Abstract

On exposure to dichlorodicyanoquinone in wet dichloromethane at room temperature equatorial 4-O-benzyl ethers are removed with moderate selectivity in the presence of other benzyl ethers in glycopyranosides and glycothiopyranosides.
Introduction
The p-methoxybenzyl (PMB) ethers and their somewhat less acid sensitive congeners, the 2-naphthylmethyl (Nap) ethers, are staple protecting groups in organic synthesis in general, and especially in oligosaccharide synthesis, owing to their facile oxidative cleavage with either dichlorodicyanoquinone (DDQ) or ceric ammonium nitrate (CAN).1–6 Simple benzyl ethers are not inert to these oxidative cleavage conditions but typically react significantly more slowly,5–16 enabling the highly selective cleavage of the PMB or Nap ethers in their presence.1,2 By way of illustration we have collated some recent examples of this type from our own laboratory,17–23 along with an oxidative cyclization,17 and a pertinent literature example1 in Table 1.
Table 1.
Examples of Selective Cleavage
| Entry | Structure | Transformation, % yield |
|---|---|---|
| 117 |

|
R = Nap → R = H, 85% |
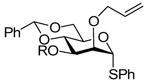
| 218 |
|
R = PMB → R = H, 93% |
| 318 |
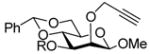
|
R = PMB → R = H, 95% |
| 419 |
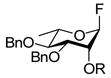
|
R = Nap → R = H, 54% |
| 520 |
|
R = PMB → R = H, 83% |
| 621 |

|
R = PMB → R = H, n = 0, 97%; n = 1, 91%; n = 2, 85%; n = 3, 80% n = 4, 85% |
| 722 |
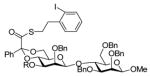
|
R = Nap → R = H, 83% |
| 823 |
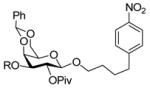
|
R = PMB → R = H, 85% |
| 917 |
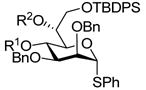
|
R1 = PMB, R2 = H → R1-R2 = PMPCH,a 85% |
| 101 |

|
R = Nap → R = H, 80% |
PMPCH = p-methoxybenzylidene
Accordingly, we were surprised when, in the course of an ongoing synthesis, we encountered significant cleavage of a benzyl ether when removing a vicinal Nap ether with DDQ (Table 2, entry 1). Intrigued by this unusual observation we carried out a more detailed investigation, the results of which are presented here.
Table 2.
Oxidative Cleavage Reactions with DDQ
| Entry | Substrate | Conditions | Products (% yield) |
|---|---|---|---|
| 1 |

4 |
0 °C, 30 min, rt, 3 h |
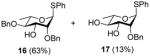
|
| 2 |
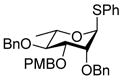
9 |
0 °C, 30 min, rt, 1.5 h |
|
| 3 |
16 |
0 °C, 30 min, rt, 5 h
0 °C, 30 min, 3 h 3 h (0.3 eq DDQ) |
|
| 4 |
18 |
0 °C, 30 min, rt, 5 h |

|
| 5 |
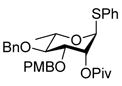
10 |
0 °C, 30 min, rt, 4 h |
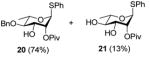
|
| 6 |
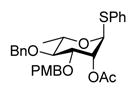
11 |
0 °C, 30 min, rt, overnight
0 °C, 30 min, rt, 4 h |

|
| 7 |
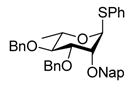
12 |
0 °C, 30 min, rt, 2.5 h |
|
| 8 |
15 |
0 °C, 30 min, rt, 2 h |
|
| 9 |

24 |
0 °C, 30 min, rt, 3 h
3 h (0.3 eq DDQ) |
|
| 10 |
26 |
0 °C, 30 min, rt, 3 h |
|
Results
Substrate Preparation
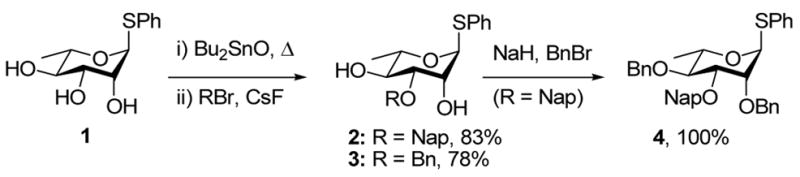
Treatment of triol 124 with dibutyltin oxide in toluene under Dean Stark conditions gave an intermediate stannylene acetal, which, on quenching with either 2-bromomethylnaphthalene or benzyl bromide in the presence of cesium fluoride, gave the 3-O-naphthylmethyl and 3-O-benzyl ethers 2 and 3, respectively. Exhaustive benzylation of 2 then gave the differentially protected substrate 4 (Scheme 1).
Scheme 1.
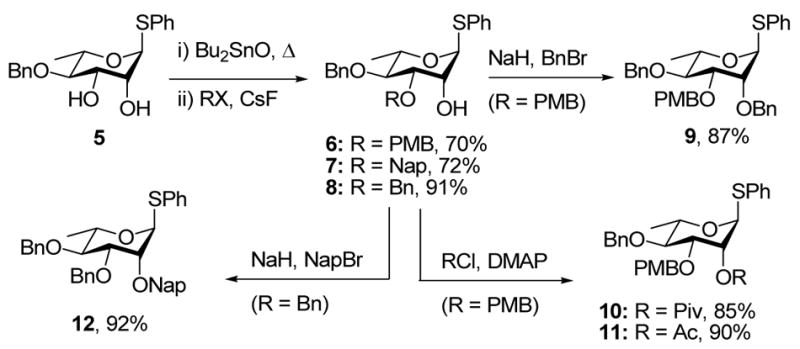
Reaction of the dibutylstannylene acetal derived from the known 4-O-benzyl ether 525 with p-methoxybenzyl chloride in the presence of cesium fluoride and tetrabutylammonium bromide afforded the 4-O-benzyl-3-O-p-methoxybenzyl ether 6, while reaction with naphthylmethyl bromide or benzyl bromide gave products 7 and 8,26 respectively (Scheme 2). The alcohol 6 was then converted to the fully protected systems 9, 10, and 11, under standard conditions, whereas reaction of the 3,4-di-O-benzyl ether 8 with naphthylmethyl bromide and sodium hydride provided 12 (Scheme 2).
Scheme 2.
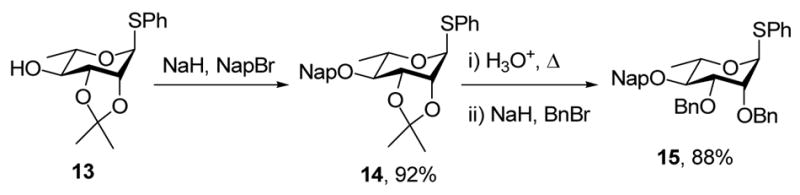
Naphthylmethylation of acetonide 1327 gave 14, which was heated to reflux in a mixture of acetic acid and aqueous dioxane followed, without isolation of the intermediate 2,3-diol, by exposure to sodium hydride and benzyl bromide to give the 2,3-di-O-benzyl-4-O-naphthylmethyl system 15 (Scheme 3).
Scheme 3.
Oxidative Cleavage Reactions
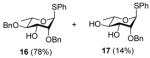
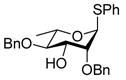
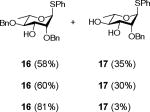
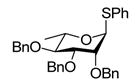
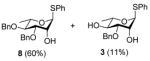
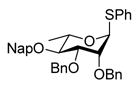
Exposure of the 2,4-di-O-benzyl-3-O-naphthylmethyl system 4 to DDQ in wet dichloromethane at 0 °C for 30 mins, followed by stirring at room temperature for 3 h gave 63% of the anticipated mono-ol 16, along with 13% of diol 1728 (Table 2, entry 1). Comparable results were obtained with the corresponding 2,4-di-O-benzyl-3-O-p-methoxybenzyl ether 9 (Table 2, entry 2). When the alcohol 16 was subjected to the standard reaction conditions, the diol 17 was again formed in amounts depending on the reaction conditions and stoichiometry employed (Table 2, entry 3). When the 2,3,4-tri-O-benzyl ether 1829 was similarly treated, a complex reaction mixture was obtained in which the product arising from selective removal of the 4-O-benzyl ether 19 predominated significantly (Table 2, entry 4). With the 2-O-pivalate ester 10, and the 2-O-acetate 11, the pattern was continued with cleavage of the 3-O-p-methoxybenzyl ether, accompanied by partial loss of the benzyl ether at the 4-O-position (Table 2, entries 5 and 6). Analysis of the initial reaction mixture from the reaction of 11 with DDQ was complicated by migration of the acetate group, but was facilitated by saponification. With the 3,4-di-O-benzyl-2-O-naphthylmethyl system 12, regioisomeric with the initial substrate 4, partial cleavage of the 4-O-benzyl ether was again observed (Table 2, entry 7). However, the trend did not extend to the third regioisomer in the series, the 2,3-di-O-benzyl-4-O-naphthylmethyl system 15, where the oxidative cleavage reaction was much cleaner and only minor amounts of debenzylated products were observed under the standard conditions (Table 2, entry 8).
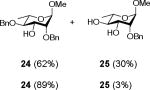
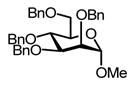
The methyl 2,4-di-O-benzyl rhamnopyranoside 2430 (Table 2, entry 9) performed much as the corresponding S-phenyl thioglycoside 16 (Table 2, entry 3) with considerable cleavage of the 4-O-benzyl ether depending on the stoichiometry employed. Finally, methyl 2,3,4,6-tetra-O-benzyl-α-D-mannopyranoside 2631 was explored as a substrate for the oxidative cleavage reaction. All four possible mono-ols were obtained from this reaction, but it is noteworthy that the 4-ol 2732 is by far the most significant product with a yield surpassing that 2-, 3-, and 6-ols combined (Table 2, entry 10).
Discussion
It is apparent from the entirety of results presented in Table 2 that the oxidative cleavage of benzyl ethers located on the 4-position of rhamno- and mannopyranosides are cleaved oxidatively with DDQ significantly more easily than benzyl ethers located at other positions around the ring. With hindsight the moderate yield recorded for the example of entry 4 of Table 1 can most likely be ascribed to the same phenomenon. From entry 3 of Table 2 it is clear that the oxidative cleavage of the 4-O-benzyl ether is an inherent character of that group and does not require the presence of the Nap or PMB ethers, that is the mechanism must involve direct oxidation of the benzyl ether rather than hole transfer from a more readily oxidized neighboring group.33,34 Likewise, entries 9 and 10 of Table 2 reveal that the thioglycoside is not the site of the initial oxidation step. Comparison of entries 9 and 10 of Table 2 indicates that the absence of a C6-O bond is not a requirement for the selective removal of the 4-O-benzyl ether.
While we have restricted our study to the rhamno- and mannopyranosyl systems of greatest interest to our laboratory, useful indications may also be gleaned from the literature. Thus, as set out in Table 1, entry 10, Matta and coworkers reported the selective cleavage of a 3-O-Nap ether in the presence of a 4-O-benzyl ether in the galactopyranose series,1 leading to the implication that an axial benzyloxy group at the 4-position of a pyranose is less readily oxidized than the equatorial counterpart. This postulate is reinforced by the apparent need for more forcing conditions: 30 equivalents of DDQ and 10 h at room temperature, to obtain a 70% yield in the cleavage of a 4-O-benzyl ether from an α-fucopyranoside noted in the course of a total synthesis of polycavernoside A.13 While we have not studied the glucopyranose series here, we do expect that selective cleavage of 4-O-benzyl ethers will be a feature of that system based on our results so far.
Overall, we are led to the conclusion that the selective cleavage of the 4-O-benzyl ether derives from the fixed antiperiplanar nature of the C4-O4 and C5-O1 bonds, which maximizes the electron-withdrawing properties of the latter, just as the disarming effect of a 4,6-O-alkylidene acetals in glycosylation reactions is attributed to the enforcement of the more electron-withdrawing tg conformation.35,36 Selective protection strategies are key features of the enormous majority of work in the carbohydrate and oligosaccharide fields,37–43 and it is possible that with optimization perbenzylation and selective debenzylation at O4 might provide a short, cost-effective entry into the 4-OH systems.
Experimental Section
General procedure for the deprotection with DDQ
To a solution of the substrate (0.03 M) in a mixture of CH2Cl2/H2O (17/1), DDQ (2.3 eq or 0.3 eq) was added at 0 °C. After 30 min, the reaction mixture was warmed to room temperature and further stirred for the specified time (Table 2) before it was diluted with CH2Cl2 and quenched with sat. aq. Na2CO3. The organic phase was separated, washed (sat. aq. Na2CO3), dried (Na2SO4), and concentrated. The crude reaction mixture was filtered through a pad of silica gel (with ethyl acetate as an eluent) and then purified by chromatography on SiO2.
Reaction of S-Phenyl 2,4-di-O-benzyl-3-O-(2-naphthylmethyl)-α-L-thiorhamnopyranoside (4) with DDQ
Compound 4 (737 mg, 1.28 mmol) was deprotected with 2.3 eq of DDQ (667 mg, 2.9 mmol) for 3 h. Purification by radial chromatography (SiO2, hexanes to 2:3 ethyl acetate:hexanes) gave 16 (351 mg, 63%), and 17 (59 mg, 13%).
Reaction of S-Phenyl 2,4-di-O-benzyl-3-O-(4-methoxybenzyl)-α-L-thiorhamnopyranoside (9) with DDQ
Compound 9 (88 mg, 0.16 mmol) was deprotected with 2.3 eq of DDQ (83 mg, 0.365 mmol) for 1.5 h. Purification by radial chromatography (SiO2, hexanes to 2:3 ethyl acetate:hexanes) gave 16 (54 mg, 78%), and 17 (8 mg, 14%).
Reaction of S-Phenyl 2,4-di-O-benzyl-α-L-thiorhamnopyranoside (16) with DDQ
Compound 16 (64 mg, 0.15 mmol) was treated with 2.3 eq of DDQ (77 mg, 0.34 mmol) for 5 h. Radial chromatography (SiO2, hexanes to 2:3 ethyl acetate:hexanes) gave unreacted 16 (37 mg, 58%), and 17 (18 mg, 35%). When 16 (27 mg, 0.06 mmol) was treated for 3 h with 2.3 eq of DDQ (32 mg, 0.14 mmol), 16 (16 mg, 60%), and 17 (6 mg, 30%) were obtained. With 16 (29 mg, 0.07 mmol) and 0.3 eq DDQ (5 mg, 0.14 mmol) for 3 h, 16 (23 mg, 81%), and 17 (0.7 mg, 3%) were obtained.
Reaction of 2,3,4-Tri-O-benzyl-α-L-thiorhamnopyranoside (18) with DDQ
Compound 1829 (117 mg, 0.22 mmol) was treated with 2.3 eq of DDQ (83 mg, 0.365 mmol) for 5 h. Radial chromatography (SiO2, hexanes to 3:7 ethyl acetate:hexanes) gave unreacted 18 (32 mg, 28%), 16 (6 mg, 6%), 19 (31 mg, 32%), a mixture of 19 and 8 (12 mg, 12%, 1H NMR: 19/8 = 2/1), 3 (3 mg, 4%), and 17 (3 mg, 4%).
Reaction of S-Phenyl 4-O-benzyl-3-O-(4-methoxybenzyl)-2-O-pivaloyl-α-L-thiorhamnopyranoside (10) with DDQ
Compound 10 (96 mg, 0.17 mmol) was deprotected with 2.3 eq of DDQ (91 mg, 0.40 mmol) for 4 h. Radial chromatography (SiO2, hexanes to 3:7 ethyl acetate:hexanes) gave 20 (55 mg, 74%), and 21 (8 mg, 13%).
Reaction of S-Phenyl 2-O-acetyl 4-O-benzyl-3-O-(4-methoxybenzyl)-α-L-thiorhamnopyranoside (11) with DDQ
Compound 11 (50 mg, 0.10 mmol) was deprotected with 2.3 eq of DDQ (91 mg, 0.40 mmol) overnight. Radial chromatography (SiO2, hexanes to 1:1 ethyl acetate:hexanes) gave a mixture of S-phenyl 3-O-acetyl-4-O-benzyl-α-L-thiorhamnopyranoside and S-phenyl 2-O-acetyl-4-O-benzyl-αL-thiorhamnopyranoside (26.2 mg, 69%), and a mixture of S-phenyl 3-O-acetyl-α-L-thiorhamnopyranoside and S-phenyl 2-O-acetyl-α-L-thiorhamnopyranoside (9 mg, 30%). The first mixture (26 mg, 0.07 mmol) was dissolved in 1/1 MeOH/Et2O mixture (2 mL) and 1.0 M NaOMe in MeOH (14 μL, 0.014 mmol) was added. The reaction mixture was stirred for 1 h and then neutralized with Amberlyst 15® ion exchange resin until pH 6, filtered, and concentrated to give pure 5 (23 mg, quant.). The second mixture (9 g, 0.03 mmol) was treated analogously and pure 1 (8 mg, quant.) was obtained. When 11 (110 mg, 0.22 mmol) was deprotected with 2.3 eq of DDQ (113 mg, 0.50 mmol) for 4 h, unreacted 11 (2 mg, 2%), a mixture of S-phenyl 3-O-acetyl-4-O-benzyl-α-L-thiorhamnoside and S-phenyl 2-O-acetyl-4-O-benzyl-α-L-thiorhamnoside (69 mg, 82%), and mixture of S-phenyl 3-O-acetyl-α-L-thiorhamnoside and S-phenyl 2-O-acetyl-α-L-thiorhamnoside (8 g, 12%) were obtained. These mixtures were deacetylated as described above to provide 5 (61 mg, quant.), and 11 (7 mg, quant).
Reaction of S-Phenyl 3,4-di-O-benzyl-2-O-(2-naphthylmethyl)-α-L-thiorhamnopyranoside (12) with DDQ
Compound 12 (69 mg, 0.12 mmol) was deprotected with 2.3 eq of DDQ (62 mg, 0.27 mmol) for 2.5 h. Radial chromatography (SiO2, hexanes to 3:7 ethyl acetate:hexanes) gave 8 (41 mg, 60%), and 3 (5 mg, 11%).
Reaction of S-Phenyl 2,3-di-O-benzyl-4-O-(2-naphthylmethyl)-α-L-thiorhamnopyranoside (15) with DDQ
Compound 15 (104 mg, 0.18 mmol) was deprotected with 2.3 eq of DDQ (94 mg, 0.42 mmol) for 2 h. Radial chromatography (SiO2, hexanes to 2:3 ethyl acetate:hexanes) gave 19 (67 mg, 85%), 3 (4 mg, 6%), and 17 (2 mg, 3%).
Reaction of Methyl 2,4-di-O-benzyl-α-L-rhamnopyranoside (24) with DDQ
Compound 2430 (50 mg, 0.1 mmol) was treated with 2.3 eq of DDQ (51 mg, 0.022 mmol) for 3 h. Radial chromatography (SiO2, hexanes to 1:1 ethyl acetate:hexanes) gave unreacted 24 (33 mg, 62%), and 25 (12 mg, 30%).
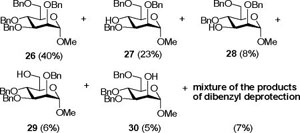
Reaction of Methyl 2,3,4,6-tetra-O-benzyl-α-D-mannopyranoside (26) with DDQ
Compound 2631 (131 mg, 0.24 mmol) was treated with 2.3 eq of DDQ (123 mg, 0.54 mmol) for 3 h. Radial chromatography (SiO2, hexanes to 1:1 ethyl acetate:hexanes) gave unreacted 26 (52 mg, 40%), a mixture of 27 and 28 (34 mg, 31%, 27/28 = 2.8/1), a mixture of 29 and 30 (12 mg, 11%, 29/30 = 1.1/1), and an inseparable mixture of more polar products of from cleavage of two benzyl ethers (6 mg, 7%).
S-Phenyl 2,4-di-O-benzyl-α-L-thiorhamnopyranoside (16)
White solid. M.p. 74–75 °C. [α]24D -108 (c 0.7, CHCl3). 1H NMR δ: 7.46 – 7.51 (m, 2H), 7.28 – 7.45 (m, 13H), 5.61 (d, J = 0.9 Hz, 1H), 4.97 (d, J = 11.0 Hz, 1H), 4.78 (d, J = 11.7 Hz, 1H), 4.72 (d, J = 11.0 Hz, 1H), 4.58 (d, J = 11.7 Hz, 1H), 4.18 – 4.26 (m, 1H), 3.98 – 4.07 (m, 2H), 3.46 (t, J = 9.1 Hz, 1H), 2.51 (d, J = 8.8 Hz, 1H), 1.40 (d, J = 6.2 Hz, 3H); 13C NMR δ: 138.5, 137.4, 134.5, 131.6, 129.1, 128.7, 128.5, 128.2, 128.1, 128.0, 127.8, 127.5, 85.0, 82.5, 80.1, 75.2, 72.5, 72.2, 68.7, 18.0; FABHRMS Calcd for C26H28NaO4S [M+Na]+: 459.1606. Found 459.1613.
S-Phenyl 2-O-benzyl-α-L-thiorhamnopyranoside (17)
This compound had spectral data in accordance with that reported in literature.28
S-Phenyl 2,3-di-O-benzyl-α-L-thiorhamnopyranoside (19)
[α]20D -12 (c 0.4, CHCl3). 1H NMR δ: 7.27 – 7.47 (m, 15H), 5.58 (d, J = 1.3 Hz, 1H), 4.71 (d, J = 12.1 Hz, 1H), 4.53 – 4.60 (m, 2H), 4.45 (d, J = 11.7 Hz, 1H), 4.10 – 4.17 (m, 1H), 4.04 (dd, J = 2.9, 1.5 Hz, 1H), 3.82 (t, J = 9.2 Hz, 1H), 3.66 (dd, J = 9.4, 3.0 Hz, 1H), 2.42 (d, J = 1.5 Hz, 1H), 1.37 (d, J = 6.2 Hz, 3H); 13C NMR δ: 137.8, 134.7, 131.4, 129.1, 128.7, 128.5, 128.1, 128.0, 127.9, 127.4, 85.8, 79.7, 75.7, 72.1, 71.9, 71.6, 69.7, 17.8; ESIHRMS Calcd for C26H28NaO4S [M+Na]+: 459.1606. Found 459.1613.
S-Phenyl 4-O-benzyl-2-O-pivaloyl-α-L-thiorhamnopyranoside (20)
White solid. M.p. 82–83 °C. [α]24D -125 (c 1, CHCl3). 1H NMR δ: 7.46 – 7.49 (m, 2H), 7.25 – 7.41 (m, 8H), 5.37 (s, 1H), 5.35 (dd, J = 3.1, 1.5 Hz, 1H), 4.83 (d, J = 11.4 Hz, 1H), 4.77 (d, J = 11.2 Hz, 1H), 4.22 – 4.29 (m, 1H), 4.08 – 4.13 (m, 1H), 3.40 (t, J = 9.4 Hz, 1H), 2.18 (d, J = 4.8 Hz, 1H), 1.37 (d, J = 6.2 Hz, 3H), 1.25 (s, 9H); 13C NMR δ: 178.0, 138.1, 133.9, 132.1, 129.1, 128.7, 128.2, 128.1, 127.8, 86.0, 81.6, 75.2, 73.9, 71.0, 68.7, 39.2, 27.2, 18.1; ESIHRMS Calcd for C24H30NaO5S [M+Na]+: 453.1712. Found 453.1711.
S-Phenyl 2-O-pivaloyl-α-L-thiorhamnopyranoside (21)
[α]24D - 108 (c 0.2, CHCl3). 1H NMR δ: 7.44 – 7.51 (m, 2H), 7.26 – 7.35 (m, 3H), 5.39 (d, J = 1.3 Hz, 1H), 5.33 (dd, J = 3.3, 1.5 Hz, 1H), 4.17 – 4.24 (m, 1H), 3.95 – 3.99 (m, 1H), 3.54 (t, J = 9.4 Hz, 1H), 2.43 (br. s., 1H), 2.29 (br. s., 1H), 1.35 (d, J = 6.2 Hz, 3H), 1.23 (s, 9H); 13C NMR δ: 178.3, 133.7, 132.1, 129.2, 127.8, 86.1, 74.0, 73.7, 71.3, 69.1, 39.2, 27.2, 17.6; ESIHRMS Calcd for C17H24NaO5S [M+Na]+: 363.1242. Found 363.1238.
Methyl 2-O-benzyl-α-L-rhamnopyranoside (25),44,45 Methyl 2,3,6-tri-O-benzyl-α-D-mannopyranoside (27),46 Methyl 2,4,6-tri-O-benzyl-α-D-mannopyranoside (28),47 Methyl 2,3,4-triO-benzyl-α-D-mannopyranoside (29),48 and Methyl 3,4,6-tri-O-benzyl-α-D-mannopyranoside (30)49 all had spectral data consistent with the literature.
Supplementary Material
Full experimental details for substrate preparation and copies of spectra of all compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
We thank the NIH (GM62160) for support of this work, and an anonymous reviewer for insight into the origin of the phenomenon.
References
- 1.Xia J, Abbas SA, Locke RD, Piskorz CFLAJ, Matta KL. Tetrahedron Lett. 2000;41:169–173. [Google Scholar]
- 2.Liao WDLR, Matta KL. Chem Commun. 2000:369–370. [Google Scholar]
- 3.Wuts PGM. In: Handbook of Reagents for Organic Synthesis: Reagents for Glycoside, Nucleotide, and Peptide Synthesis. Crich D, editor. Wiley; Chichester: 2005. pp. 425–428. [Google Scholar]
- 4.Csávás M, Szabó ZB, Borbás A, Lipták A. In: Handbook of Reagents for Organic Synthesis: Reagents for Glycoside, Nucleotide, and Peptide Synthesis. Crich D, editor. Wiley; Chichester: 2005. p. 459.p. 460. [Google Scholar]
- 5.Greene TW, Wuts PGM. Protective Groups in Organic Synthesis. 3. Wiley; New York: 1999. [Google Scholar]
- 6.Kocienski PJ. Protecting Groups. 3. Thieme; Stuttgart: 2005. [Google Scholar]
- 7.Tanaka T, Oikawa Y, Nakajima N, Hamada T, Yonemitsu O. Chem Pharm Bull. 1987;35:2203–2208. [Google Scholar]
- 8.Horita K, Nagato S, Oikawa Y, Yonemitsu O. Tetrahedron Lett. 1987;28:3253–3256. [Google Scholar]
- 9.Sviridov AF, Ermolenko MS, Yashunsky DV, Borodkin VS, Kochetkov NK. Tetrahedron Lett. 1987;28:3839–3842. [Google Scholar]
- 10.Vedejs E, Buchanan RA, Watanabe Y. J Am Chem Soc. 1989;111:8430–8438. [Google Scholar]
- 11.Ikemoto N, Schreiber SL. J Am Chem Soc. 1992;114:2524–2536. [Google Scholar]
- 12.Meng D, Bertinato P, Balog A, Su DS, Kamenecka T, Sorensen EJ, Danishefsky SJ. J Am Chem Soc. 1997;111:10073–10092. [Google Scholar]
- 13.Fujiwara K, Murai AMY-Y, Yasumoto T. J Am Chem Soc. 1998;120:10770–10771. [Google Scholar]
- 14.Crimmins MT, Choy AL. J Am Chem Soc. 1999;121:5653–5660. [Google Scholar]
- 15.Zhu B, Panek JS. Org Lett. 2000;2:2575–2578. doi: 10.1021/ol006104w. [DOI] [PubMed] [Google Scholar]
- 16.Crimmins MT, Emmitte KA. J Am Chem Soc. 2001;123:1533–1534. doi: 10.1021/ja005892h. [DOI] [PubMed] [Google Scholar]
- 17.Crich D, Banerjee A. J Am Chem Soc. 2006;128:8078–8086. doi: 10.1021/ja061594u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crich D, Jayalath P, Hutton TK. J Org Chem. 2006;71:3064–3070. doi: 10.1021/jo0526789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crich D, Hutton TK, Banerjee A, Jayalath P, Picione J. Tetrahedron: Asymmetry. 2005;16:105–119. [Google Scholar]
- 20.Crich D, Li W, Li H. J Am Chem Soc. 2004;126:15081–15086. doi: 10.1021/ja0471931. [DOI] [PubMed] [Google Scholar]
- 21.Crich D, Banerjee A, Yao Q. J Am Chem Soc. 2004;126:14930–14934. doi: 10.1021/ja047194t. [DOI] [PubMed] [Google Scholar]
- 22.Crich D, Yao Q. J Am Chem Soc. 2004;126:8232–8236. doi: 10.1021/ja048070j. [DOI] [PubMed] [Google Scholar]
- 23.Crich D, Li H. J Org Chem. 2002;67:4640–4646. doi: 10.1021/jo0108818. [DOI] [PubMed] [Google Scholar]
- 24.Groneberg RD, Miyazaki T, Stylianides NA, Schulze TJ, Stahl W, Schreiner EP, Suzuki T, Iwabuchi Y, Smith AL, Nicolaou KC. J Am Chem Soc. 1993;115:7593–7611. [Google Scholar]
- 25.Pozsgay V. J Org Chem. 1998;63:5983–5999. doi: 10.1021/jo980660a. [DOI] [PubMed] [Google Scholar]
- 26.Lau R, Schüle G, Schwaneberg U, Ziegler T. Liebigs. 1995:1745–1754. [Google Scholar]
- 27.Pozsgay V. Carbohydr Res. 1992;235:295–302. doi: 10.1016/0008-6215(92)80098-l. [DOI] [PubMed] [Google Scholar]
- 28.Maloney DJ, Hecht SM. Org Lett. 2005;7:1097–1099. doi: 10.1021/ol0500463. [DOI] [PubMed] [Google Scholar]
- 29.Furukawa JI, Kobayashi S, Nomizu M, Nishi N, Sakairi N. Tetrahedron Lett. 2000;41:3453–3457. [Google Scholar]
- 30.Pozsgay V. Carbohydr Res. 1979;69:284–286. [Google Scholar]
- 31.Girard C, Miramon ML, de Solminihac T, Herscovici J. Carbohydr Res. 2002;337:1769–1774. doi: 10.1016/s0008-6215(02)00206-9. [DOI] [PubMed] [Google Scholar]
- 32.Schie CR, Tzeng ZH, Kulkarni SS, Uang BJ, Hsu CY, Hung SC. Angew Chem Int Ed. 2005;44:1665–1668. doi: 10.1002/anie.200462172. [DOI] [PubMed] [Google Scholar]
- 33.For examples of hole (radical cation) transfer and delocalization and its consequences see the extensive work on the oxidative cleavage of DNA see Wagenknecht H-A, editor. Charge Transfer in DNA; From Mechanism to Application. Wiley-VCH; Weinheim: 2005. and footnote 34.
- 34.Giese B. Top Curr Chem. 2004;236:27–44. [Google Scholar]
- 35.Jensen HH, Nordstrom M, Bols M. J Am Chem Soc. 2004;126:9205–9213. doi: 10.1021/ja047578j. [DOI] [PubMed] [Google Scholar]
- 36.Jensen HH, Bols M. Acc Chem Res. 2006;39:259–265. doi: 10.1021/ar050189p. [DOI] [PubMed] [Google Scholar]
- 37.Grindley TB. In: Modern Methods in Carbohydrate Chemistry. Khan SH, O’Neill RA, editors. Harwood Academic; Amsterdam: 1996. pp. 225–250. [Google Scholar]
- 38.Green LG, Ley SV. In: Carbohydrates in Chemistry and Biology. Ernst B, Hart GW, Sinaÿ P, editors. Vol. 1. Wiley-VCH; Weinheim: 2000. pp. 427–448. [Google Scholar]
- 39.Bols M. Carbohydrate Building Blocks. Wiley; New York: 1996. [Google Scholar]
- 40.Crich D, editor. Handbook of Reagents for Organic Synthesis: Reagents for Glycoside, Nucleotide, and Peptide Synthesis. Wiley; Chichester: 2005. [Google Scholar]
- 41.Garegg PJ. In: Preparative Carbohydrate Chemistry. Hanessian S, editor. Dekker; New York: 1993. pp. 53–67. [Google Scholar]
- 42.Calinaud P, Gelas J. In: Preparative Carbohydrate Chemistry. Hanessian S, editor. Dekker; New York: 1997. pp. 3–33. [Google Scholar]
- 43.David S. In: Preparative Carbohydrate Chemistry. Hanessian S, editor. Dekker; New York: 1997. pp. 69–83. [Google Scholar]
- 44.Toman R, Rosik J, Zikmund M. Carbohydr Res. 1982;103:165–169. [Google Scholar]
- 45.Liptak A, Nanasi P, Neszmelyi A, Wagner H. Tetrahedron. 1980;36:1261–1268. [Google Scholar]
- 46.Shie CR, Tzeng ZH, Kulkarni SS, Uang BJ, Hsu CY, Hung SC. Angew Chem Int Ed. 2005;44:1665–1668. doi: 10.1002/anie.200462172. [DOI] [PubMed] [Google Scholar]
- 47.Cappa A, Marcantoni E, Torregiani E, Bartoli G, Bellucci MC, Bosco M, Sambri L. J Org Chem. 1999;64:5696–5699. doi: 10.1021/jo990014r. [DOI] [PubMed] [Google Scholar]
- 48.Belakhov V, Dovgolevsky E, Rabkin E, Shulami S, Shoham Y, Baasov T. Carbohydr Res. 2004;339:385–392. doi: 10.1016/j.carres.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 49.Marzabadi CH, Spilling CD. J Org Chem. 1993;58:3761–3766. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Full experimental details for substrate preparation and copies of spectra of all compounds. This material is available free of charge via the Internet at http://pubs.acs.org.


