Abstract
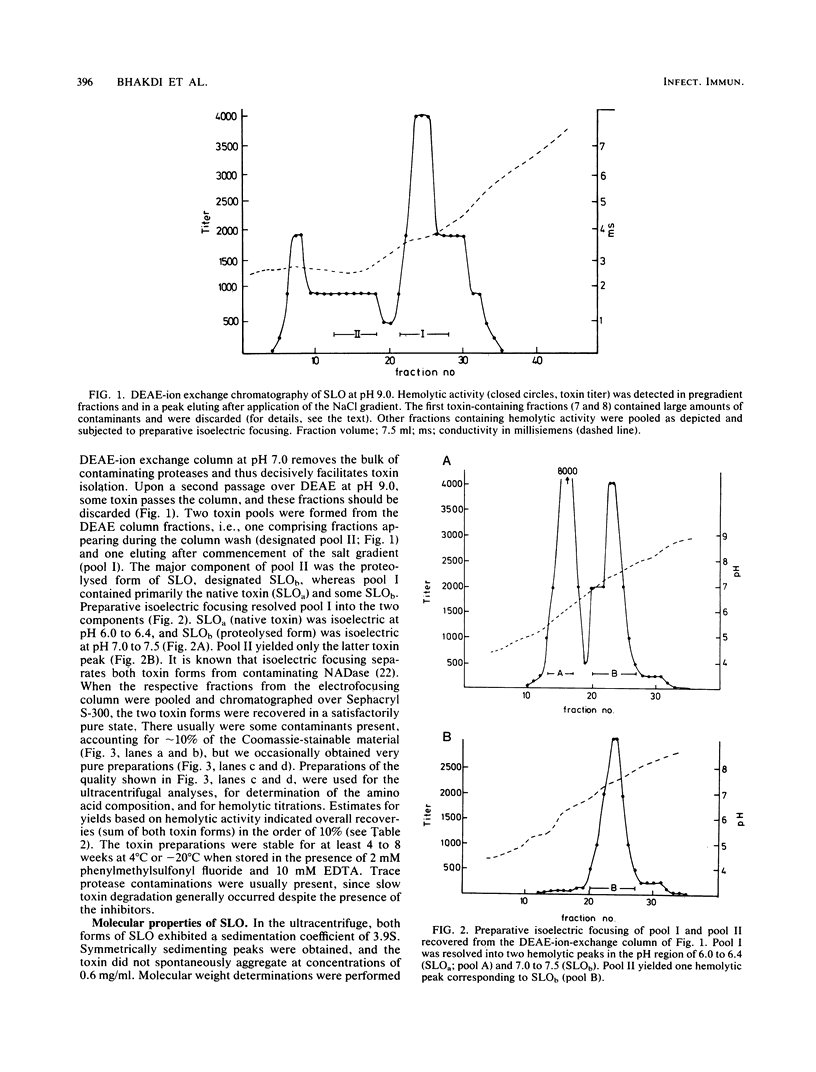
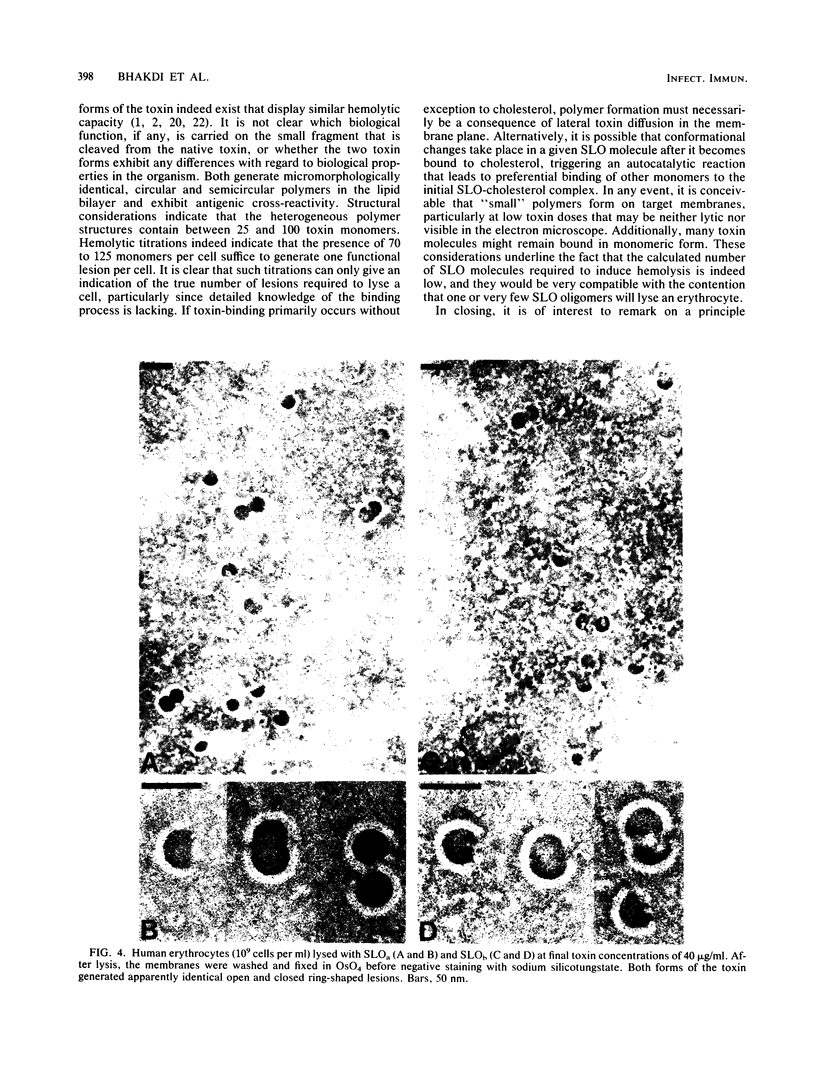
Streptolysin-O was isolated from culture supernatants of group-A beta-hemolytic streptococci (Richards strain) by ammonium sulfate and polyethylene glycol precipitation, DEAE-ion exchange chromatography, preparative isoelectric focusing, and chromatography on Sephacryl S-300. Two forms of the toxin possessing similar hemolytic capacity were identified. The native toxin was a single polypeptide chain devoid of amino sugars with a sedimentation coefficient of 3.9S and a molecular weight of 69,000, and was isoelectric at pH 6.0 to 6.4. Partial degradation of the native toxin occurred during the isolation procedure, yielding a hemolytically active polypeptide with a molecular weight of 57,000 and a pI of 7.0 to 7.5. Both forms of the toxin generated the typical, heterogeneous, open and closed ring-structured channels in erythrocyte membranes. Structural considerations indicated that between 25 and 100 monomer toxin molecules constituted the individual ultrastructurally recognizable channels. Hemolytic titrations indicated that the presence of 70 to 125 toxin molecules per erythrocyte was required to generate an average of one functional lesion per cell. The data are consistent with the concept that one or very few streptolysin-O channels will cause hemolysis.
Full text
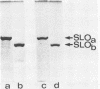
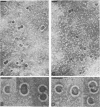
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alouf J. E., Raynaud M. Purification and some properties of streptolysin O. Biochimie. 1973;55(10):1187–1193. doi: 10.1016/s0300-9084(74)80322-6. [DOI] [PubMed] [Google Scholar]
- Alouf J. E. Streptococcal toxins (streptolysin O, streptolysin S, erythrogenic toxin). Pharmacol Ther. 1980;11(3):661–717. doi: 10.1016/0163-7258(80)90045-5. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Muhly M., Füssle R. Correlation between toxin binding and hemolytic activity in membrane damage by staphylococcal alpha-toxin. Infect Immun. 1984 Nov;46(2):318–323. doi: 10.1128/iai.46.2.318-323.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham L., Duncan J. L. Approximate dimensions of membrane lesions produced by streptolysin S and streptolysin O. Biochim Biophys Acta. 1983 Mar 23;729(1):115–122. doi: 10.1016/0005-2736(83)90462-5. [DOI] [PubMed] [Google Scholar]
- Calandra G. B., Theodore T. S. Cellular location of streptolysin O. Infect Immun. 1975 Oct;12(4):750–753. doi: 10.1128/iai.12.4.750-753.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dourmashkin R. R., Rosse W. F. Morphologic changes in the membranes of red blood cells undergoing hemolysis. Am J Med. 1966 Nov;41(5):699–710. doi: 10.1016/0002-9343(66)90031-3. [DOI] [PubMed] [Google Scholar]
- Duncan J. L. Characteristics of streptolysin O hemolysis: kinetics of hemoglobin and 86rubidium release. Infect Immun. 1974 Jun;9(6):1022–1027. doi: 10.1128/iai.9.6.1022-1027.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. L., Schlegel R. Effect of streptolysin O on erythrocyte membranes, liposomes, and lipid dispersions. A protein-cholesterol interaction. J Cell Biol. 1975 Oct;67(1):160–174. doi: 10.1083/jcb.67.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN H., BARROW P., GOLDBERG B. Effect of antibody and complement on permeability control in ascites tumor cells and erythrocytes. J Exp Med. 1959 Nov 1;110:699–713. doi: 10.1084/jem.110.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harboe N., Ingild A. Immunization, isolation of immunoglobulins, estimation of antibody titre. Scand J Immunol Suppl. 1973;1:161–164. doi: 10.1111/j.1365-3083.1973.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Kehoe M., Timmis K. N. Cloning and expression in Escherichia coli of the streptolysin O determinant from Streptococcus pyogenes: characterization of the cloned streptolysin O determinant and demonstration of the absence of substantial homology with determinants of other thiol-activated toxins. Infect Immun. 1984 Mar;43(3):804–810. doi: 10.1128/iai.43.3.804-810.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz T., Schmidt K. H. Improved methodology for the analysis of mixtures by band centrifugation. Quantitative determination of components in protein mixtures. J Biol Stand. 1981 Jan;9(1):51–65. doi: 10.1016/s0092-1157(81)80065-0. [DOI] [PubMed] [Google Scholar]
- SEARS D. A., WEED R. I., SWISHER S. N. DIFFERENCES IN THE MECHANISM OF IN VITRO IMMUNE HEMOLYSIS RELATED TO ANTIBODY SPECIFICITY. J Clin Invest. 1964 May;43:975–985. doi: 10.1172/JCI104983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shany S., Grushoff P. S., Bernheimer A. W. Physical separation of streptococcal nicotinamide adenine dinucleotide glycohydrolase from streptolysin O. Infect Immun. 1973 May;7(5):731–734. doi: 10.1128/iai.7.5.731-734.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth C. J., Fehrenbach F. J. Isoelectric analysis of haemolysins and enzymes from streptococci of groups A, C and G. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Dec;82(6):860–870. doi: 10.1111/j.1699-0463.1974.tb02384.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranum-Jensen J., Bhakdi S. Freeze-fracture analysis of the membrane lesion of human complement. J Cell Biol. 1983 Sep;97(3):618–626. doi: 10.1083/jcb.97.3.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VINOGRAD J., BRUNER R., KENT R., WEIGLE J. Band-centrifugation of macromolecules and viruses in self-generating density gradients. Proc Natl Acad Sci U S A. 1963 Jun;49:902–910. doi: 10.1073/pnas.49.6.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Epps D. E., Andersen B. R. Isolation of streptolysin O by preparative polyacrylamide gel electrophoresis. Infect Immun. 1973 Mar;7(3):493–495. doi: 10.1128/iai.7.3.493-495.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Epps D. E., Andersen B. R. Streptolysin O: sedimentation coefficient and molecular weight determinations. J Bacteriol. 1969 Oct;100(1):526–527. doi: 10.1128/jb.100.1.526-527.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]