Abstract
Background: Short sleep is associated with obesity and may alter the endocrine regulation of hunger and appetite.
Objective: We tested the hypothesis that the curtailment of human sleep could promote excessive energy intake.
Design: Eleven healthy volunteers [5 women, 6 men; mean ± SD age: 39 ± 5 y; mean ± SD body mass index (in kg/m2): 26.5 ± 1.5] completed in random order two 14-d stays in a sleep laboratory with ad libitum access to palatable food and 5.5-h or 8.5-h bedtimes. The primary endpoints were calories from meals and snacks consumed during each bedtime condition. Additional measures included total energy expenditure and 24-h profiles of serum leptin and ghrelin.
Results: Sleep was reduced by 122 ± 25 min per night during the 5.5-h bedtime condition. Although meal intake remained similar (P = 0.51), sleep restriction was accompanied by increased consumption of calories from snacks (1087 ± 541 compared with 866 ± 365 kcal/d; P = 0.026), with higher carbohydrate content (65% compared with 61%; P = 0.04), particularly during the period from 1900 to 0700. These changes were not associated with a significant increase in energy expenditure (2526 ± 537 and 2390 ± 369 kcal/d during the 5.5-h and 8.5-h bedtime periods, respectively; P = 0.58), and we found no significant differences in serum leptin and ghrelin between the 2 sleep conditions.
Conclusions: Recurrent bedtime restriction can modify the amount, composition, and distribution of human food intake, and sleeping short hours in an obesity-promoting environment may facilitate the excessive consumption of energy from snacks but not meals.
INTRODUCTION
Genetic factors are thought to underlie individual susceptibility to obesity, whereas nongenetic factors determine the phenotypic expression of the disease (1, 2). The rising rates of obesity in the United States have been associated with considerable environmental, social, and behavioral changes that promote overeating and inactivity (3). Driven by the demands and opportunities of modern life, a growing number of Americans report reduced sleep times (4). The potential importance of this trend for public health is highlighted by epidemiologic data, which indicate that self-reported sleep of <6 h per night is associated with increased adiposity (5, 6). Some, but not all, of the available prospective studies in adults have also linked short sleep duration with greater risk of weight gain and obesity (6, 7). An important question raised by these reports is whether reduced sleep duration contributes directly to the mechanisms of unhealthy weight gain or reflects the presence of other relevant but unrecognized risk factors and pathways of reverse causation (5, 6).
Existing cross-sectional data suggest that the association of short sleep with obesity may be accompanied by changes in peripheral concentrations of the orexigenic hormone ghrelin and the anorexigenic hormone leptin (8, 9). In addition, lower leptin and higher ghrelin concentrations and increased hunger and appetite have been reported after short-term sleep restriction of healthy men (10). If chronically activated by the lack of sufficient sleep, such hormone changes have been hypothesized to promote overeating and raise the risk of obesity. Although an increase in food intake has been documented in rodents subjected to partial sleep deprivation, these experimental interventions are also accompanied by markedly enhanced energy expenditure and weight loss (11–13). To date, no studies have measured the effect of recurrent sleep curtailment on the components of human energy intake and expenditure, and the validity of the increased energy intake hypothesis remains uncertain.
In the present study, we examined the effects of recurrent bedtime restriction by 3 h per night on food intake, energy expenditure, and 24-h concentrations of leptin and ghrelin in healthy, middle-aged adults. Because habitual sleep curtailment is an increasingly common aspect of the Western lifestyle characterized by physical inactivity and overeating, these studies were carried out under controlled laboratory conditions of sedentary living with ad libitum access to palatable food. We hypothesized that recurrent bedtime curtailment would be accompanied by an increased intake of energy from meals and snacks.
SUBJECTS AND METHODS
Participants
Sedentary men and women aged 34–49 y with a body mass index (in kg/m2) between 24 and 29 and self-reported sleep duration of 6.5–8.5 h/d were recruited through local newspaper advertisements and by word of mouth. Volunteers were excluded from participation for any of the following: self-reported sleep problems (Pittsburgh Sleep Quality Index score >10), night work, variable sleep habits, or habitual daytime naps; physically demanding occupations or regular exercise; depressed mood (Center for Epidemiologic Studies of Depression score >15); excessive intake of alcohol (>14 drinks/wk for men; >7 for women) or caffeine (>300 mg/d); smoking; use of prescription medications or over-the-counter drugs affecting sleep or metabolism; or abnormal findings on medical history, physical exam, or laboratory screening tests (including a 75-g oral glucose challenge and one night of full polysomnography). Only nonpregnant women were studied, and data collection was scheduled during the first half of the woman's menstrual cycle. Eleven subjects (5 women, 6 men) including 5 white, 4 African American, and 2 Hispanic individuals completed the study. All research volunteers gave written informed consent and were paid for their participation.
Study protocol
The study protocol was approved by the institutional review boards of the Universities of Chicago and Wisconsin. Each subject completed two 14-d study periods with scheduled bedtimes of 5.5 or 8.5 h per night in random order ≥3 mo apart. To achieve bedtimes of 5.5 and 8.5 h without shifts in circadian phase, the usual lights-off and wakeup times of the subjects were moved proportionally closer together or further apart. Subjects were studied in the controlled environment of the University of Chicago sleep research laboratory, which offers individual accommodations similar to those of a comfortable hotel room with a queen-sized bed and has built-in infrastructure for video monitoring and sleep recording. Six subjects started with the 5.5-h bedtime condition, and 5 subjects were studied under 8.5-h bedtimes first. Participants spent most waking hours indoors engaged in leisurely activities or home-office-type work and had free access to a telephone, desktop computer, television, videos, reading material, and the Internet. On average, subjects spent no more than 30 min/d outside of the laboratory on the university campus. No naps were allowed, and individual safety and compliance were monitored continuously by our research staff. Before and after each 14-d study, the participants remained at bed rest for 48 h with identical caloric intake including oral and intravenous doses of glucose at 0900 and identical carbohydrate-rich meals at 1400 and 1900 as previously described (14). During the last 24 h of this period, blood was sampled every 30 min starting at 2000. Bedtimes were 7 h per night before each study, and 5.5 or 8.5 h per night afterward according to the assigned sleep condition.
Energy intake
A registered dietitian interviewed all participants to determine their food preferences and to exclude the presence of any eating disorders and developed nutritionally balanced, individual meal plans consisting of breakfast, lunch, dinner, and a selection of palatable snacks and soft drinks. During each bedtime condition, the subjects received the same customized 3-d meal sequence including typical Western foods on a rotating basis. Breakfast was served at 0800–0900 and included such items as eggs, bacon, or sausage; toast, bagel, pancakes, or waffles; jelly, peanut butter, or cream cheese; cereal; fruit; yogurt; juice; milk; coffee; and tea. Lunch was served at 1300–1400 and could be a hot or cold entrée (eg, hot or cold sandwich, pizza, pasta, or meat; a vegetable; and starch) along with soup or salad, soft drink, and dessert. Dinner was served at 1830–1930 and was usually a hot meat, poultry, or fish entrée with a vegetable and starch, in addition to salad, uncaffeinated beverage, and dessert. Subjects were allowed one caffeinated beverage with breakfast and one with lunch as needed to match their usual consumption of caffeine at home. Meals were served in excess to allow ad libitum intake of energy. Food was weighed before and after each meal to determine actual consumption. In addition, study participants had unlimited access to a snack bar in their room, which was kept stocked with soft drinks and the same individually customized assortment of 10 snacks during each study period. The snacks included salty snacks (eg, pretzels, chips and dip, cheese and crackers, popcorn, and nuts), sweets (eg, snack bars, muffins, cookies, pudding, ice cream, and candy), fresh and dried fruits, yogurt, raw vegetables and dip, and uncaffeinated beverages (eg, milk, juice, soda, and water). Items consumed from the snack bar were weighed, and disappearance from the inventory was recorded twice every 24 h to determine the intake of calories during the day (0700–1900) and at night (1900–0700). Total snack consumption of one of the subjects was measured only once daily at 0700. The caloric content and macronutrient composition of all meals and snacks, consumed between 0700 on day 1 and 0700 on day 14 of each bedtime condition was calculated by using Nutritionist IV software (version 4.1; Axxya Systems, Stafford, TX).
Energy expenditure
For the measurement of total energy expenditure, study participants ingested 18O- and 2H-labeled water (2.0 and 0.14 g/kg total body water, respectively) on the first morning of each bedtime condition, and their next 3 urine voids were collected. Two more urine voids were collected in the morning of day 14. Samples were analyzed by isotope-ratio mass spectrometry at the University of Wisconsin in Madison as previously described (15, 16). Resting metabolic rate was measured under basal conditions by indirect calorimetry (Vmax Encore 29; Sensormedics, Yorba Linda, CA) after awakening on day 14 of each bedtime condition by using standard methods (16). Subsequently, the subjects consumed a customized standard breakfast and their resting metabolic rate was measured for the next 4 h. The area under the 4-h postprandial curve minus the resting metabolic rate was divided by the caloric content of breakfast and multiplied by 100 to obtain an estimate of the thermic effect of food (in % of energy intake). Activity energy expenditure was determined by subtracting the resting metabolic rate and the thermic effect of food from the total energy expenditure of the subjects. The physical activity level of the subjects was calculated as the ratio between their total energy expenditure and resting metabolic rate.
Measurement of body weight and composition
Body composition was measured by dual-energy X-ray absorptiometry (DXA) before and after each bedtime condition on the same instrument (Prodigy; Lunar, Madison, WI). Technical problems resulted in the loss of data from one subject at the end of the short sleep condition. Body weight was measured with a digital medical scale (Scale-Tronix Inc, Wheaton, IL) in the morning before each DXA scan. Height was measured at screening with a Harpenden stadiometer (Holtain Ltd, Crosswell, United Kingdom). Body fat was determined by multiplying body weight by the percentage of body fat from the DXA scan. The bone mineral content by DXA and body fat were subtracted from body weight to determine fat-free soft tissue mass.
Sleep recording
Sleep was recorded by using a Neurofax-1100 EEG Acquisition System (Nihon Kohden America Inc, Foothill Ranch, CA). Before enrollment, the subjects underwent a night of full laboratory polysomnography for habituation and to exclude the presence of primary sleep pathology. Records were scored in 30-s epochs of wake, movement, stage 1, stage 2, stage 3, stage 4, and rapid eye movement sleep. Respiratory events, periodic leg movements, and arousals were defined according to established clinical criteria, and subjects with a respiratory disturbance index >10 or any sleep movement disorder were excluded from participation. Only electroencephalographic, electrooculographic, and electromyographic channels were recorded on subsequent study nights. The data from each subject during both study conditions were scored by the same sleep technician. Total sleep time was calculated as the sum of all epochs scored as sleep. Sleep efficiency was calculated as the percentage of scheduled time in bed that was scored as sleep.
Hormone assays
Serum leptin was measured by radioimmunoassay (Linco Research, St Charles, MO). We also measured total ghrelin by radioimmunoassay (Linco Research) in 9 of the subjects.
Data analysis
t Tests accounting for the crossover design of the study (17) were used to compare the intake of energy from meals and snacks during each bedtime condition. To control for any difference in body weight at the beginning of each study, these comparisons were repeated with the use of a generalized estimating equation (GEE) regression model (18) with baseline body weight and treatment period as time-varying covariates. Correlation analysis was used to explore the relation between energy intake and body weight. Secondary measures, such as sleep parameters, macronutrient distribution, and metabolic hormone concentrations, were compared between the 2 study conditions by using paired t tests. Finally, a GEE regression model that included the consumption of calories from meals and snacks, physical-activity-related energy expenditure, bedtime condition, and treatment period (first or second study) as time-varying covariates was used to explore the factors that might explain the considerable variability in individual propensity for weight gain during these studies. All energy intake and expenditure variables in this model were expressed relative to the resting metabolic rate of the subjects. Analyses were performed by using Stata (version 10; StataCorp, College Station, TX) and SPSS (version 11; SPSS Inc, Chicago, IL). Values in the text are reported as means ± SDs. Statistical significance was defined as P < 0.05.
RESULTS
Sleep duration
Subject characteristics at the time of enrollment are summarized in Table 1. The average amount of sleep during the 5.5-h bedtime condition was reduced by 122 ± 25 min per night compared with that during the 8.5-h condition (Table 2).
TABLE 1.
Participant characteristics1
| Characteristic | Value |
| Age (y) | 39 ± 5 |
| Body weight (kg) | 75.8 ± 9.4 |
| Height (cm) | 168.8 ±10.6 |
| BMI (kg/m2) | 26.5 ± 1.5 |
| Self-reported sleep duration (h:min/night) | 7:35 ± 0:42 |
| PSQI global score | 2 ± 2 |
| RDI (events/h) | 3 ± 3 |
| CES-D score | 3 ± 5 |
All values are mean ± SD (n = 11). PSQI, Pittsburgh Sleep Quality Index; RDI, respiratory disturbance index (number of apneas and hypopneas per hour of sleep); CES-D, Center for Epidemiologic Studies of Depression 20-item scale.
TABLE 2.
Average sleep duration and architecture during each study period1
| 8.5-h bedtime | 5.5-h bedtime | Change 5.5–8.5-h bedtime | |
| Total sleep time (h:min) | 7:13 ± 0:26 | 5:11 ± 0:07 | −2:02 ± 0:25 |
| Lights-off time (h:min) | 23:16 ± 00:39 | 0:31 ± 00:49 | 1:19 ± 0:25 |
| Wakeup time (h:min) | 7:42 ± 00:39 | 6:02 ± 00:49 | −1:39 ± 0:24 |
| Sleep onset latency (min) | 20 ± 9 | 8 ± 3 | −12 ± 8 |
| Wake after sleep onset (min) | 61 ± 24 | 14 ± 6 | −46 ± 22 |
| Sleep efficiency (%) | 85 ± 5 | 94 ± 2 | 9 ± 5 |
| Stage 1 sleep (min) | 30 ± 15 | 16 ± 6 | −14 ± 15 |
| Stage 2 sleep (min) | 246 ± 40 | 177 ± 44 | −69 ± 29 |
| Stages 3 + 4 sleep (min) | 51 ± 37 | 45 ± 36 | −6 ± 16 |
| REM sleep (min) | 106 ± 23 | 74 ± 22 | −32 ± 22 |
All values are group means ± SD (n = 11). REM, rapid eye movement. P < 0.01 for all paired t test comparisons, except for stage 1 sleep (P = 0.01) and stages 3 + 4 sleep (P = 0.23).
Energy consumption
There were no significant differences in the consumption (Table 3) or distribution of energy from meals between the 2 study periods: breakfast, lunch, and dinner accounted for, respectively, 31.7 ± 6.1%, 32.8 ± 2.6%, and 35.6 ± 5.4% of meal calories during the 5.5-h bedtime period and 30.5 ± 5.7%, 33.6 ± 4.5%, and 35.9 ± 5.4% during the 8.5-h bedtime condition. The macronutrient content of meals was also similar: carbohydrate, fat, and protein contributed, respectively, 52.4 ± 4.1%, 33.6 ± 4.0%, and 14.0 ± 2.0% of meal calories during the 5.5-h bedtime period and 53.0 ± 5.8%, 33.1 ± 4.3%, and 13.9 ± 3.3% during the 8.5-h bedtime condition. In contrast, bedtime restriction was accompanied by increased consumption of energy from snacks (P = 0.026; Table 3) and a shift toward more carbohydrate (64.5 ± 6.7% compared with 61.0 ± 6.3% of energy; P = 0.04) and relatively less fat (29.6 ± 5.6% compared with 32.2 ± 5.6% of energy; P = 0.08) and protein (5.9 ± 2.1% compared with 6.7 ± 2.8% of energy; P = 0.08) in the macronutrient content of ingested snacks. Although the difference in the intake of calories from snacks between 0700 and 1900 was not statistically significant (P = 0.18; Table 3), bedtime curtailment was accompanied by an increased consumption of snacks during the period from 1900 to 0700 (P < 0.01).
TABLE 3.
Energy balance, body weight, and adiposity1
| 8.5-h bedtime | 5.5-h bedtime | Difference 5.5–8.5 h | |
| Energy intake from meals (kcal/d) | 2536 ± 943 | 2611 ± 873 | 76 ± 368 |
| Energy intake from snacks (kcal/d) | 866 ± 365 | 1087 ± 541 | 221 ± 2832 |
| Snack intake from 0700–1900 (kcal, n = 10) | 624 ± 355 | 737 ± 374 | 113 ± 226 |
| Snack intake from 1900–0700 (kcal, n = 10) | 236 ± 227 | 371 ± 272 | 136 ± 1603 |
| Total energy intake (TEI, kcal/d) | 3402 ± 974 | 3699 ± 903 | 297 ± 4404 |
| Total energy expenditure (TEE, kcal/d) | 2390 ± 369 | 2526 ± 537 | 136 ± 437 |
| Resting metabolic rate (RMR, kcal/d) | 1617 ± 267 | 1655 ± 321 | 38 ± 162 |
| Thermic effect of food (% of energy intake) | 6.6 ± 1.8 | 7.3 ± 1.6 | 0.7 ± 1.6 |
| Activity energy expenditure (kcal/d) | 546 ± 221 | 599 ± 427 | 53 ± 400 |
| Physical activity level (TEE/RMR) | 1.49 ± 0.16 | 1.54 ± 0.28 | 0.05 ± 0.27 |
| Energy balance (TEI minus TEE, kcal/d) | 1012 ± 744 | 1173 ± 764 | 161 ± 346 |
| Initial body weight (kg) | 75.0 ± 9.0 | 77.3 ± 10.2 | 2.2 ± 3.45 |
| Initial body fat (kg) | 24.3 ± 6.9 | 25.6 ± 5.9 | 1.3 ± 2.5 |
| Initial fat-free soft tissue mass (kg) | 47.8 ± 11.7 | 48.8 ± 12.2 | 1.0 ± 1.65 |
| 14-d Change in body weight (kg) | 2.1 ± 2.1 | 1.9 ± 1.6 | −0.2 ± 1.3 |
| 14-d Change in body fat (kg, n = 10) | 1.5 ± 1.0 | 1.7 ± 0.8 | 0.2 ± 0.6 |
| 14-d Change in fat-free soft tissue (kg, n = 10) | 0.6 ± 1.5 | 0.3 ± 1.3 | −0.3 ± 1.1 |
All values are mean ± SD (n = 11 unless otherwise specified).
P = 0.026 controlling for baseline weight and treatment period.
P < 0.01 controlling for baseline weight and treatment period.
P = 0.04 accounting only for the crossover design, but not significant (P = 0.46) when baseline weight and treatment period were controlled for in the generalized estimating equation model.
P = 0.06 by paired t test.
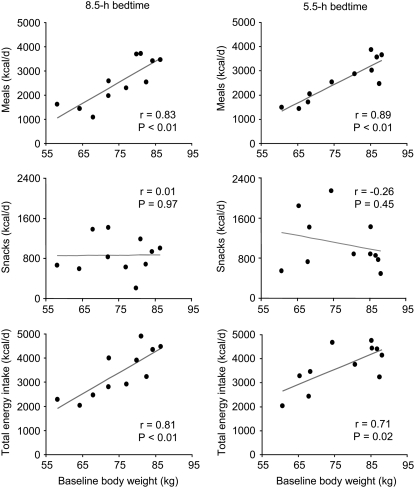
Because body weight is an important determinant of energy needs, we examined the relation between ingested calories and the initial body weight of the subjects during the 5.5- and 8.5-h bedtime conditions. Body weight correlated strongly with the intake of energy from meals, but was not related to the consumption of calories from snacks (Figure 1). Results remained unchanged when fat-free soft tissue mass was used instead of initial body weight (data not shown). Given these findings, the total caloric intake of the subjects (meals and snacks combined) during each sleep condition was compared in 2 different ways. Accounting only for the crossover design of the study, total energy intake was significantly higher during the period of bedtime curtailment (P = 0.04). This difference was due primarily to the increased consumption of calories from snacks (P = 0.04) and not meals (P = 0.49). When study design and initial body weight were controlled for, the difference in total energy intake between the 2 bedtime conditions was no longer significant (P = 0.46), whereas the difference in consumption of calories from snacks remained significant (P = 0.026; Table 3).
FIGURE 1.
Average daily energy intake from meals (top panels), snacks (middle panels), and meals plus snacks (bottom panels) as a function of the initial body weight of the 11 subjects during the 8.5-h (left) and 5.5-h bedtime (right) conditions. Each panel shows a corresponding best-fit line along with the Spearman's correlation coefficient (r) and its P value.
Energy expenditure and balance
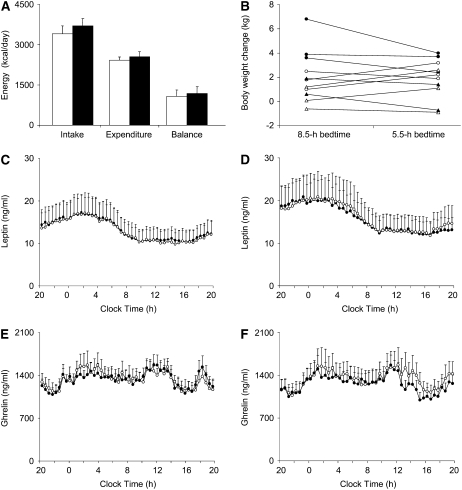
There were no statistically significant differences in total energy expenditure and its components, including activity energy expenditure, thermic effect of food, and resting metabolic rate, between the 2 bedtime conditions (Table 3). The physical activity level of the subjects (ie, total energy expenditure divided by the resting metabolic rate) averaged 1.54 ± 0.28 with 5.5-h and 1.49 ± 0.16 with 8.5-h bedtimes (P = 0.58), and their ratios of overall energy intake relative to resting metabolic rate were 2.23 ± 0.32 and 2.08 ± 0.34, respectively (P = 0.13). The surplus of energy intake in the group as a whole during both sleep conditions (Figure 2A) was the result of markedly different contributions from the individual study participants: some subjects repeatedly showed a strong propensity to overeat and gain weight irrespective of the presence or absence of sleep loss, whereas others exhibited little, if any, change in body weight (Figure 2B). In an exploratory analysis, both the novelty of exposure to the experimental environment and the excessive consumption of calories from meals were significant predictors of individual weight gain during the study (Table 4). There was also a trend for the intake of energy from snacks to contribute to individual weight gain, whereas the opposing effects of daily physical activity and extended wakefulness were not statistically significant (Table 4).
FIGURE 2.
A: Mean (±SE) daily energy balance of 11 subjects during the 8.5-h (open bars) and 5.5-h (solid bars) bedtime conditions. B: Individual variability in weight gain during each 14-d study period. Data points connected with a line reflect the change in body weight of the same individual during the 8.5-h (left) and 5.5-h (right) bedtime conditions. Triangles represent female and circles male subjects. Solid symbols: subjects who completed the 8.5-h bedtime intervention first; open symbols: subjects who started with the 5.5-h bedtime condition first. C: Mean (±SE) 24-h serum leptin concentrations before the start of the 8.5-h (open circles) and 5.5-h (solid circles) bedtime conditions (n = 11). D: Mean (±SE) 24-h leptin concentrations at the end of the 8.5-h (open circles) and 5.5-h (solid circles) bedtime conditions (n = 11). E: Mean (±SE) 24-h serum ghrelin concentrations before the start of the 8.5-h (open circles) and 5.5-h (solid circles) bedtime conditions (n = 9). F: Mean (±SE) 24-h ghrelin concentrations at the end of the 8.5-h (open circles) and 5.5-h (solid circles) bedtime conditions (n = 9).
TABLE 4.
Predictors of individual weight gain1
| Variable | β | 95% CI | P value |
| Initial study period | 0.86 | (0.21, 1.51) | 0.01 |
| MEI/RMR2 | 0.32 | (0.13, 0.52) | <0.01 |
| SEI/RMR2 | 0.24 | (−0.02, 0.50) | 0.07 |
| Physical activity level (TEE/RMR)2 | −0.17 | (−0.39, 0.05) | 0.12 |
| 5.5-h bedtime condition | −0.54 | (−1.26, 0.19) | 0.15 |
MEI, energy intake from meals; SEI, energy intake from snacks; TEE, total energy expenditure; RMR, resting metabolic rate. Results are from a generalized estimating equation model based on 11 subjects with 2 observations each (during the 5.5- and the 8.5-h bedtime conditions).
For a 0.1-unit increase in the ratio.
Metabolic hormones
Mean 24-h leptin concentrations before the 5.5- and 8.5-h bedtime conditions were 13.3 ± 10.3 and 13.0 ± 11.8 ng/mL, respectively (P = 0.76; Figure 2C). The results were similar when the respective baseline values were expressed relative to the initial adiposity of the participants: 0.487 ± 0.337 and 0.481 ± 0.373 ng · mL−1·kg body fat−1 (P = 0.66). Twenty-four-hour leptin concentrations increased in a similar fashion by 2.6 ± 3.7 and 3.1 ± 4.5 ng/mL at the end of the 5.5- and 8.5-h bedtime interventions (P = 0.66), and the corresponding mean concentrations (15.8 ± 12.3 and 16.1 ± 15.4 ng/mL; P = 0.83; Figure 2D) were similarly matched to the final adiposity of the subjects in both the presence and the absence of sleep loss (0.568 ± 0.404 and 0.577 ± 0.512 ng · mL−1 · kg body fat−1; P = 0.87). The 24-h concentrations of total ghrelin did not change significantly during the 5.5- or the 8.5-h bedtime intervention (P = 0.45) and remained comparable both before (1295 ± 339 and 1262 ± 409 pg/mL; P = 0.65; Figure 2E) and after (1242 ± 457 and 1311 ± 572 pg/mL; P = 0.53; Figure 2F) each respective study period.
DISCUSSION
The present study examined whether the curtailment of human sleep in an environment that promoted overeating and inactivity would be accompanied by an increased intake of energy from meals and snacks. Using a protocol of recurrent bedtime restriction, we were able to change the sleep duration of the study participants from >7 h/d, which in epidemiologic studies corresponds with the sleep category with lowest prevalence of excess adiposity, to <5.5 h/d, which falls in a category associated with an increased risk of obesity (5, 6). As intended, the physical activity levels of the participants during both sleep conditions were well within the range of sedentary humans (19). Our results show that recurrent bedtime restriction in the setting of ad libitum access to palatable food is accompanied by an increased consumption of excess calories from snacks without a statistically significant change in the intake of energy from meals.
The concomitant monitoring of energy expenditure and peripheral leptin and ghrelin concentrations allowed us to assess the role of several factors that have been hypothesized to link the lack of sufficient sleep with increased energy intake. Previous studies in rodents showed that partial sleep deprivation is associated with hyperphagia accompanied by markedly increased energy expenditure and weight loss (11–13). In contrast, the direct measurements of energy expenditure during the short sleep condition of our study indicate that the increased consumption of snacks by the subjects was not related to a comparable rise in their energy needs (Table 3). The differences in the resting metabolic rate and the thermic effect of food between the 2 sleep conditions of this experiment were small and were not significant. The existing studies of total sleep deprivation in humans further indicate that compared with relaxed wakefulness, a night of restful sleep saves relatively little energy (20, 21). The possibility that daily exposure to 3 extra hours of wakefulness may be accompanied by increased out-of-bed physical activity (22) also did not have a large effect on the energy budget of our sedentary subjects (Table 3). Overall, the small changes in energy expenditure during the 5.5-h bedtime condition were not sufficient to offset the observed rise in consumption of excess calories derived mostly from snacks (Table 3). Clearly, such changes in the energy balance of susceptible individuals could exacerbate their risk of weight gain and obesity (3); however, the apparent modest size of any such effect indicates that much larger and longer studies will be needed to define the effect of sleep restriction on these clinical endpoints.
Cross-sectional observations of lower leptin and higher ghrelin concentrations in persons with short sleep duration (8, 9) have led to the hypothesis that such hormone changes are likely to promote overeating and lead to an increased risk of obesity. However, a recent clinical trial provided conflicting results (23), raising the possibility that single measurements of leptin and ghrelin in epidemiologic studies (8, 9) may be influenced by systemic differences in the diurnal profiles of food intake and peripheral metabolic hormones in short sleepers (24, 25). The finding of increased energy intake from snacks, but not meals, and the lack of significant changes in ghrelin (26) during the short sleep condition of our study (Figure 2F) are also at odds with the existing hypothesis. Likewise, in the setting of ad libitum energy intake, we did not find differences in the 24-h leptin concentrations between the 2 bedtime conditions: final leptin concentrations increased along with the accumulation of a positive energy balance and reflected the adiposity of the subjects equally well irrespective of the presence or absence of sleep loss (Figure 2D). Two laboratory studies, designed to provide energy intake near weight maintenance levels, indicate that total sleep deprivation does not lower 24-h leptin concentrations (25, 27). In contrast, lower leptin, higher ghrelin, and increased hunger and appetite were found in lean men exposed to short-term sleep curtailment and mild caloric restriction in the form of intravenous glucose (10). When combined, these observations raise the possibility that acute sleep loss may amplify the human neuroendocrine response to caloric restriction and enhance the defense of affected individuals against disruptions in their food supply.
What, then, are the factors that underlie the increased intake of energy from snacks in our experiment? The results of the study support the concept that the control of human energy balance can be easily compromised by the propensity of many individuals to overeat in the setting of sedentary living with unlimited food availability (28, 29). Under these circumstances, the excessive consumption of calories from meals and snacks was augmented by the novelty of the experimental environment and emerged as an important predictor of individual weight gain (Table 4). These observations support the concept that nonhomeostatic factors could play a considerable role in determining human feeding behavior (30). Because bedtime curtailment resulted in more extended exposure (by 3 h/d) to palatable food, this factor may have contributed to the observed increase in energy consumption. Such possibility is supported by the larger 54% relative increase in snack intake during the nighttime period of the 5.5-h bedtime condition, which included most of the extra waking hours, compared with the 18% relative increase during the day, when the exposure to food between the 2 sleep conditions was more similar (Table 3). Although these changes may share some similarity with certain aspects of the night eating syndrome (31), our subjects exhibited a much more consolidated pattern of sleep during the 5.5-h compared with the 8.5-h bedtime intervention (Table 2) and did not wake up to eat during the periods of restricted sleep.
Finally, the increase in the carbohydrate content of ingested snacks during the 5.5-h bedtime condition suggests that sleep loss itself may have an effect on human energy intake. Enhanced carbohydrate consumption and preference for sweets have already been reported in psychologically demanding circumstances (32). More recently, the discovery of a new group of orexin-containing neurons in the hypothalamus has led to the description of an integrated network of pathways that regulate mammalian arousal, waking, and feeding behavior (33). These neurons respond to sleep loss and metabolic signals such as glucose and can modulate the control of reward and motivation (34). Some animal data already raise the possibility that changes in this system may contribute to the association between chronic metabolic disorders and insufficient sleep (35). Our findings indicate that alterations in the balance between sleep and wakefulness can modify the amount, composition, and distribution of human food intake and suggest that sleeping short hours in modern societies may aggravate the problem of excessive energy consumption.
In summary, bedtime restriction in an environment that promotes overeating and inactivity was accompanied by increased intake of calories from snacks with higher carbohydrate content without a statistically significant change in the consumption of energy from meals. In the absence of comparable changes in energy expenditure and differences in serum leptin and ghrelin, alternative mechanisms, such as more prolonged exposure to palatable food and sleep-loss-related changes in reward seeking and motivation, may underlie these changes in feeding behavior. The presence of considerable individual differences in the propensity to consume excess calories from snacks also raises the possibility that chronic bedtime curtailment may have more deleterious metabolic consequences in persons with such preexisting susceptibility. Although this hypothesis is consistent with epidemiologic reports showing an association of self-reported short sleep with more frequent snacking and increased risk of obesity (36, 37), our discussion is based on the detailed evaluation of a small number of subjects over a limited period of time in the laboratory. Additional studies will be needed to examine the effect of habitual sleep curtailment on human food intake and energy metabolism under free-living conditions.
Acknowledgments
The study involved >300 inpatient days in the laboratory of Eve Van Cauter at the University of Chicago. We gratefully acknowledge Eve Van Cauter's contribution to the conceptual design of the study and her comments on the results and an initial draft of this article. We thank our volunteers for their participation and the staff of the University of Chicago General Clinical Research Center, Sleep Research Laboratory, Endocrinology Clinic Bone Densitometry Facility, and Diabetes Research and Training Center for their skilled technical assistance.
The authors' responsibilities were as follows—PDP and DAS: study concept and design; AVN, JMK, JI, KK, DAS, and PDP: data acquisition, analysis, or interpretation; PDP: drafting of the manuscript; AVN, JMK, JI, KK, and DAS: critical revision of the text. The authors had no conflicts of interest related to this work.
REFERENCES
- 1.Friedman JM. Modern science versus the stigma of obesity. Nat Med 2004;10:563–9 [DOI] [PubMed] [Google Scholar]
- 2.Bouchard C. The biological predisposition to obesity: beyond the thrifty genotype scenario. Int J Obes 2007;31:1337–9 [DOI] [PubMed] [Google Scholar]
- 3.Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science 2003;299:853–5 [DOI] [PubMed] [Google Scholar]
- 4.Basner M, Fomberstein KM, Razavi FM, et al. American time use survey: sleep time and its relationship to waking activities. Sleep 2007;30:1085–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cappuccio FP, Taggart FM, Kandala NB, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep 2008;31:619–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;16:643–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stranges S, Cappuccio FP, Kandala NB, et al. Cross-sectional versus prospective associations of sleep duration with changes in relative weight and body fat distribution: the Whitehall II Study. Am J Epidemiol 2008;167:321–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taheri S, Lin E, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med 2004;1:e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaput JP, Despres JP, Bouchard C, Tremblay A. Short sleep duration is associated with reduced leptin levels and increased adiposity: results from the Quebec family study. Obesity (Silver Spring) 2007;15:253–61 [DOI] [PubMed] [Google Scholar]
- 10.Spiegel K, Tasali E, Penev P, Van Cauter E. Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 2004;141:846–50 [DOI] [PubMed] [Google Scholar]
- 11.Rechtschaffen A, Bergmann BM, Everson CA, Kushida CA, Gilliland MA. Sleep deprivation in the rat: X. Integration and discussion of the findings. 1989. Sleep 2002;25:68–87 [PubMed] [Google Scholar]
- 12.Everson CA, Crowley WR. Reductions in circulating anabolic hormones induced by sustained sleep deprivation in rats. Am J Physiol Endocrinol Metab 2004;286:E1060–70 [DOI] [PubMed] [Google Scholar]
- 13.Koban M, Swinson KL. Chronic REM-sleep deprivation of rats elevates metabolic rate and increases UCP1 gene expression in brown adipose tissue. Am J Physiol 2005;289:E68–74 [DOI] [PubMed] [Google Scholar]
- 14.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet 1999;354:1435–9 [DOI] [PubMed] [Google Scholar]
- 15.Schoeller DA, Ravussin E, Schutz Y, Acheson KJ, Baertschi P, Jequier E. Energy expenditure by doubly labeled water: validation in humans and proposed calculation. Am J Physiol 1986;250:R823–30 [DOI] [PubMed] [Google Scholar]
- 16.Schoeller DA, Hnilicka JM. Reliability of the doubly labeled water method for the measurement of total daily energy expenditure in free-living subjects. J Nutr 1996;126:348S–54S [PubMed] [Google Scholar]
- 17.Hills M, Armitage P. The two-period cross-over clinical trial. Br J Clin Pharmacol 1979;8:7–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986;42:121–30 [PubMed] [Google Scholar]
- 19.Hayes M, Chustek M, Heshka S, Wang Z, Pietrobelli A, Heymsfield SB. Low physical activity levels of modern Homo sapiens among free-ranging mammals. Int J Obes (Lond) 2005;29:151–6 [DOI] [PubMed] [Google Scholar]
- 20.Fontvieille AM, Rising R, Spraul M, Larson DE, Ravussin E. Relationship between sleep stages and metabolic rate in humans. Am J Physiol 1994;267:E732–7 [DOI] [PubMed] [Google Scholar]
- 21.Zhang K, Sun M, Werner P, et al. Sleeping metabolic rate in relation to body mass index and body composition. Int J Obes Relat Metab Disord 2002;26:376–83 [DOI] [PubMed] [Google Scholar]
- 22.Zepelin H, Rechtschaffen A. Mammalian sleep, longevity, and energy metabolism. Brain Behav Evol 1974;10:425–70 [DOI] [PubMed] [Google Scholar]
- 23.Littman AJ, Vitiello MV, Foster-Schubert K, et al. Sleep, ghrelin, leptin and changes in body weight during a 1-year moderate-intensity physical activity intervention. Int J Obes (Lond) 2007;31:466–75 [DOI] [PubMed] [Google Scholar]
- 24.Birketvedt GS, Florholmen J, Sundsfjord J, et al. Behavioral and neuroendocrine characteristics of the night-eating syndrome. JAMA 1999;282:657–63 [DOI] [PubMed] [Google Scholar]
- 25.Mullington JM, Chan JL, Van Dongen HP, et al. Sleep loss reduces diurnal rhythm amplitude of leptin in healthy men. J Neuroendocrinol 2003;15:851–4 [DOI] [PubMed] [Google Scholar]
- 26.Ravussin E, Tschop M, Morales S, Bouchard C, Heiman ML. Plasma ghrelin concentration and energy balance: overfeeding and negative energy balance studies in twins. J Clin Endocrinol Metab 2001;86:4547–51 [DOI] [PubMed] [Google Scholar]
- 27.Shea SA, Hilton MF, Orlova C, Ayers RT, Mantzoros CS. Independent circadian and sleep/wake regulation of adipokines and glucose in humans. J Clin Endocrinol Metab 2005;90:2537–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schoeller DA. Balancing energy expenditure and body weight. Am J Clin Nutr 1998;68:956S–61S [DOI] [PubMed] [Google Scholar]
- 29.Hill JO, Wyatt HR. Role of physical activity in preventing and treating obesity. J Appl Physiol 2005;99:765–70 [DOI] [PubMed] [Google Scholar]
- 30.Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron 2002;36:199–211 [DOI] [PubMed] [Google Scholar]
- 31.O'Reardon JP, Peshek A, Allison KC. Night eating syndrome: diagnosis, epidemiology and management. CNS Drugs 2005;19:997–1008 [DOI] [PubMed] [Google Scholar]
- 32.Christensen L. The effect of carbohydrates on affect. Nutrition 1997;13:503–14 [DOI] [PubMed] [Google Scholar]
- 33.Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci 2007;8:171–81 [DOI] [PubMed] [Google Scholar]
- 34.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature 2005;437:556–9 [DOI] [PubMed] [Google Scholar]
- 35.Horvath TL, Gao XB. Input organization and plasticity of hypocretin neurons: possible clues to obesity's association with insomnia. Cell Metab 2005;1:279–86 [DOI] [PubMed] [Google Scholar]
- 36.Imaki M, Hatanaka Y, Ogawa Y, Yoshida Y, Tanada S. An epidemiological study on relationship between the hours of sleep and life style factors in Japanese factory workers. J Physiol Anthropol Appl Human Sci 2002;21:115–20 [DOI] [PubMed] [Google Scholar]
- 37.Patel SR, Malhotra A, White DP, Gottlieb DJ, Hu FB. Association between reduced sleep and weight gain in women. Am J Epidemiol 2006;164:947–54 [DOI] [PMC free article] [PubMed] [Google Scholar]