipram to radiation and temozolomide resulted in tumor regression (Figure 5C, D). After 12 weeks of this therapy, bioluminescence declined to approximately 1% of the starting values. Together, these data indicate that Rolipram provides a unique therapeutic effect that promotes tumor regression and enhances survival.
Figure 5. Addition of Rolipram to standard radiation therapy and temozolomide results in tumor regression.
Intracranial tumor growth was measured by serial bioluminescence imaging. Shown is the mean photon flux over time for the highly effective treatments (see Figure 3) as compared to control. (A) Radiation and temozolomide, (B) Rolipram and temozolomide, (C) Rolipram, radiation and temozolomide, (D) Comparison of Rolipram, radiation and temozolomide to the current standard of care, radiation and temozolomide.
Discussion
In this study, we show that PDE4A1 overexpression stimulates brain tumor growth in vivo, while inhibition of PDE4 suppresses tumor growth and augments the anti-tumor effects of chemo- and radiation therapies. PDE4 has previously been shown to be widely expressed in a number of different human tumor cell lines (4). In addition, we now show that PDE4A is also expressed in astrocytomas, medulloblastomas, oligodendrogliomas, ependymomas and meningiomas, indicating that PDE4A activity may be essential to the growth of all brain tumors.
To better define the role of PDE4 in brain tumor biology, we overexpressed PDE4A1, a brain specific isoform, in U87 glioblastoma and Daoy medulloblastoma cells (26). Stimulation of growth occurred in both brain tumor models. U87 cells were derived from a malignant astrocytoma. These tumors typically carry mutations in the MAPK, PI3K, Rb and MDM2/p53 pathways (28) and, consistent with this, U87 cells possess deletion of p14/p16 and PTEN genes (29). Daoy cells were derived from a desmoplastic medulloblastoma. Medulloblastomas are characterized by mutational activation of the sonic hedgehog, Wnt, and Myc pathways (30). Daoy cells also carry a mutation of TP53 (31). Despite these differences in molecular profile, PDE4A1 overexpression stimulated both U87 and Daoy growth in vivo. These data suggest that PDE4A1 effects were not dependent upon the locus into which the transgene inserted and were not specific to pathways activated by mutation in these tumors. Instead, these results indicate that PDE4A1 is a positive regulator of brain tumor growth.
It will be important to determine whether the cAMP-hydrolyzing activity of PDE4A1 is essential to its growth promoting effects. Previously, we and others have suggested that low levels of cAMP stimulate brain tumor growth (3, 12, 32–34). The current study lends additional support to this model as the effects of PDE4A1 overexpression and Rolipram are most likely the result of changes in cAMP levels. The work of Houslay and others has shown that PDE4s play multiple roles in intracellular signaling. These roles include the regulation of receptor desensitization and G protein switching, as well as PKA and Erk activation (5). There are at least 20 different isoforms of PDE4 that can be inhibited with Rolipram. Each contains a unique N-terminal region that appears to regulate specific compartmentalized functions (6). For example, the N-terminal regions of PDE4A4 and PDE4A5 interact with the SH3 homology domains of Src family kinases, while the N terminal region of PDE4A1 facilitates membrane insertion, particularly into the trans-Golgi membrane (35). Thus overexpression of even catalytically inactive PDE4 isoforms can alter signaling functions by displacing active endogenous PDE4 from necessary binding sites and functioning as dominant negative constructs (8). The PDE4A1 isoform lacks the regulatory and protein-protein interacting domains that are found in longer PDE4 isoforms (5, 35). Thus, the growth-promoting effects of PDE4A1 overexpression are most readily attributed to increased cAMP hydrolysis as evidenced by the observed decrease in levels of cAMP.
In this regard, the localization of PDE4A1 to the membranes of the Golgi apparatus and Golgi-derived vesicles is of interest. While global changes in cAMP were detected in our cells and xenografts overexpressing PDE4A1, PDE4 effects can also be limited to regulating local cAMP dependent functions. For instance, targeted deletion of PDE4B revealed that it, and not PDE4A or PDE4D was necessary for regulating TLR signaling in macrophages (9). The effect of PDE4B loss on macrophage responses to LPS stimulation was dependent upon protein kinase A activation but was not associated with global changes in cAMP levels. These data suggest that localization of PDE4B is necessary to regulate cAMP levels in a critical subcompartment for TLR signaling. Thus it will be essential to identify Golgi-localized mediators of PDE4A1 effects on tumor growth and to determine whether elevation of cAMP levels antagonizes their growth promoting functions.
Elevation of cAMP as a therapeutic strategy for cancer has previously been examined, but was limited by the excessive toxicity of the early pleiotropic phosphodiesterase inhibitors (36, 37). Specific phosphodiesterase inhibitors such as Rolipram are better tolerated at therapeutic doses (38) and exhibit strong anti-tumor effects. (10–12). Prior studies suggested a therapeutic role for Rolipram in the treatment of brain tumors and colon cancer (3, 12). To our knowledge, this is the first report to demonstrate a survival advantage in response to Rolipram treatment over an extended preclinical trial.
The potential importance of Rolipram therapy was most evident upon analysis of bioluminescence data pertaining to the highly effective therapies of radiation and temozolomide, Rolipram and temozolomide, and Rolipram, radiation, and temozolomide. Though the initial pattern of tumor growth was similar among these three groups, the value of Rolipram treatment became most evident at 12 weeks of therapy. Therapy with either Rolipram and temozolomide or radiation and temozolomide resulted in cessation of further tumor growth, but tumors remained viable as evidenced by their persistent bioluminescent signal. In contrast, treatment with Rolipram in conjunction with radiation and temozolomide resulted in nearly complete loss of bioluminescence, indicating tumor regression.
Human studies have shown that a major determinant of response to alkylator therapy like temozolomide is the expression and activity of the DNA repair enzyme methyl glutamyl methyl transferase (MGMT) (39). In patients with reduced MGMT expression through promoter methylation, there is a greater response to temozolomide therapy. Thus one possible mechanism whereby Rolipram enhances the effects of temozolomide and radiation might be the inhibition of MGMT. Consistent with published studies we found that Daoy and U87 cells express MGMT (40) but this was unaffected by Rolipram treatment in vitro (data not shown). Thus the mechanism by which Rolipram augments the activity of radiation and temozolomide may be distinct from modulating DNA alkylation.
The present study strongly indicates that PDE4 inhibitors should be evaluated in clinical trials for brain tumors. Our data suggest that PDE4 inhibition may be the key adjunct to standard chemotherapy and radiation therapy to promote brain tumor regression. Furthermore, the equivalence of combined radiation and temozolomide to Rolipram and temozolomide suggests that PDE inhibitors might prove to be suitable alternatives for radiation when combined with chemotherapy. This may be especially important for young patients for whom irradiation is not an option due to the detrimental effects radiation has on their developing brains.
The potential for Rolipram to be a useful agent in brain tumor therapy is emphasized by its ability to penetrate into and treat diseases of the central nervous system. However, clinical application of Rolipram might also be limited by its toxicities. In multiple clinical trials for depression and multiple sclerosis, Rolipram was relatively well tolerated with nausea and vomiting the most commonly reported side effect (38). However, in long-term toxicity studies in rats, high dose Rolipram therapy was also associated with prolactinomas and mammary adenocarcinoma (41). While serious, these side effects are not substantially different from the nausea, vomiting and secondary cancer risks of commonly used treatments for brain tumors like radiation therapy and temozolomide. Therefore, if Rolipram demonstrated a unique therapeutic effect its known toxicities would not preclude its use in the treatment of malignant brain tumors. However, newer PDE4 subfamily-specific inhibitors may prove equal or more efficacious and less toxic (6, 42). Thus, additional investigation into whether other PDE4 isoforms can also regulate brain tumor growth, and whether subfamily specific inhibitors exhibit equal efficacy to Rolipram is essential.
Supplementary Material
Acknowledgments
The authors thank Erich Kiehl for assistance with mice irradiation, Michael Geske for help with statistical analysis, Mahil Rao for designing lentiviral constructs and Kristin Brown for editorial assistance. We also wish to thank Drs. Jonathan Gitlin and Louis Muglia for critical reading of the manuscript. We would like to thank the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, MO, for the use of the High Speed Cell Sorter Core.
Financial Support: The Children’s Brain Tumor Foundation and The Pediatric Brain Tumor Foundation (JBR), Pilot Study Funding from the Molecular Imaging Center at Washington University School of Medicine and the NIH P50 CA94056 (DPW), NCI Cancer Center Support Grant #P30 CA91842 (High Speed Cell Sorter Core), NIH Neuroscience Blueprint Core Grant P30 NS057105 to Washington University (Viral Vectors Core), NIH R21 CA 108677 (MicroRT©).
References
- 1.DeAngelis LM. Chemotherapy for brain tumors--a new beginning. N Engl J Med. 2005;352:1036–8. doi: 10.1056/NEJMe058010. [DOI] [PubMed] [Google Scholar]
- 2.Wen PY, Kesari S, Drappatz J. Malignant gliomas: strategies to increase the effectiveness of targeted molecular treatment. Expert Rev Anticancer Ther. 2006;6:733–54. doi: 10.1586/14737140.6.5.733. [DOI] [PubMed] [Google Scholar]
- 3.Yang L, Jackson E, Woerner BM, Perry A, Piwnica-Worms D, Rubin JB. Blocking CXCR4-Mediated Cyclic AMP Suppression Inhibits Brain Tumor Growth In vivo. Cancer Res. 2007;67:651–8. doi: 10.1158/0008-5472.CAN-06-2762. [DOI] [PubMed] [Google Scholar]
- 4.Marko D, Pahlke G, Merz KH, Eisenbrand G. Cyclic 3′,5′-nucleotide phosphodiesterases: potential targets for anticancer therapy. Chem Res Toxicol. 2000;13:944–8. doi: 10.1021/tx000090l. [DOI] [PubMed] [Google Scholar]
- 5.Houslay MD, Adams DR. PDE4 cAMP phosphodiesterases: modular enzymes that orchestrate signalling cross-talk, desensitization and compartmentalization. Biochem J. 2003;370:1–18. doi: 10.1042/BJ20021698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lynch MJ, Hill EV, Houslay MD. Intracellular targeting of phosphodiesterase-4 underpins compartmentalized cAMP signaling. Curr Top Dev Biol. 2006;75:225–59. doi: 10.1016/S0070-2153(06)75007-4. [DOI] [PubMed] [Google Scholar]
- 7.Lynch MJ, Baillie GS, Mohamed A, et al. RNA silencing identifies PDE4D5 as the functionally relevant cAMP phosphodiesterase interacting with beta arrestin to control the protein kinase A/AKAP79-mediated switching of the beta2-adrenergic receptor to activation of ERK in HEK293B2 cells. J Biol Chem. 2005;280:33178–89. doi: 10.1074/jbc.M414316200. [DOI] [PubMed] [Google Scholar]
- 8.McCahill A, McSorley T, Huston E, et al. In resting COS1 cells a dominant negative approach shows that specific, anchored PDE4 cAMP phosphodiesterase isoforms gate the activation, by basal cyclic AMP production, of AKAP-tethered protein kinase A type II located in the centrosomal region. Cell Signal. 2005;17:1158–73. doi: 10.1016/j.cellsig.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Jin SL, Lan L, Zoudilova M, Conti M. Specific role of phosphodiesterase 4B in lipopolysaccharide-induced signaling in mouse macrophages. J Immunol. 2005;175:1523–31. doi: 10.4049/jimmunol.175.3.1523. [DOI] [PubMed] [Google Scholar]
- 10.Drees M, Zimmermann R, Eisenbrand G. 3′,5′-Cyclic nucleotide phosphodiesterase in tumor cells as potential target for tumor growth inhibition. Cancer Res. 1993;53:3058–61. [PubMed] [Google Scholar]
- 11.Merz KH, Marko D, Regiert T, Reiss G, Frank W, Eisenbrand G. Synthesis of 7- benzylamino-6-chloro-2-piperazino-4-pyrrolidinopteridine and novel derivatives free of positional isomers. Potent inhibitors of cAMP-specific phosphodiesterase and of malignant tumor cell growth. J Med Chem. 1998;41:4733–43. doi: 10.1021/jm981021v. [DOI] [PubMed] [Google Scholar]
- 12.McEwan DG, Brunton VG, Baillie GS, Leslie NR, Houslay MD, Frame MC. Chemoresistant KM12C colon cancer cells are addicted to low cyclic AMP levels in a phosphodiesterase 4-regulated compartment via effects on phosphoinositide 3-kinase. Cancer Res. 2007;67:5248–57. doi: 10.1158/0008-5472.CAN-07-0097. [DOI] [PubMed] [Google Scholar]
- 13.Chen TC, Wadsten P, Su S, et al. The type IV phosphodiesterase inhibitor rolipram induces expression of the cell cycle inhibitors p21(Cip1) and p27(Kip1), resulting in growth inhibition, increased differentiation, and subsequent apoptosis of malignant A-172 glioma cells. Cancer Biol Ther. 2002;1:268–76. doi: 10.4161/cbt.80. [DOI] [PubMed] [Google Scholar]
- 14.Sullivan M, Rena G, Begg F, Gordon L, Olsen AS, Houslay MD. Identification and characterization of the human homologue of the short PDE4A cAMP-specific phosphodiesterase RD1 (PDE4A1) by analysis of the human HSPDE4A gene locus located at chromosome 19p13.2. Biochem J. 1998;333:693–703. doi: 10.1042/bj3330693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shakur Y, Pryde JG, Houslay MD. Engineered deletion of the unique N-terminal domain of the cyclic AMP-specific phosphodiesterase RD1 prevents plasma membrane association and the attainment of enhanced thermostability without altering its sensitivity to inhibition by rolipram. Biochem J. 1993;292:677–86. doi: 10.1042/bj2920677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scotland G, Houslay MD. Chimeric constructs show that the unique N-terminal domain of the cyclic AMP phosphodiesterase RD1 (RNPDE4A1A; rPDE-IVA1) can confer membrane association upon the normally cytosolic protein chloramphenicol acetyltransferase. Biochem J. 1995;308:673–81. doi: 10.1042/bj3080673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baillie GS, Huston E, Scotland G, et al. TAPAS-1, a novel microdomain within the unique N-terminal region of the PDE4A1 cAMP-specific phosphodiesterase that allows rapid, Ca2+-triggered membrane association with selectivity for interaction with phosphatidic acid. J Biol Chem. 2002;277:28298–309. doi: 10.1074/jbc.M108353200. [DOI] [PubMed] [Google Scholar]
- 18.Huston E, Houslay TM, Baillie GS, Houslay MD. cAMP phosphodiesterase-4A1 (PDE4A1) has provided the paradigm for the intracellular targeting of phosphodiesterases, a process that underpins compartmentalized cAMP signalling. Biochem Soc Trans. 2006;34:504–9. doi: 10.1042/BST0340504. [DOI] [PubMed] [Google Scholar]
- 19.Rubin JB, Kung AL, Klein RS, et al. A small-molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. Proc Natl Acad Sci U S A. 2003;100:13513–8. doi: 10.1073/pnas.2235846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith MC, Luker KE, Garbow JR, et al. CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res. 2004;64:8604–12. doi: 10.1158/0008-5472.CAN-04-1844. [DOI] [PubMed] [Google Scholar]
- 21.Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295:868–72. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- 22.Gross S, Piwnica-Worms D. Real-time imaging of ligand-induced IKK activation in intact cells and in living mice. Nat Methods. 2005;2:607–14. doi: 10.1038/nmeth779. [DOI] [PubMed] [Google Scholar]
- 23.Kiehl EL, Stojadinovic S, Malinowski KT, et al. Conformal murine CNS radiotherapy using microRT. 15th International Conference on the use of Computers in Radiation Therapy; 2007 June 4 -7, 2007; Toronto, ON. 2007. [Google Scholar]
- 24.Woerner BM, Warrington NM, Kung AL, Perry A, Rubin JB. Widespread CXCR4 activation in astrocytomas revealed by phospho-CXCR4-specific antibodies. Cancer Res. 2005;65:11392–9. doi: 10.1158/0008-5472.CAN-05-0847. [DOI] [PubMed] [Google Scholar]
- 25.Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- 26.McPhee I, Cochran S, Houslay MD. The novel long PDE4A10 cyclic AMP phosphodiesterase shows a pattern of expression within brain that is distinct from the long PDE4A5 and short PDE4A1 isoforms. Cell Signal. 2001;13:911–8. doi: 10.1016/s0898-6568(01)00217-0. [DOI] [PubMed] [Google Scholar]
- 27.McPhee I, Pooley L, Lobban M, Bolger G, Houslay MD. Identification, characterization and regional distribution in brain of RPDE-6 (RNPDE4A5), a novel splice variant of the PDE4A cyclic AMP phosphodiesterase family. Biochem J. 1995;310:965–74. doi: 10.1042/bj3100965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kleihues P, Burger PC, Collins VP, Newcomb EW, Ohgaki H, Cavenee WK. Glioblastoma. In: Kleihues PCWK, editor. World Health organization Classification of Tumours: Pathology and genetics of tumours of the central nervous system. Lyon: IARC Press; 2000. [Google Scholar]
- 29.Ishii N, Maier D, Merlo A, et al. Frequent co-alterations of TP53, p16/CDKN2A, p14ARF, PTEN tumor suppressor genes in human glioma cell lines. Brain Pathol. 1999;9:469–79. doi: 10.1111/j.1750-3639.1999.tb00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su X, Gopalakrishnan V, Stearns D, et al. Abnormal expression of REST/NRSF and Myc in neural stem/progenitor cells causes cerebellar tumors by blocking neuronal differentiation. Mol Cell Biol. 2006;26:1666–78. doi: 10.1128/MCB.26.5.1666-1678.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.U HS, Banaie A, Rigby L, Chen J. Mutant p53 may selectively suppress glial specific proteins in pluripotential human neuroectodermal tumor cells. Neurosci Lett. 1998;244:41–6. doi: 10.1016/s0304-3940(98)00061-5. [DOI] [PubMed] [Google Scholar]
- 32.Furman MA, Shulman K. Cyclic AMP and adenyl cyclase in brain tumors. J Neurosurg. 1977;46:477–83. doi: 10.3171/jns.1977.46.4.0477. [DOI] [PubMed] [Google Scholar]
- 33.Racagni G, Pezzotta S, Giordana MT, et al. Cyclic nucleotides in experimental and human brain tumors. J Neurooncol. 1983;1:61–7. doi: 10.1007/BF00153643. [DOI] [PubMed] [Google Scholar]
- 34.Warrington NM, Woerner BM, Daginakatte GC, et al. Spatiotemporal differences in CXCL12 expression and cyclic AMP underlie the unique pattern of optic glioma growth in neurofibromatosis type 1. Cancer Res. 2007;67:8588–95. doi: 10.1158/0008-5472.CAN-06-2220. [DOI] [PubMed] [Google Scholar]
- 35.Huston E, Gall I, Houslay TM, Houslay MD. Helix-1 of the cAMP-specific phosphodiesterase PDE4A1 regulates its phospholipase-D-dependent redistribution in response to release of Ca2+ J Cell Sci. 2006;119:3799–810. doi: 10.1242/jcs.03106. [DOI] [PubMed] [Google Scholar]
- 36.Anderson JA, Woodcock TM, Harty JI, Knott AW, Edwards MJ. The effects of oral pentoxifylline on interleukin-2 toxicity in patients with metastatic renal cell carcinoma. Eur J Cancer. 1995;31A:714–7. doi: 10.1016/0959-8049(94)00507-2. [DOI] [PubMed] [Google Scholar]
- 37.Stewart DJ, Dahrouge S, Agboola O, Girard A. Cranial radiation and concomitant cisplatin and mitomycin-C plus resistance modulators for malignant gliomas. J Neurooncol. 1997;32:161–8. doi: 10.1023/a:1005788121043. [DOI] [PubMed] [Google Scholar]
- 38.Zeller E, Stief HJ, Pflug B, Sastre-y-Hernandez M. Results of a phase II study of the antidepressant effect of rolipram. Pharmacopsychiatry. 1984;17:188–90. doi: 10.1055/s-2007-1017435. [DOI] [PubMed] [Google Scholar]
- 39.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 40.Chakravarti A, Erkkinen MG, Nestler U, et al. Temozolomide-mediated radiation enhancement in glioblastoma: a report on underlying mechanisms. Clin Cancer Res. 2006;12:4738–46. doi: 10.1158/1078-0432.CCR-06-0596. [DOI] [PubMed] [Google Scholar]
- 41.Nishiyama S, Okudaira M, Saito N. Mechanisms of rolipram-induced increase in the incidence of mammary adenocarcinoma: histopathological study of a 104- week oral carcinogenicity study in female Sprague-Dawley rats. Arch Toxicol. 2006;80:88–97. doi: 10.1007/s00204-005-0016-6. [DOI] [PubMed] [Google Scholar]
- 42.Houslay MD, Schafer P, Zhang KY. Keynote review: phosphodiesterase-4 as a therapeutic target. Drug Discov Today. 2005;10:1503–19. doi: 10.1016/S1359-6446(05)03622-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.