Abstract
Background
Observational studies suggested that a diet high in fruits and vegetables, both of which are rich with antioxidants, may prevent cancer development. However, findings from randomized trials of the association between antioxidant use and cancer risk have been mostly negative.
Methods
From 8171 women who were randomly assigned in the Women's Antioxidant Cardiovascular Study, a double-blind, placebo-controlled 2 × 2 × 2 factorial trial of vitamin C (500 mg of ascorbic acid daily), natural-source vitamin E (600 IU of α-tocopherol every other day), and beta carotene (50 mg every other day), 7627 women who were free of cancer before random assignment were selected for this study. Diagnoses and deaths from cancer at a specific site were confirmed by use of hospital reports and the National Death Index. Cox proportional hazards regression models were used to assess hazard ratios (represented as relative risks [RRs]) of common cancers associated with use of antioxidants, either individually or in combination. Subgroup analyses were conducted to determine if duration of use modified the association of supplement use with cancer risk. All statistical tests were two-sided.
Results
During an average 9.4 years of treatment, 624 women developed incident invasive cancer and 176 women died from cancer. There were no statistically significant effects of use of any antioxidant on total cancer incidence. Compared with the placebo group, the RRs were 1.11 (95% confidence interval [CI] = 0.95 to 1.30) in the vitamin C group, 0.93 (95% CI = 0.79 to 1.09) in the vitamin E group, and 1.00 (95% CI = 0.85 to 1.17) in the beta carotene group. Similarly, no effects of these antioxidants were observed on cancer mortality. Compared with the placebo group, the RRs were 1.28 (95% CI = 0.95 to 1.73) in the vitamin C group, 0.87 (95% CI = 0.65 to 1.17) in the vitamin E group, and 0.84 (95% CI = 0.62 to 1.13) in the beta carotene group. Duration and combined use of the three antioxidants also had no effect on cancer incidence and cancer death.
Conclusions
Supplementation with vitamin C, vitamin E, or beta carotene offers no overall benefits in the primary prevention of total cancer incidence or cancer mortality.
CONTEXT AND CAVEATS
Prior knowledge
Although some observational studies suggest that a diet high in fruits and vegetables and thus antioxidants may be associated with a reduced risk of cancer, randomized trials have not supported this association.
Study design
Randomized controlled trial using a factorial design with hazard ratios from Cox regression models to compare intervention effects.
Contribution
This study, which was designed to have 80% power to detect a 30% reduction in the overall risk of cancer, suggests that long-term dietary supplementation with any combination of the antioxidants vitamin C, vitamin E, or beta carotene does not reduce the risk of cancer or the risk of dying from cancer.
Implications
There is no basis for a recommendation that individuals increase dietary levels of antioxidants as a means of reducing cancer risk.
Limitations
This study had very limited statistical power to investigate any effect of dietary antioxidants on the risk of specific cancers.
From the Editors
A large number of epidemiologic studies have suggested that a diet high in fruits, vegetables, and other foods derived from plants is associated with a lower risk of cancer (1–8). Antioxidant nutrients such as ascorbic acid, α-tocopherol, and the carotenoids (eg, beta carotene) that are present in plant-derived foods may have a major preventive role against carcinogenesis (9), and it has been suggested that they may suppress tumor cell growth and induce tumor cell apoptosis (10–12). Antioxidants also counterbalance the production of reactive oxygen species (ROS), which may cause oxidative damage to cells and modify cell growth regulatory pathways, leading to enhanced risk for carcinogenesis (13–15).
Over the past three decades, numerous clinical trials (16–30) have evaluated the efficacy of antioxidants and minerals, used singly or in various combinations, in the prevention of cancers and other chronic diseases. However, their findings have been inconsistent, and results of most of them (16–27) did not support a benefit of vitamin or mineral supplements in primary cancer prevention. Several recent meta-analyses also showed no reduction in risk for cancer development with antioxidant vitamins or beta carotene, with the exception of a possible reduction in cancer mortality with selenium supplementation (31–35). The negative findings may be attributable to the short exposure to interventions, with most trials lasting only 4–6 years (36): because malignant cancer cells can take decades to develop, many trials may have been too short to demonstrate any benefit (36). Another possible explanation is that most trials have evaluated the effects of high doses of only one or two antioxidants (36–39), although experimental data have suggested that antioxidants act not only individually but also cooperatively to achieve optimal effects on cancer prevention. Moreover, heterogeneity of the study populations may have contributed to the divergence of the findings, with the possibility that antioxidants could be beneficial among populations more likely to experience oxidative stress (40).
In this randomized factorial trial of an average 9 years of supplementation and follow-up among women at high risk of cardiovascular disease, we systematically evaluated the individual and combined effects of three antioxidant supplements, ascorbic acid, vitamin E, and beta carotene, in the primary prevention of cancer incidence and mortality.
Subjects and Methods
Study Design and Population
The Women's Antioxidant Cardiovascular Study (WACS) (ClinicalTrials.gov identifier NCT00000541) is a randomized, double-blind, placebo-controlled, 2 × 2 × 2 factorial trial evaluating the benefits and risks of synthetic vitamin C (500 mg of ascorbic acid daily), natural-source vitamin E (600 IU of α-tocopherol every other day), and beta carotene (50 mg of Lurotin every other day) among women at high risk for cardiovascular disease (CVD). Vitamin C and beta carotene were supplied by BASF Corp (Wyandotte, MI) and vitamin E was supplied by (Cognis Corp, LaGrange, Il). Approximately 2–3 years after random assignment to the antioxidant arms, a folic acid–vitamins B6 and B12 component was added to the trial, yielding a four-arm factorial trial. The WACS trial was approved by the Institutional Review Board of the Brigham and Women's Hospital (Boston, MA). The trial's independent Data and Safety Monitoring Board met annually to review the results. Written informed consent was obtained from all participants.
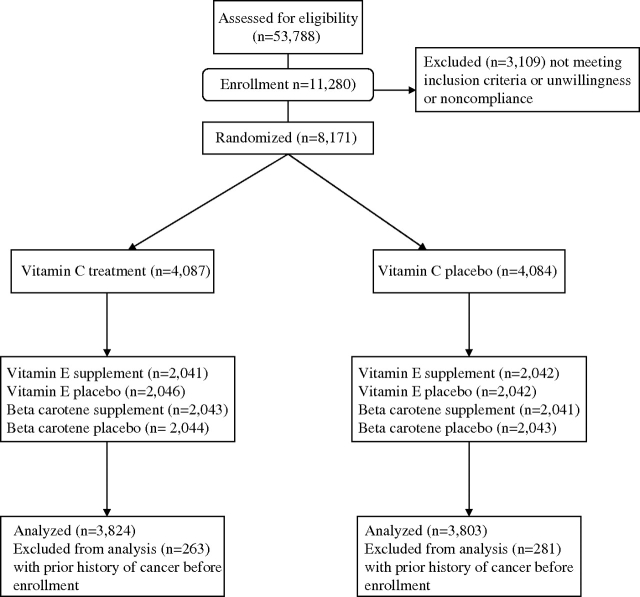
The design of the trial has been described in detail (41,42). Briefly, between September 1992 and May 1995, 53 788 women from a pool of more than 450 000 respondents to an invitation packet of the Women's Health Study (25,43,44) were considered likely to be eligible for the study and were sent the WACS invitational mailing. Women were considered to be eligible to participate in the WACS if they were at least 40 years of age; were postmenopausal or not intending to become pregnant; and had known CVD or at least three of the following cardiac risk factors: hypertension, high cholesterol level, diabetes, parental history of myocardial infarction, or obesity (ie, body mass index ≥30 kg/m2). Women were excluded if they had a self-reported history of cancer (except nonmelanoma skin cancer) within the past 10 years, had active liver disease or cirrhosis, had chronic kidney failure, were current users of anticoagulants, or were unwilling to avoid out-of-study use of vitamins A, C, and E and beta carotene at intakes exceeding the recommended daily allowance during the trial.
Between June 1995 and October 1996, a total of 8171 women were randomly assigned according to a 2 × 2 × 2 factorial design. Results for the trial's primary endpoints of major cardiovascular events have been reported previously (42). We excluded women from this analysis who reported having had a diagnosis of cancer (n = 544) more than 10 years ago because the etiologic factors for primary and recurrent cancer incidence are likely different (45–47). As a result, 7627 women (93.3%) free of cancer at enrollment were included in this analysis for the primary prevention of cancer incidence and mortality (Figure 1). We had more than 90% power, with a two-sided test (α = .05), to detect the individual effect of vitamins C and E and beta carotene with a 30% reduction in the risk for total cancer incidence. Power to detect the individual effect of vitamins C and E and beta carotene with a 40% reduction in the risk for cancer death was more than 80%.
Figure 1.
Flow diagram of participants in the Women's Antioxidant Cardiovascular Study.
Study Treatment and Follow-up
After random assignment, participants received a supply of monthly calendar packs containing antioxidant supplements or placebo as well as follow-up questionnaires on compliance, potential adverse effects, occurrence of endpoints, and risk factors that were sent every 6 months for the first year and annually thereafter. Study treatment and endpoint ascertainment were continued in a blinded fashion through January 31, 2005, the scheduled end of the trial. In July 2006, follow-up and validation of reported endpoints were complete and morbidity and mortality follow-up were completed for 93% and more than 99% of subjects, respectively (42).
Compliance for use of the three antioxidants has been reported elsewhere (42). Compliance, defined as having taken at least two thirds of the study capsules, was 76% at 4 years and 68% at 8 years for each antioxidant. The average compliance throughout the trial was 73%. Outside use of the three antioxidant supplements for at least 4 d/mo was ranged from 2% to 13% at both 4 and 8 years and was not different between supplement and placebo groups. No statistically significant difference in side effects between the supplement and placebo groups was observed (42).
Endpoints
Women reported the occurrence of cancer through follow-up questionnaires, letters, or telephone calls. Deaths were reported by family members or postal authorities or through the National Death Index. For women who reported a diagnosis of cancer and for those who had died, we sought permission from participants or the next of kin if deceased to acquire medical records and pathology reports, which were then reviewed by the WACS Endpoints Committee of physicians blinded to the treatment assignment. Reports of cancer were confirmed with pathology or cytology reports (94%) or, when a pathology or cytology review was not conducted, cancer was confirmed by clinical and radiological or laboratory marker evidence (eg, elevated CA 125). The primary cancer outcome was any invasive cancer, excluding nonmelanoma skin cancer. Only confirmed cancer outcomes were included in the analysis.
Statistical Analysis
Analyses were conducted for the primary endpoint of total invasive as well as site-specific cancers. If a woman developed more than one primary cancer (excluding nonmelanoma skin cancer), the first following random assignment was used in these analyses.
We used Cox proportional hazards regression models to calculate hazard ratios (that were presented as relative risks [RRs]) and 95% confidence intervals (CIs). Models included main effect terms of the three antioxidants and age. Tests of proportionality of the relative risks over time were performed with an interaction term of treatment with the logarithm of time in the Cox models. The proportionality assumption was not violated for total invasive cancers (P ≥ .11 for all three antioxidants) or cancer death (P ≥ .26).
We additionally performed analyses of the multiplicative interactions among the three antioxidants with cancer incidence and mortality. We first tested interactions between pairs of the three antioxidants in the models with adjustment for age and main effect terms of the three antioxidants. We also tested interactions among all three antioxidants in the model with adjustment for age, main effect terms of the three antioxidants, and interaction terms between any pairs of the three antioxidants. To evaluate whether duration of supplement use may affect the risk of cancer development, we also conducted subgroup analysis according to duration of supplementation (1–5 and >5 years after random assignment). Moreover, we assessed the effect modification by variables that may induce oxidative stress, including smoking status (never, past, and current), alcohol consumption (<15 g/d or <1 drink/d vs ≥15 g/d or ≥1 drink/d), and body mass index (BMI; <25 or ≥25 kg/m2) (48–50), by use of multiplicative interaction terms between subgroup indicators and supplement assignment and testing for trends for ordinal subgroup categories.
To investigate the effect of compliance, we carried out a sensitivity analysis that censored women at the time when they stopped taking at least two thirds of their study medications, reported taking outside supplements containing study agents, or had missing compliance information. All statistical analyses were conducted by SAS version 9 (SAS Institute, Cary, NC), with a two-sided test with a threshold of .05 for statistical significance.
Results
Baseline characteristics were similar between the supplement and placebo groups (Table 1). The mean age (±SD) of all women (N = 7627) was 60.4 ± 8.8 years. Most of the participants were postmenopausal (77% or 5909 women) and overweight or obese (77% or 5848 women), and few (27% or 2067 women) reported having taken multivitamins at baseline. The average baseline intakes of antioxidants, assessed by a semiquantitative food frequency questionnaire in all women, including those of vitamins A, C, and E, were greater than the amounts recommended by the recommended daily allowance, according to the 2005 Dietary Guidelines (51). However, no statistically significant difference in baseline intakes of these antioxidants was observed between the supplement and placebo groups. The distribution of risk factors for cancers assessed at baseline including BMI, physical activity, current smoking, hormone therapy use, use of screening tests, and family history of cancer was similar in participants receiving supplements or placebo (Table 1). Approximately half of the participants in the supplement and placebo groups reported having used a nonsteroidal anti-inflammatory drug or aspirin during the past month (data not shown). The average duration of follow-up from random assignment to the end of the trial was 9.4 years (range = 8.3–10.1 years).
Table 1.
Baseline characteristics of women free of cancer according to the assignment groups in the Women's Antioxidant Cardiovascular Study*
| Vitamin C |
Vitamin E |
Beta carotene |
||||
| Characteristic | Active | Placebo | Active | Placebo | Active | Placebo |
| No. of participants | 3824 | 3803 | 3791 | 3836 | 3807 | 3820 |
| Mean age, y (SD) | 60.4 (9) | 60.4 (9) | 60.4 (9) | 60.4 (9) | 60.4 (9) | 60.4 (9) |
| Race, % | ||||||
| Caucasian | 94.0 | 93.9 | 93.9 | 93.9 | 93.4 | 94.4 |
| African American | 3.3 | 3.3 | 3.3 | 3.4 | 3.7 | 3.0 |
| Other or unknown | 2.7 | 2.8 | 2.8 | 2.7 | 2.9 | 2.6 |
| Smoking status, % | ||||||
| Never | 43.5 | 43.0 | 43.0 | 43.4 | 43.6 | 42.9 |
| Current | 14.8 | 15.8 | 15.4 | 15.3 | 14.9 | 15.7 |
| Past | 41.7 | 41.2 | 41.6 | 41.3 | 41.5 | 41.4 |
| Mean BMI, kg/m2 (SD) | 30.3 (7) | 30.4 (7) | 30.3 (7) | 30.4 (7) | 30.3 (7) | 30.4 (7) |
| Mean physical activity, kcal/wk (SD) | 846 (1167) | 866 (1167) | 845 (1153) | 867 (1208) | 848 (1214) | 864 (1147) |
| History of diabetes, % | 19.1 | 19.0 | 18.8 | 19.3 | 19.1 | 19.0 |
| Family history of cancer, % | 29.4 | 27.6 | 28.1 | 29.0 | 27.5 | 29.6 |
| Postmenopause, % | 77.6 | 77.4 | 77.2 | 77.8 | 77.8 | 77.2 |
| Hormone therapy use, % | ||||||
| Never | 33.2 | 34.5 | 33.0 | 34.6 | 34.3 | 33.4 |
| Past use | 15.3 | 14.9 | 15.1 | 15.1 | 14.9 | 15.2 |
| Current E + P use | 13.7 | 14.1 | 14.1 | 13.7 | 13.9 | 13.8 |
| Current E-alone use | 34.3 | 32.8 | 34.2 | 32.9 | 33.2 | 33.9 |
| No. of mammogram screening, % | ||||||
| 0 | 2.1 | 2.6 | 2.5 | 2.2 | 2.2 | 2.5 |
| 1–2 | 30.3 | 30.1 | 29.5 | 30.9 | 30.7 | 29.7 |
| 3–4 | 34.1 | 33.1 | 33.5 | 33.7 | 32.8 | 34.4 |
| ≥5 | 33.5 | 34.2 | 34.5 | 33.2 | 34.3 | 33.4 |
| Colonoscopy/sigmoidoscopy during the past year, % | 11.1 | 12.6 | 11.6 | 12.1 | 11.7 | 12.0 |
| Current multivitamin use, % | 26.7 | 27.9 | 27.4 | 27.1 | 27.0 | 27.5 |
| Mean dietary intake/d (SD) | ||||||
| Total alcohol intake†, g | 3.3 (8) | 3.4 (8) | 3.3 (8) | 3.3 (8) | 3.4 (8) | 3.3 (8) |
| Total energy intake†, kcal | 1733 (560) | 1717 (543) | 1727 (551) | 1724 (552) | 1728 (552) | 1723 (550) |
| Total fat intake†, g | 58.3 (24) | 57.8 (23) | 58.1 (23) | 58.0 (24) | 58.1 (23) | 58.0 (23) |
| Total vitamin C intake†, mg | 232 (227) | 233 (230) | 238 (241) | 227 (215) | 232 (221) | 233 (235) |
| Total vitamin E intake†, IU | 124 (196) | 129 (203) | 127 (202) | 125 (196) | 129 (201) | 123 (197) |
| Total carotene intake†, IU | 10 762 (8542) | 10 579 (8559) | 10 754 (8520) | 10 591 (8580) | 10 675 (8357) | 10 667 (8738) |
| Total vitamin A intake†, IU | 13 936 (9665) | 13 711 (9578) | 13 917 (9580) | 13 733 (9663) | 13 821 (9447) | 13 826 (9793) |
E + P = estrogen plus progestin; E-alone = estrogen alone.
Included 7209 women who provided sufficient dietary information and had plausible total energy intake (600–3500 kcal/d).
Primary Cancer Incidence and Cancer Mortality
During the trial period, 624 women had a confirmed diagnosis of invasive cancer, excluding nonmelanoma skin cancer. The major sites of cancer were the breast (n = 257), lung (n = 74), and colorectum (n = 44, 31 colon and 13 rectum). The total number of cancer deaths was 176. There were no statistically significant effects of use of any antioxidant on total cancer incidence (Table 2). Compared with the placebo group, the RRs were 1.11 (95% CI = 0.95 to 1.30) in the vitamin C supplement group, 0.93 (95% CI = 0.79 to 1.09) in the vitamin E group, and 1.00 (95% CI = 0.85 to 1.17) in the beta carotene group. Similarly, no effects of these antioxidants were observed on cancer mortality (Table 2). Compared with the placebo group, the RRs were 1.28 (95% CI = 0.95 to 1.73) in the vitamin C group, 0.87 (95% CI = 0.65 to 1.17) in the vitamin E group, and 0.84 (95% CI = 0.62 to 1.13) in the beta carotene group.
Table 2.
Total or site-specific cancer incidence and cancer mortality in the Women's Antioxidant Cardiovascular Study*
| Vitamin C |
Vitamin E |
Beta carotene |
|||||||
| No. of case subjects |
No. of case subjects |
No. of case subjects |
|||||||
| Incidence or mortality | Active | Placebo | RR (95% CI)† | Active | Placebo | RR (95% CI)† | Active | Placebo | RR (95% CI)† |
| Total cancer | 329 | 295 | 1.11 (0.95 to 1.30) | 300 | 324 | 0.93 (0.79 to 1.09) | 311 | 313 | 1.00 (0.85 to 1.17) |
| Breast | 135 | 122 | 1.11 (0.87 to 1.41) | 127 | 130 | 0.98 (0.77 to 1.25) | 129 | 128 | 1.01 (0.79 to 1.30) |
| Lung | 48 | 26 | 1.84 (1.14 to 2.97) | 41 | 33 | 1.25 (0.79 to 1.97) | 41 | 33 | 1.26 (0.80 to 1.99) |
| Colorectum | 19 | 25 | 0.76 (0.42 to 1.38) | 17 | 27 | 0.63 (0.34 to 1.15) | 25 | 19 | 1.32 (0.73 to 2.39) |
| Pancreas | 14 | 6 | 2.32 (0.89 to 6.04) | 10 | 10 | 1.00 (0.41 to 2.39) | 11 | 9 | 1.24 (0.51 to 2.99) |
| Uterine | 23 | 27 | 0.85 (0.49 to 1.49) | 20 | 30 | 0.67 (0.38 to 1.18) | 28 | 22 | 1.27 (0.73 to 2.23) |
| Ovary | 10 | 12 | 0.84 (0.36 to 1.93) | 8 | 14 | 0.58 (0.24 to 1.37) | 12 | 10 | 1.20 (0.52 to 2.78) |
| Non-Hodgkin lymphoma | 16 | 16 | 0.99 (0.50 to 1.99) | 14 | 18 | 0.77 (0.39 to 1.56) | 10 | 22 | 0.46 (0.22 to 0.97) |
| Other cancers‡ | 36 | 29 | 1.24 (0.76 to 2.02) | 33 | 32 | 1.03 (0.64 to 1.68) | 29 | 36 | 0.81 (0.50 to 1.33) |
| Cancer death | 99 | 77 | 1.28 (0.95 to 1.73) | 82 | 94 | 0.87 (0.65 to 1.17) | 80 | 96 | 0.84 (0.62 to 1.13) |
Hazard ratios are presented as relative risks (RRs) and 95% confidence intervals (CIs).
RR is the rate in the treatment group compared with the rate in the placebo group for each agent.
Included cancers of the thyroid, melanoma, leukemia, kidney, and bladder, with the number of cases ranging between 10 and 20.
When evaluating site-specific cancers, we found that the vitamin E supplement group had a reduced (albeit not to a statistically significant extent) risk for colorectal cancer development compared with the placebo group (RR = 0.63, 95% CI = 0.34 to 1.15, P = .13), due largely to a reduced risk of colon cancer (RR = 0.41, 95% CI = 0.10 to 0.89, P = .02). However, there was no statistically significant association of vitamin E supplementation with rectal or other cancers. Beta carotene supplementation was associated with a reduced risk of incident non-Hodgkin lymphoma (RR = 0.46, 95% CI = 0.22 to 0.97, P = .04). There was no evidence that beta carotene had statistically significant associations with the risk of other cancers. We also did not find evidence for an association of vitamin C supplementation with reduced risk of any cancers. The vitamin C supplement group had a higher incidence rate for lung cancer (RR = 1.84, 95% CI = 1.14 to 2.97, P = .01) compared with the placebo group. The associations of the three antioxidants with the risk of breast tumors in situ were also not statistically significant (data not shown).
There were no statistically significant interactions between pairs of the antioxidants or among all three antioxidants for total cancer incidence or death (see total years in Figure 2), and no statistically significant interactions were observed for specific cancers (data not shown). We conducted a sensitivity analysis by censoring women who did not meet our compliance criteria and found that the results were not appreciably changed for total cancer incidence or death (data not shown).
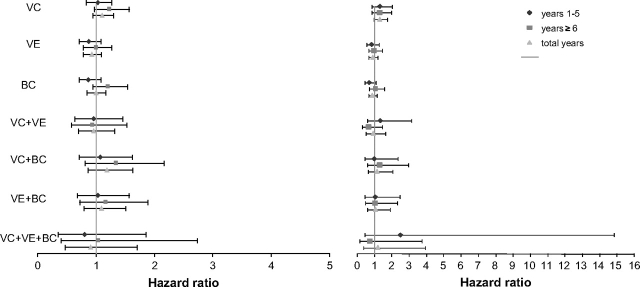
Figure 2.
Hazard ratios and 95% confidence intervals of total cancer incidence (left) and cancer mortality (right) according to duration of treatment in the Women's Antioxidant Cardiovascular Study. VC = vitamin C supplement; VE = vitamin E supplement; BC = β-carotene supplement. Hazard ratio is the rate in the treatment group compared with the rate in the placebo group for single and combined agents.
Duration of Antioxidant Supplementation
When separating the supplementation and follow-up time into two periods, the early (year 1 to year 5) and later (year 6 to year 10) periods of supplementation, we observed no statistically significant associations of the three antioxidants (taken singly or combined) with total cancer incidence or mortality for either period (Figure 2). However, there was a nonstatistically significant increase for either period in cancer mortality in the vitamin C supplement group (Figure 2).
Subgroup Analysis
We further evaluated whether the variables that may induce oxidative stress modified associations of the three antioxidants with total cancer incidence and cancer death. There was a modification by cigarette smoking on vitamin E supplementation and cancer mortality of borderline statistical significance (P value for interaction = .08). Women in the vitamin E supplement group who currently smoked or had smoked in the past had lower rates of cancer death (for current smokers, RR = 0.80, 95% CI = 0.45 to 1.41; and for past smokers, RR = 0.62, 95% CI = 0.39 to 0.99), in contrast to those who never smoked (RR = 1.50, 95% CI = 0.87 to 2.57). In addition, women of normal BMI (<25 kg/m2) in the vitamin C supplement group had marginally higher rates of cancer death (RR = 2.00, 95% CI = 1.12 to 3.58, P = .02) (Table 3). However, there was no statistically significant effect of vitamin C on the risk of cancer death among women with high BMI (≥25 kg/m2) (Table 3). The effects of the three antioxidants on cancer mortality were not modified by alcohol consumption (Table 3). The modification by cigarette smoking, alcohol consumption, and BMI was also not statistically significant for the effects of the three antioxidants on total cancer incidence (Table 3).
Table 3.
Total cancer incidence and cancer mortality according to randomization status in the Women's Antioxidant Cardiovascular Study*
| Vitamin C |
Vitamin E |
Beta carotene |
||||||||||
| No. of case subjects |
No. of case subjects |
No. of case subjects |
||||||||||
| Active | Placebo | RR (95% CI)† | Pinteraction‡ | Active | Placebo | RR (95% CI)† | Pinteraction‡ | Active | Placebo | RR (95% CI)† | Pinteraction‡ | |
| Total cancer | ||||||||||||
| Smoking | .68 | .46 | .80 | |||||||||
| Never | 125 | 117 | 1.08 (0.84 to 1.39) | 123 | 119 | 1.04 (0.81 to 1.34) | 122 | 120 | 1.00 (0.78 to 1.28) | |||
| Past | 147 | 127 | 1.13 (0.89 to 1.43) | 126 | 148 | 0.85 (0.67 to 1.07) | 135 | 139 | 0.99 (0.78 to 1.25) | |||
| Current | 57 | 51 | 1.19 (0.82 to 1.74) | 51 | 57 | 0.93 (0.64 to 1.36) | 54 | 54 | 1.08 (0.74 to 1.58) | |||
| Alcohol consumption | .65 | .66 | .42 | |||||||||
| Low (≤15 g/d or ≤1 drink per d) | 306 | 273 | 1.11 (0.94 to 1.30) | 277 | 302 | 0.92 (0.78 to 1.08) | 286 | 293 | 0.98 (0.83 to 1.16) | |||
| High (>15 g/d or >1 drink per d) | 23 | 22 | 1.28 (0.71 to 2.30) | 23 | 22 | 1.04 (0.58 to 1.88) | 25 | 20 | 1.25 (0.69 to 2.25) | |||
| Body mass index | .33 | .16 | .76 | |||||||||
| <25 mg/m2 | 85 | 66 | 1.29 (0.94 to 1.78) | 63 | 88 | 0.75 (0.54 to 1.04) | 81 | 70 | 1.05 (0.76 to 1.45) | |||
| ≥25 mg/m2 | 244 | 229 | 1.07 (0.89 to 1.28) | 237 | 236 | 0.98 (0.82 to 1.18) | 230 | 243 | 0.99 (0.83 to 1.19) | |||
| Cancer death | ||||||||||||
| Smoking | .25 | .08 | .75 | |||||||||
| Never | 28 | 27 | 1.06 (0.62 to 1.80) | 33 | 22 | 1.50 (0.87 to 2.57) | 25 | 30 | 0.82 (0.48 to 1.39) | |||
| Past | 42 | 31 | 1.29 (0.81 to 2.05) | 28 | 45 | 0.62 (0.39 to 0.99) | 33 | 40 | 0.84 (0.53 to 1.33) | |||
| Current | 29 | 19 | 1.65 (0.93 to 2.95) | 21 | 27 | 0.80 (0.45 to 1.41) | 22 | 26 | 0.94 (0.53 to 1.65) | |||
| Alcohol consumption | .97 | .41 | .76 | |||||||||
| Low (≤15 g/d or ≤1 drink per d | 93 | 71 | 1.29 (0.94 to 1.75) | 75 | 89 | 0.84 (0.62 to 1.14) | 74 | 90 | 0.83 (0.61 to 1.13) | |||
| High (>15 g/d or >1 drink per d) | 6 | 6 | 1.22 (0.39 to 3.80) | 7 | 5 | 1.46 (0.46 to 4.63) | 6 | 6 | 0.99 (0.32 to 3.06) | |||
| Body mass index | .08 | .53 | .65 | |||||||||
| <25 mg/m2 | 44 | 17 | 2.00 (1.12 to 3.58) | 21 | 30 | 0.74 (0.42 to 1.29) | 26 | 25 | 0.93 (0.54 to 1.62) | |||
| ≥25 mg/m2 | 65 | 60 | 1.08 (0.76 to 1.54) | 61 | 64 | 0.92 (0.65 to 1.31) | 54 | 71 | 0.80 (0.56 to 1.14) | |||
Hazard ratios are presented as relative risks (RRs) and 95% confidence intervals (CIs).
RR is the rate in the treatment group compared with the rate in the placebo group for each agent.
Two-sided, based on the chi-square test for interaction.
Discussion
In this randomized trial of vitamins C and E and beta carotene supplementation among women at high risk for cardiovascular events, we observed no overall associations of the three antioxidant supplements, taken singly or combined, with total cancer incidence or mortality. Duration of supplementation also did not appear to alter the associations of these supplements with risk of cancer or mortality due to cancer.
In melanoma cells, vitamin C treatment effectively decreased the expression of cyclooxygenase-2 and type I insulin-like growth factor receptor, resulting in suppression of cell proliferation (10). Treatment with antioxidants such as vitamin E and beta carotene was also shown to induce cell apoptosis in breast cancer cells by restoring transforming growth factor-β and Fas (or CD95) signaling pathways (11) or increasing the expression of peroxisome proliferator–activated receptor gamma (12). Antioxidants also counterbalance the production of ROS and thus prevent the harmful effects of these oxygen intermediates on cellular nucleic acids, lipids, and proteins (13,15). Growth regulatory pathways that are involved in cell proliferation and apoptosis are sensitive to ROS, and these unavoidable byproducts of aerobic respiration may in some circumstances lead to inappropriate cell signaling and carcinogenesis (14).
Previous observational studies that assessed the relationship between total intakes of antioxidant nutrients and cancer prevention have yielded inconsistent results. Several case–control studies have reported an inverse association between antioxidant intakes and cancer risk, but cohort studies have generally observed little association. A meta-analysis that reported risk reduction for esophageal and gastric cardia adenocarcinoma with higher intakes of vitamins E and C and beta carotene was based primarily on case–control studies (52). Similarly, another meta-analysis concluded that vitamin C or beta carotene intakes were inversely associated with breast cancer risk, but the inverse association was mainly driven by case–control studies (6). By contrast, other pooled analyses of prospective studies have not shown an association of vitamins E and C or beta carotene intakes with risk of incident cancers of the breast (53), colorectum (54), lung (55), or ovary (56). Therefore, the cumulative data from observational studies offer limited, if any, support for an inverse association between antioxidant intakes and risk for cancer development.
Numerous randomized trials have also been conducted to investigate the effects of antioxidants in the prevention of cancers, but the findings have been mostly disappointing (16–27). A meta-analysis that pooled data from 12 randomized trials of antioxidants and primary cancer prevention showed no reduction in risk for total cancer incidence with antioxidant supplementation (RR = 0.99, 95% CI = 0.94 to 1.04) (35). With respect to individual types of antioxidants, the authors of this meta-analysis concluded that vitamin E did not reduce overall cancer (RR = 0.99, 95% CI = 0.94 to 1.04), but beta carotene supplementation was associated with a slight increase in cancer incidence (RR = 1.06, 95% CI = 1.00 to 1.12), with the increased risk mostly seen in smokers (RR = 1.10, 95% CI = 1.03 to 1.18) (35). Similarly, meta-analyses of the association of antioxidants and minerals with incidence of cancer at specific sites indicated no beneficial effects of antioxidants or minerals. Two meta-analyses (31,32) on incident gastrointestinal cancers found no statistically significant effects of supplementation with beta carotene; vitamins A, C, and E; and selenium (alone or in combination) [from Bjelakovic et al. (31), pooled RRs = 0.90, 95% CI = 0.77 to 1.05; and from Bjelakovic et al. (32), pooled RRs = 0.94, 95% CI = 0.83 to 1.06]. Another meta-analysis of eight randomized trials also reported no statistically significant effects of beta carotene; vitamins A, C, or E; or selenium supplements, alone or in combination, on the prevention of colorectal adenoma (pooled RR = 0.82, 95% CI = 0.60 to 1.1) (33). Consistent with these analyses, we also found no effects of vitamins C and E and beta carotene supplementation in reducing the risk for total cancer events.
Systematic reviews of antioxidant trials also found no reduction in cancer mortality with antioxidant supplementation. The meta-analysis of 12 randomized trials of vitamin or mineral supplements described above reported no association between antioxidants and cancer mortality (pooled RR = 1.03, 95% CI = 0.92 to 1.15) (35). Specifically, no reduction in cancer mortality was found with vitamin E (RR = 1.04, 95% CI = 0.94 to 1.04) or beta carotene (RR = 1.16, 95% CI = 0.98 to 1.37) supplementation (35). However, a possible risk reduction in cancer mortality was observed for the mineral supplement selenium (RR = 0.78, 95% CI = 0.65 to 0.94) (35). A pooled analysis of five studies from the two Linxian trials (the Linxian General Population Trial and the Linxian Dysplasia Trial) also did not present strong evidence that vitamin C combined with molybdenum improves cancer survival (RR = 1.06, 95% CI = 0.92 to 1.21) (34). Two recent meta-analyses have concluded that there may be an increase in total mortality with increasing antioxidant use (31,57). A review of 19 clinical trials reported a dose–response relationship between vitamin E and risk for total death (57). A later review comprising 47 low-bias risk (high methodological quality) trials reported that supplementation with vitamin E or beta carotene increased total mortality (RR = 1.04, 95% CI = 1.01 to 1.07, and RR = 1.07, 95% CI = 1.02 to 1.11, respectively), but a modification of risk estimates by dosage was evident only with beta carotene supplementation (58). By contrast, supplementation with vitamin C did not influence total mortality (58). In this population, we observed no association of supplementation with vitamin C or E or beta carotene with fatal cancer events.
A recent meta-analysis of four large randomized trials suggested that beta carotene supplementation may be associated with elevated lung cancer risk among current smokers (RR = 1.24, 95% CI = 1.10 to 1.39) but not among past smokers (RR = 1.10, 95% CI = 0.84 to 1.45) (59). In our population with a low prevalence of smoking, we observed no effect of beta carotene on lung cancer. Nonetheless, we found that vitamin C was associated with an elevated risk for lung cancer incidence. Two previous trials of vitamin C combined with other antioxidants reported no effects on incidence of respiratory cancers (16,24). The Linxian General Population Trial also reported no statistically significant effect of vitamin C combined with molybdenum on lung cancer death (RR = 1.01, 95% CI = 0.73 to 1.39) (60). In observational studies, no association between total vitamin C intake and lung cancer risk was reported in a pooled analysis of nine prospective studies (RR comparing the highest vs lowest quintile = 0.97, 95% CI = 0.78 to 1.22, P for trend = .82) (55). It has been suggested that vitamin C may act as a pro-oxidant and promote oxidative damage to DNA when its local concentrations are high (61,62). However, the overall evidence thus far suggests that there is no substantial oxidative DNA damage in humans who ingest high amounts of vitamin C (63,64). Our results that indicate an association between vitamin C supplementation and lung cancer may be due to chance. Nevertheless, a possible increase in lung cancer risk among women with vitamin C supplementation warrants further investigation in analyses of data from other completed randomized trials.
In our trial, neither duration of treatment nor combination of the three antioxidant supplements had effects on overall fatal or nonfatal cancer events. Thus, our results are in agreement with the recent review of randomized trials indicating that total mortality was not affected by duration of supplementation and single or combined antioxidant regimens (58).
Several other factors besides duration and the combination of supplements could affect the efficacy of antioxidants. For instance, lifestyle behaviors may modify an individual's response, in terms of cancer risk, to antioxidant supplementation. Findings from the Linxian General Population Trial (65), which showed a possible reduction in cancer incidence with vitamin and mineral supplementation in a poorly nourished population, suggest that antioxidant supplementation is most likely beneficial for individuals who are low or deficient for various nutrients. By contrast, most studies, including ours, were conducted among well-nourished populations that had sufficient intakes of antioxidants (58). In addition, there may be a sex-specific difference in the effect of antioxidants on cancer risk. In the Supplémentation en Vitamines et Minéraux Antioxydants (SUVIMAX) trial, antioxidant supplementation was associated with a lower cancer incidence in men, but not in women (16). Although men in the SUVIMAX trial may have benefited from antioxidant supplements due to their lower baseline levels of antioxidants (16), subsequent findings of an inverse association between baseline serum antioxidant levels and total cancer incidence in men but not in women suggest that factors other than baseline antioxidant levels may play a role in mediating any effects on carcinogenesis (66). Different antioxidant effects attributable to gender also could have contributed to the null results in trials conducted among female populations, including ours.
Limitations of the WACS trial include the lack of complete follow-up and compliance. However, when we conducted sensitivity analyses that excluded women who did not meet the compliance criteria, we observed no major changes in the results. Another limitation of this study is that it may not be appropriate to apply our results, obtained from a population at high risk for CVD, to the general population. Nevertheless, our results are mostly consistent with previous findings from high-risk populations. We also had limited power to evaluate the combined effects of the three antioxidants on site-specific incident cancers. For example, there was less than 40% power to detect a 30% reduction in the risk for major site-specific cancers such as the breast, colorectum, or lung with supplementation with the three antioxidants. However, we have tested the combined effects on total cancer incidence with nearly 80% power for detection of the same magnitude of risk reduction and did not find any statistically significant effects resulting from the combined supplements. Finally, although this randomized trial of antioxidants is one of the few trials with long duration of treatment, it may still be of insufficient duration to assess effects on cancer incidence, given the long latency for cancer.
In conclusion, findings from the WACS trial suggest that there are no overall benefits or risks of vitamins C and E and beta carotene supplementation in the primary prevention of total cancer incidence or cancer mortality.
Funding
Grant HL46959 from the National Heart, Lung, and Blood Institute and grants CA112529 and CA126846 from the National Cancer Institute.
Footnotes
The study sponsor had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to publish. J. M. Gaziano has received pills and packaging for another study from Wyeth Pharmaceuticals.
We acknowledge the invaluable contributions of the WACS staff, including Jean MacFadyen, Eleanor Danielson, Marilyn Chown, Shamikhah Curry, Margarette Haubourg, Felicia Zangi, Tony Laurinaitis, Geneva McNair, Philomena Quinn, Harriet Samuelson, Ara Sarkissian, Natalya Gometskaya, and M. V. Moorthy. We also thank the cancer endpoints reviewers including Wendy Chen, I-Min Lee, and Shumin Zhang. Finally, we are indebted to the dedicated WACS participants.
References
- 1.Weisburger JH. Nutritional approach to cancer prevention with emphasis on vitamins, antioxidants, and carotenoids. Am J Clin Nutr. 1991;53(suppl 1):226S–237S. doi: 10.1093/ajcn/53.1.226S. [DOI] [PubMed] [Google Scholar]
- 2.van Poppel G. Epidemiological evidence for beta-carotene in prevention of cancer and cardiovascular disease. Eur J Clin Nutr. 1996;50(suppl 3):S57–S61. [PubMed] [Google Scholar]
- 3.La Vecchia C, Altieri A, Tavani A. Vegetables, fruit, antioxidants and cancer: a review of Italian studies. Eur J Nutr. 2001;40(6):261–267. doi: 10.1007/s394-001-8354-9. [DOI] [PubMed] [Google Scholar]
- 4.Genkinger JM, Platz EA, Hoffman SC, Comstock GW, Helzlsouer KJ. Fruit, vegetable, and antioxidant intake and all-cause, cancer, and cardiovascular disease mortality in a community-dwelling population in Washington County, Maryland. Am J Epidemiol. 2004;160(12):1223–1233. doi: 10.1093/aje/kwh339. [DOI] [PubMed] [Google Scholar]
- 5.Lunet N, Valbuena C, Vieira AL, et al. Fruit and vegetable consumption and gastric cancer by location and histological type: case-control and meta-analysis. Eur J Cancer Prev. 2007;16(4):312–327. doi: 10.1097/01.cej.0000236255.95769.22. [DOI] [PubMed] [Google Scholar]
- 6.Gandini S, Merzenich H, Robertson C, Boyle P. Meta-analysis of studies on breast cancer risk and diet: the role of fruit and vegetable consumption and the intake of associated micronutrients. Eur J Cancer. 2000;36(5):636–646. doi: 10.1016/s0959-8049(00)00022-8. [DOI] [PubMed] [Google Scholar]
- 7.Pavia M, Pileggi C, Nobile CG, Angelillo IF. Association between fruit and vegetable consumption and oral cancer: a meta-analysis of observational studies. Am J Clin Nutr. 2006;83(5):1126–1134. doi: 10.1093/ajcn/83.5.1126. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez CA, Pera G, Agudo A, et al. Fruit and vegetable intake and the risk of stomach and oesophagus adenocarcinoma in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) Int J Cancer. 2006;118(10):2559–2566. doi: 10.1002/ijc.21678. [DOI] [PubMed] [Google Scholar]
- 9.Ames BN. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983;221(4617):1256–1264. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- 10.Lee SK, Kang JS, Jung da J, et al. Vitamin C suppresses proliferation of the human melanoma cell SK-MEL-2 through the inhibition of cyclooxygenase-2 (COX-2) expression and the modulation of insulin-like growth factor II (IGF-II) production. J Cell Physiol. 2008;216(1):180–188. doi: 10.1002/jcp.21391. [DOI] [PubMed] [Google Scholar]
- 11.Yu W, Sanders BG, Kline K. RRR-alpha-tocopheryl succinate-induced apoptosis of human breast cancer cells involves Bax translocation to mitochondria. Cancer Res. 2003;63(10):2483–2491. [PubMed] [Google Scholar]
- 12.Cui Y, Lu Z, Bai L, Shi Z, Zhao WE, Zhao B. β-Carotene induces apoptosis and up-regulates peroxisome proliferator-activated receptor gamma expression and reactive oxygen species production in MCF-7 cancer cells. Eur J Cancer. 2007;43(17):2590–2601. doi: 10.1016/j.ejca.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 13.Seifried HE, Anderson DE, Sorkin BC, Costello RB. Free radicals: the pros and cons of antioxidants. Executive summary report. J Nutr. 2004;134(11):3143S–3163S. doi: 10.1093/jn/134.11.3143S. [DOI] [PubMed] [Google Scholar]
- 14.Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol. 2004:239–267. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- 15.Seifried HE, Anderson DE, Fisher EI, Milner JA. A review of the interaction among dietary antioxidants and reactive oxygen species. J Nutr Biochem. 2007;18(9):567–579. doi: 10.1016/j.jnutbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Hercberg S, Galan P, Preziosi P, et al. The SU.VI.MAX Study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med. 2004;164(21):2335–2342. doi: 10.1001/archinte.164.21.2335. [DOI] [PubMed] [Google Scholar]
- 17.Clark LC, Combs GF, Jr, Turnbull BW, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA. 1996;276(24):1957–1963. [PubMed] [Google Scholar]
- 18.Albanes D, Malila N, Taylor PR, et al. Effects of supplemental alpha-tocopherol and beta-carotene on colorectal cancer: results from a controlled trial (Finland) Cancer Causes Control. 2000;11(3):197–205. doi: 10.1023/a:1008936214087. [DOI] [PubMed] [Google Scholar]
- 19.Beta Carotene Prevention study group the Alpha-Tocopherol. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med. 1994;330(15):1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 20.Hennekens CH, Buring JE, Manson JE, et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996;334(18):1145–1149. doi: 10.1056/NEJM199605023341801. [DOI] [PubMed] [Google Scholar]
- 21.Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334(18):1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 22.GISSI-Prevenzione Investigators. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet. 1999;354(9177):447–455. [PubMed] [Google Scholar]
- 23.Lee IM, Cook NR, Manson JE, Buring JE, Hennekens CH. Beta-carotene supplementation and incidence of cancer and cardiovascular disease: the Women's Health Study. J Natl Cancer Inst. 1999;91(24):2102–2106. doi: 10.1093/jnci/91.24.2102. [DOI] [PubMed] [Google Scholar]
- 24.Heart Protection study collaborative group. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):23–33. doi: 10.1016/S0140-6736(02)09328-5. [DOI] [PubMed] [Google Scholar]
- 25.Lee IM, Cook NR, Gaziano JM, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294(1):56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 26.Lonn E, Bosch J, Yusuf S, et al. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA. 2005;293(11):1338–1347. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 27.Plummer M, Vivas J, Lopez G, et al. Chemoprevention of precancerous gastric lesions with antioxidant vitamin supplementation: a randomized trial in a high-risk population. J Natl Cancer Inst. 2007;99(2):137–146. doi: 10.1093/jnci/djk017. [DOI] [PubMed] [Google Scholar]
- 28.Marras C, McDermott MP, Rochon PA, et al. Survival in Parkinson disease: thirteen-year follow-up of the DATATOP cohort. Neurology. 2005;64(1):87–93. doi: 10.1212/01.WNL.0000148603.44618.19. [DOI] [PubMed] [Google Scholar]
- 29.Witte KK, Nikitin NP, Parker AC, et al. The effect of micronutrient supplementation on quality-of-life and left ventricular function in elderly patients with chronic heart failure. Eur Heart J. 2005;26(21):2238–2244. doi: 10.1093/eurheartj/ehi442. [DOI] [PubMed] [Google Scholar]
- 30.Rayman M, Thompson A, Warren-Perry M, et al. Impact of selenium on mood and quality of life: a randomized, controlled trial. Biol Psychiatry. 2006;59(2):147–154. doi: 10.1016/j.biopsych.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 31.Bjelakovic G, Nikolova D, Simonetti RG, Gluud C. Antioxidant supplements for prevention of gastrointestinal cancers: a systematic review and meta-analysis. Lancet. 2004;364(9441):1219–1228. doi: 10.1016/S0140-6736(04)17138-9. [DOI] [PubMed] [Google Scholar]
- 32.Bjelakovic G, Nikolova D, Simonetti RG, Gluud C. Systematic review and meta-analysis: primary and secondary prevention of gastrointestinal cancers with antioxidant supplements. Aliment Pharmacol Ther. 2008;28(6):689–703. doi: 10.1111/j.1365-2036.2008.03785.x. [DOI] [PubMed] [Google Scholar]
- 33.Bjelakovic G, Nagorni A, Nikolova D, Simonetti RG, Bjelakovic M, Gluud C. Meta-analysis: antioxidant supplements for primary and secondary prevention of colorectal adenoma. Aliment Pharmacol Ther. 2006;24(2):281–291. doi: 10.1111/j.1365-2036.2006.02970.x. [DOI] [PubMed] [Google Scholar]
- 34.Coulter ID, Hardy ML, Morton SC, et al. Antioxidants vitamin C and vitamin e for the prevention and treatment of cancer. J Gen Intern Med. 2006;21(7):735–744. doi: 10.1111/j.1525-1497.2006.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bardia A, Tleyjeh IM, Cerhan JR, et al. Efficacy of antioxidant supplementation in reducing primary cancer incidence and mortality: systematic review and meta-analysis. Mayo Clin Proc. 2008;83(1):23–34. doi: 10.4065/83.1.23. [DOI] [PubMed] [Google Scholar]
- 36.Stanner SA, Hughes J, Kelly CN, Buttriss J. A review of the epidemiological evidence for the ‘antioxidant hypothesis’. Public Health Nutr. 2004;7(3):407–422. doi: 10.1079/phn2003543. [DOI] [PubMed] [Google Scholar]
- 37.Niki E, Noguchi N, Tsuchihashi H, Gotoh N. Interaction among vitamin C, vitamin E, and beta-carotene. Am J Clin Nutr. 1995;62(suppl 6):1322S–1326S. doi: 10.1093/ajcn/62.6.1322S. [DOI] [PubMed] [Google Scholar]
- 38.Davis CD, Uthus EO. DNA methylation, cancer susceptibility, and nutrient interactions. Exp Biol Med (Maywood) 2004;229(10):988–995. doi: 10.1177/153537020422901002. [DOI] [PubMed] [Google Scholar]
- 39.Hamilton IM, Gilmore WS, Benzie IF, Mulholland CW, Strain JJ. Interactions between vitamins C and E in human subjects. Br J Nutr. 2000;84(3):261–267. doi: 10.1017/s0007114500001537. [DOI] [PubMed] [Google Scholar]
- 40.Salganik RI. The benefits and hazards of antioxidants: controlling apoptosis and other protective mechanisms in cancer patients and the human population. J Am Coll Nutr. 2001;20(suppl 5):464S–472S. doi: 10.1080/07315724.2001.10719185. discussion 473S–475S. [DOI] [PubMed] [Google Scholar]
- 41.Bassuk SS, Albert CM, Cook NR, et al. The Women's Antioxidant Cardiovascular Study: design and baseline characteristics of participants. J Womens Health (Larchmt) 2004;13(1):99–117. doi: 10.1089/154099904322836519. [DOI] [PubMed] [Google Scholar]
- 42.Cook NR, Albert CM, Gaziano JM, et al. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: results from the Women's Antioxidant Cardiovascular Study. Arch Intern Med. 2007;167(15):1610–1618. doi: 10.1001/archinte.167.15.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352(13):1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 44.Cook NR, Lee IM, Gaziano JM, et al. Low-dose aspirin in the primary prevention of cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294(1):47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 45.Maher EA, Brennan C, Wen PY, et al. Marked genomic differences characterize primary and secondary glioblastoma subtypes and identify two distinct molecular and clinical secondary glioblastoma entities. Cancer Res. 2006;66(23):11502–11513. doi: 10.1158/0008-5472.CAN-06-2072. [DOI] [PubMed] [Google Scholar]
- 46.Smid M, Wang Y, Zhang Y, et al. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008;68(9):3108–3114. doi: 10.1158/0008-5472.CAN-07-5644. [DOI] [PubMed] [Google Scholar]
- 47.Jones AM, Mitter R, Springall R, et al. A comprehensive genetic profile of phyllodes tumours of the breast detects important mutations, intra-tumoral genetic heterogeneity and new genetic changes on recurrence. J Pathol. 2008;214(5):533–544. doi: 10.1002/path.2320. [DOI] [PubMed] [Google Scholar]
- 48.Dandona P, Mohanty P, Ghanim H, et al. The suppressive effect of dietary restriction and weight loss in the obese on the generation of reactive oxygen species by leukocytes, lipid peroxidation, and protein carbonylation. J Clin Endocrinol Metab. 2001;86(1):355–362. doi: 10.1210/jcem.86.1.7150. [DOI] [PubMed] [Google Scholar]
- 49.Kurose I, Higuchi H, Miura S, et al. Oxidative stress-mediated apoptosis of hepatocytes exposed to acute ethanol intoxication. Hepatology. 1997;25(2):368–378. doi: 10.1053/jhep.1997.v25.pm0009021949. [DOI] [PubMed] [Google Scholar]
- 50.Reilly M, Delanty N, Lawson JA, FitzGerald GA. Modulation of oxidant stress in vivo in chronic cigarette smokers. Circulation. 1996;94(1):19–25. doi: 10.1161/01.cir.94.1.19. [DOI] [PubMed] [Google Scholar]
- 51.Department of Health and Human Services, US Department of Agriculture. The Dietary Guidelines for Americans, 2005. 6th ed. Washington, DC: US Government Printing Office; 2005. [Google Scholar]
- 52.Kubo A, Corley DA. Meta-analysis of antioxidant intake and the risk of esophageal and gastric cardia adenocarcinoma. Am J Gastroenterol. 2007;102(10):2323–2330. doi: 10.1111/j.1572-0241.2007.01374.x. quiz 2331. [DOI] [PubMed] [Google Scholar]
- 53.Cho E, Spiegelman D, Hunter DJ, et al. Premenopausal intakes of vitamins A, C, and E, folate, and carotenoids, and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2003;12(8):713–720. [PubMed] [Google Scholar]
- 54.Mannisto S, Yaun SS, Hunter DJ, et al. Dietary carotenoids and risk of colorectal cancer in a pooled analysis of 11 cohort studies. Am J Epidemiol. 2007;165(3):246–255. doi: 10.1093/aje/kwk009. [DOI] [PubMed] [Google Scholar]
- 55.Cho E, Hunter DJ, Spiegelman D, et al. Intakes of vitamins A, C and E and folate and multivitamins and lung cancer: a pooled analysis of 8 prospective studies. Int J Cancer. 2006;118(4):970–978. doi: 10.1002/ijc.21441. [DOI] [PubMed] [Google Scholar]
- 56.Koushik A, Hunter DJ, Spiegelman D, et al. Intake of the major carotenoids and the risk of epithelial ovarian cancer in a pooled analysis of 10 cohort studies. Int J Cancer. 2006;119(9):2148–2154. doi: 10.1002/ijc.22076. [DOI] [PubMed] [Google Scholar]
- 57.Miller ER, 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142(1):37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 58.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297(8):842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 59.Tanvetyanon T, Bepler G. Beta-carotene in multivitamins and the possible risk of lung cancer among smokers versus former smokers: a meta-analysis and evaluation of national brands. Cancer. 2008;113(1):150–157. doi: 10.1002/cncr.23527. [DOI] [PubMed] [Google Scholar]
- 60.Kamangar F, Qiao YL, Yu B, et al. Lung cancer chemoprevention: a randomized, double-blind trial in Linxian, China. Cancer Epidemiol Biomarkers Prev. 2006;15(8):1562–1564. doi: 10.1158/1055-9965.EPI-06-0316. [DOI] [PubMed] [Google Scholar]
- 61.Podmore ID, Griffiths HR, Herbert KE, Mistry N, Mistry P, Lunec J. Vitamin C exhibits pro-oxidant properties. Nature. 1998;392(6676):559. doi: 10.1038/33308. [DOI] [PubMed] [Google Scholar]
- 62.Rehman A, Collis CS, Yang M, et al. The effects of iron and vitamin C co-supplementation on oxidative damage to DNA in healthy volunteers. Biochem Biophys Res Commun. 1998;246(1):293–298. doi: 10.1006/bbrc.1998.8592. [DOI] [PubMed] [Google Scholar]
- 63.Duarte TL, Lunec J. Review: When is an antioxidant not an antioxidant? A review of novel actions and reactions of vitamin C. Free Radic Res. 2005;39(7):671–686. doi: 10.1080/10715760500104025. [DOI] [PubMed] [Google Scholar]
- 64.Lee KW, Lee HJ, Surh YJ, Lee CY. Vitamin C and cancer chemoprevention: reappraisal. Am J Clin Nutr. 2003;78(6):1074–1078. doi: 10.1093/ajcn/78.6.1074. [DOI] [PubMed] [Google Scholar]
- 65.Blot WJ, Li JY, Taylor PR, et al. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J Natl Cancer Inst. 1993;85(18):1483–1492. doi: 10.1093/jnci/85.18.1483. [DOI] [PubMed] [Google Scholar]
- 66.Galan P, Briancon S, Favier A, et al. Antioxidant status and risk of cancer in the SU.VI.MAX study: is the effect of supplementation dependent on baseline levels? Br J Nutr. 2005;94(1):125–132. doi: 10.1079/bjn20051462. [DOI] [PubMed] [Google Scholar]