Abstract
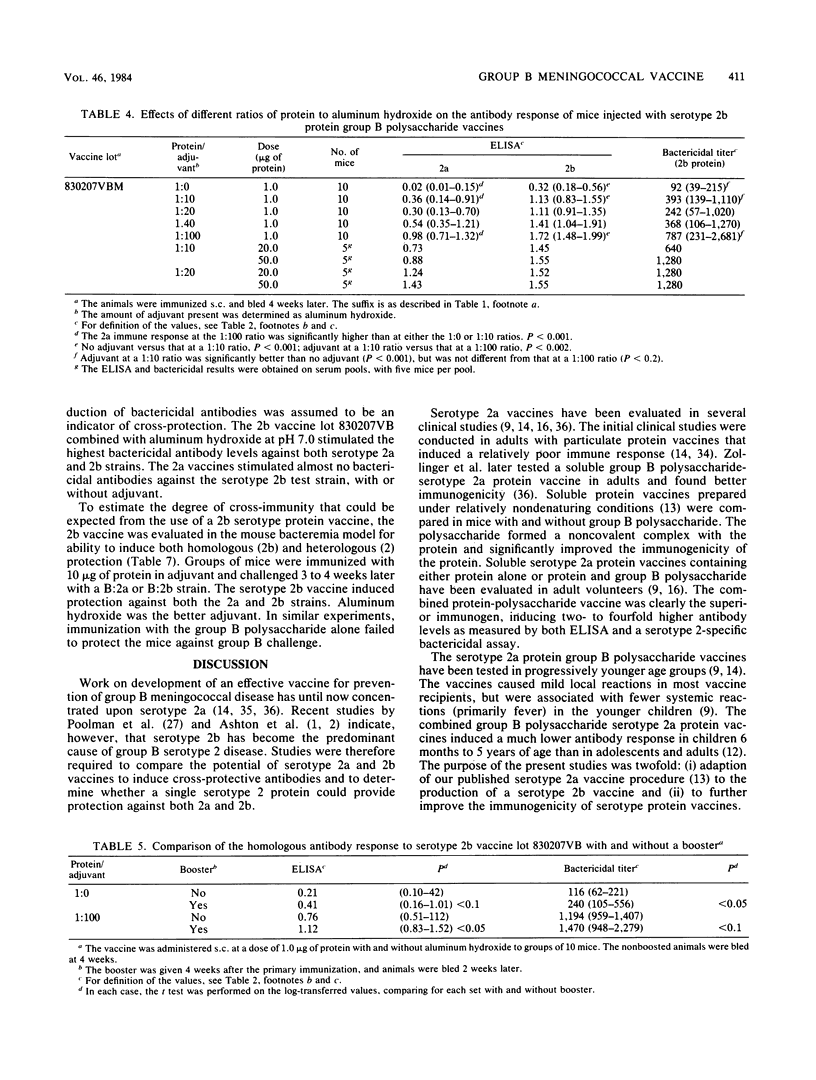
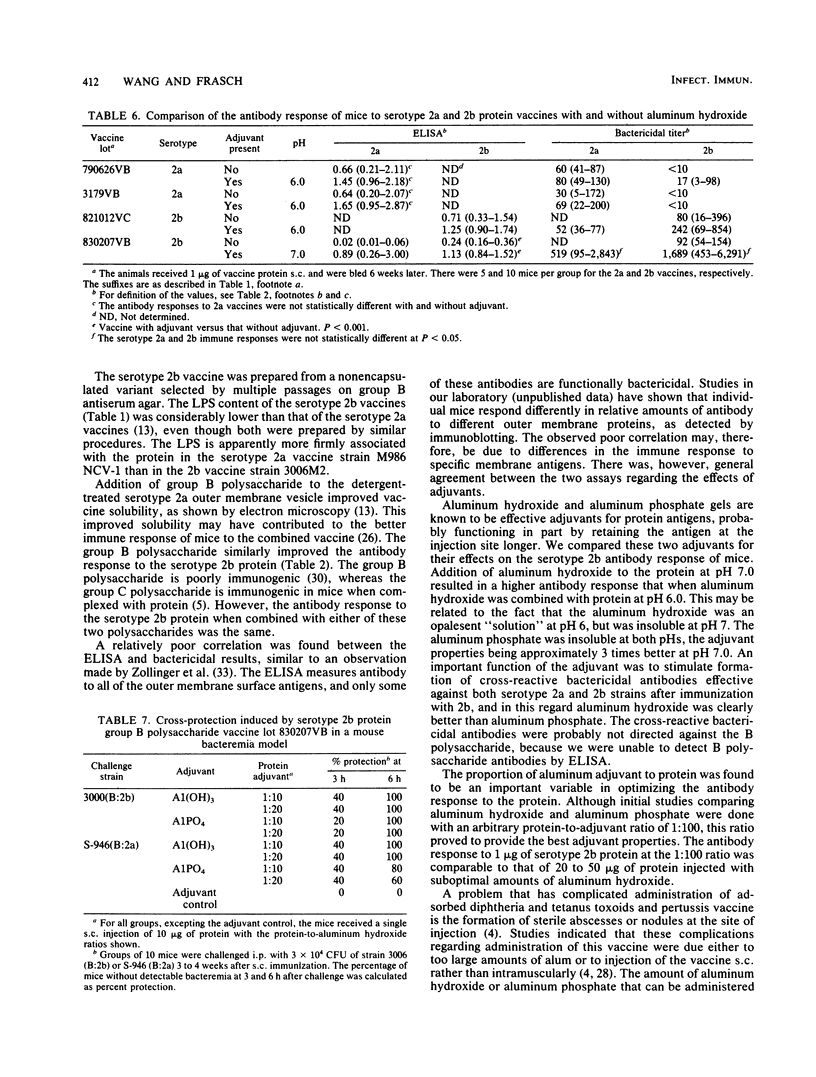
Although serotype 2 remains the predominant cause of group B Neisseria meningitidis disease in many parts of the world, most cases of this disease are now due to serotype 2b rather than 2a. For this reason, we adapted the serotype 2a vaccine method of C. E. Frasch and M. S. Peppler (Infect. Immun. 37:271-280, 1982) to the production of a serotype 2b protein vaccine. A spontaneously occurring nonencapsulated mutant of the group B serotype 2b strain 3006 was obtained by selection on group B antiserum agar. Serotype 2b outer membrane protein vaccines were prepared with less than 1% lipoplysaccharide contamination. The immunogenicity of these vaccines was evaluated in mice in the presence and absence of meningococcal group B and group C capsular polysaccharides. The group B and group C polysaccharides equally potentiated the antibody response to the serotype 2b protein. Addition of aluminum hydroxide or aluminum phosphate markedly improved the antibody response to the serotype 2b protein, but aluminum hydroxide-adjuvanted vaccines consistently elicited higher antibody levels. Aluminum hydroxide-adsorbed serotype 2a and 2b protein vaccines were evaluated for induction of cross-protective bactericidal antibodies. The 2a vaccines were 2a specific, whereas the 2b vaccines elicited antibodies strongly bactericidal for both 2a and 2b meningococcal strains and protected against bacteremia in a mouse model. It may therefore be possible to provide protection against both 2a and 2b disease by using an aluminum hydroxide-adsorbed protein vaccine containing a single serotype 2 protein component.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashton F. E., Ryan A., Diena B. B., MacKenzie A. M., Chan F. Serotypes among Neisseria meningitidis serogroups B and C strains isolated in Canada. Can J Microbiol. 1980 Dec;26(12):1480–1488. doi: 10.1139/m80-245. [DOI] [PubMed] [Google Scholar]
- Ashton F. E., Ryan J. A., Jones C., Brodeur B. R., Diena B. B. Serotypes of Neisseria meningitidis associated with an increased incidence of meningitis cases in the Hamilton area, Ontario, during 1978 and 1979. Can J Microbiol. 1983 Jan;29(1):129–136. doi: 10.1139/m83-020. [DOI] [PubMed] [Google Scholar]
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Bernier R. H., Frank J. A., Jr, Nolan T. F., Jr Abscesses complicating DTP vaccination. Am J Dis Child. 1981 Sep;135(9):826–828. doi: 10.1001/archpedi.1981.02130330036011. [DOI] [PubMed] [Google Scholar]
- Beuvery E. C., Miedema F., van Delft R. W., Haverkamp J., Leussink A. B., te Pas B. J., Teppema K. S., Tiesjema R. H. Preparation and physicochemical and immunological characterization of polysaccharide-outer membrane protein complexes of Neisseria meningitidis. Infect Immun. 1983 Apr;40(1):369–380. doi: 10.1128/iai.40.1.369-380.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven D. E., Frasch C. E. Protection against group B meningococcal disease: evaluation of serotype 2 protein vaccines in a mouse bacteremia model. Infect Immun. 1979 Oct;26(1):110–117. doi: 10.1128/iai.26.1.110-117.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Maeyer S., Seba J. M., Reginster G. Epidemiology of meningococcal meningitis in Belgium. J Infect. 1981 Mar;3(1 Suppl):63–70. doi: 10.1016/s0163-4453(81)80010-2. [DOI] [PubMed] [Google Scholar]
- Frasch C. E., Chapman S. S. Classification of Neisseria meningitidis group B into distinct serotypes. I. Serological typing by a microbactericidal method. Infect Immun. 1972 Jan;5(1):98–102. doi: 10.1128/iai.5.1.98-102.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch C. E., Chapman S. S. Classification of Neisseria meningitidis group B into distinct serotypes. II. Extraction of type-specific antigens for serotyping by precipitin techniques. Infect Immun. 1972 Aug;6(2):127–133. doi: 10.1128/iai.6.2.127-133.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch C. E., Peppler M. S. Protection against group B Neisseria meningitidis disease: preparation of soluble protein and protein-polysaccharide immunogens. Infect Immun. 1982 Jul;37(1):271–280. doi: 10.1128/iai.37.1.271-280.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch C. E., Robbins J. D. Protection against group B meningococcal disease. III. Immunogenicity of serotype 2 vaccines and specificity of protection in a guinea pig model. J Exp Med. 1978 Mar 1;147(3):629–644. doi: 10.1084/jem.147.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frøholm L. O., Berdal B. P., Bøvre K., Gaustad P., Harboe A., Holten E., Høiby E. A., Lystad A., Omland T., Frasch C. E. Meningococcal group B vaccine trial in Norway 1981--1982. Preliminary report of results available November 1982. NIPH Ann. 1983 Dec;6(2):133–138. [PubMed] [Google Scholar]
- Gold R., Artenstein M. S. Meningococcal infections. 2. Field trial of group C meningococcal polysaccharide vaccine in 1969-70. Bull World Health Organ. 1971;45(3):279–282. [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C., Liu T. Y., Artenstein M. S. Human immunity to the meningococcus. 3. Preparation and immunochemical properties of the group A, group B, and group C meningococcal polysaccharides. J Exp Med. 1969 Jun 1;129(6):1349–1365. doi: 10.1084/jem.129.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankins W. A., Gwaltney J. M., Jr, Hendley J. O., Farquhar J. D., Samuelson J. S. Clinical and serological evaluation of a meningococcal polysaccharide vaccine groups A, C, Y, and W135. Proc Soc Exp Biol Med. 1982 Jan;169(1):54–57. doi: 10.3181/00379727-169-41306. [DOI] [PubMed] [Google Scholar]
- Holten E. Serotypes of Neisseria meningitidis isolated from patients in Norway during the first six months of 1978. J Clin Microbiol. 1979 Feb;9(2):186–188. doi: 10.1128/jcm.9.2.186-188.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K., Kinoshita T., Kitajima H., Inoue K. Inhibitory effect of K-76 monocarboxylic acid, an anticomplementary agent, on the C3b inactivator system. J Immunol. 1981 Jul;127(1):104–108. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leinonen M., Frasch C. E. Class-specific antibody response to group B Neisseria meningitidis capsular polysaccharide: use of polylysine precoating in an enzyme-linked immunosorbent assay. Infect Immun. 1982 Dec;38(3):1203–1207. doi: 10.1128/iai.38.3.1203-1207.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocca L. F., del Real G., Frasch C. E. Serotypes and polyacrylamide gel electrophoresis types among disease-associated isolates of group B Neisseria meningitidis in Spain, 1976-1979. J Infect Dis. 1983 Aug;148(2):249–253. doi: 10.1093/infdis/148.2.249. [DOI] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppler M. S., Frasch C. E. Protection against group B Neisseria meningitidis disease: effect of serogroup B polysaccharide and polymyxin B on immunogenicity of serotype protein preparations. Infect Immun. 1982 Jul;37(1):264–270. doi: 10.1128/iai.37.1.264-270.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman J. T., Hopman C. T., Zanen H. C. Immunochemical characterization of Neisseria meningitidis serotype antigens by immunodiffusion and SDS-polyacrylamide gel electrophoresis immunoperoxidase techniques and the distribution of serotypes among cases and carriers. J Gen Microbiol. 1980 Feb;116(2):465–473. doi: 10.1099/00221287-116-2-465. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. Chemical analysis of major outer membrane proteins of Neisseria meningitidis: comparison of serotypes 2 and 11. J Bacteriol. 1980 Jan;141(1):169–176. doi: 10.1128/jb.141.1.169-176.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyle F. A., Artenstein M. S., Brandt B. L., Tramont E. C., Kasper D. L., Altieri P. L., Berman S. L., Lowenthal J. P. Immunologic response of man to group B meningococcal polysaccharide vaccines. J Infect Dis. 1972 Nov;126(5):514–521. doi: 10.1093/infdis/126.5.514. [DOI] [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E., Altieri P., Berman S., Lowenthal J., Artenstein M. S. Safety and immunogenicity of a Neisseria meningitidis type 2 protein vaccine in animals and humans. J Infect Dis. 1978 Jun;137(6):728–739. doi: 10.1093/infdis/137.6.728. [DOI] [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E., Griffiss J. M., Altieri P., Berman S. Complex of meningococcal group B polysaccharide and type 2 outer membrane protein immunogenic in man. J Clin Invest. 1979 May;63(5):836–848. doi: 10.1172/JCI109383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E. Importance of complement source in bactericidal activity of human antibody and murine monoclonal antibody to meningococcal group B polysaccharide. Infect Immun. 1983 Apr;40(1):257–264. doi: 10.1128/iai.40.1.257-264.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E. Outer-membrane protein and lipopolysaccharide serotyping of Neisseria meningitidis by inhibition of a solid-phase radioimmunoassay. Infect Immun. 1977 Nov;18(2):424–433. doi: 10.1128/iai.18.2.424-433.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


