Abstract
Persistent Epstein-Barr virus (EBV) infection remains asymptomatic in the majority of virus carriers, despite the potent growth transforming potential of this virus. The increased frequency of EBV associated B cell lymphomas in immune compromised individuals suggests that tumor-free chronic infection with this virus is in part due to immune control. Here we discuss the evidence that loss of selective components of EBV specific immunity might contribute to EBV associated malignancies, like Nasopharyngeal Carcinoma, Burkitt’s and Hodgkin’s lymphoma, in otherwise immune competent patients. Furthermore, we discuss how current vaccine approaches against EBV might be able to target these selective deficiencies.
Keywords: Nasopharyngeal carcinoma, Hodgkin’s lymphoma, Burkitt’s lymphoma, T cells, malaria, HIV
1. Immune escape mechanisms of latent EBV infection
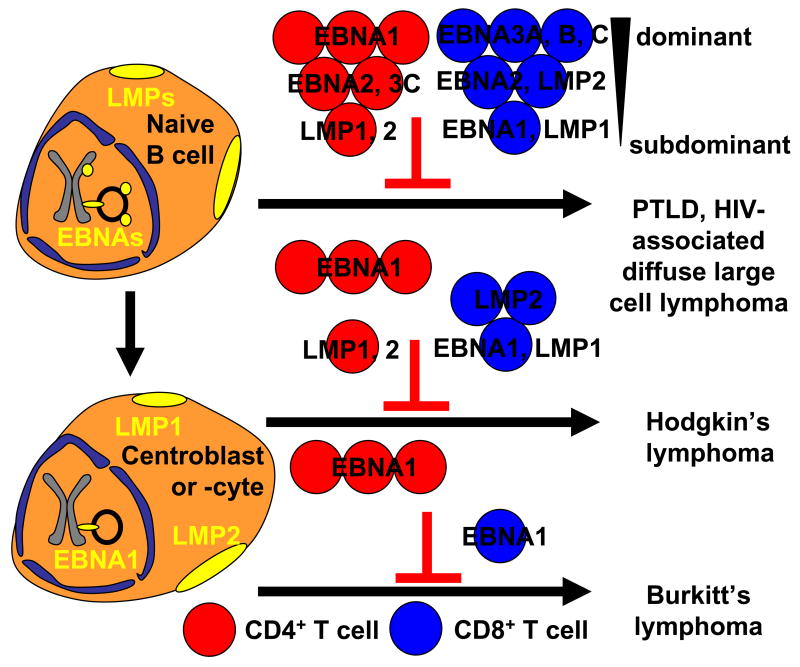
EBV is a ubiquitous γ1-Herpesvirus that infects more than 90% of the human adult population. The virus establishes persistent infection through its latency in B cells, from which it continuously reactivates lytic replication to produce infectious viral particles for transmission. While it expresses more than 80 lytic antigens, latently infected cells express up to 8 proteins and several non-translated RNAs 1. The non-translated RNAs are the BamHI A rightward transcripts (BARTs), which are thought to give rise to EBV encoded microRNAs 2, and the EBV-encoded RNAs (EBERs), which have been suggested to protect EBV infected cells from apoptosis 3. Of the six latent EBV proteins, 6 are nuclear antigens (EBNA1, 2, 3A, 3B, 3C, LP) and two are membrane proteins (LMP1 and 2). This limited antigen expression is probably one of the essential immune escape mechanisms of latent EBV infection, while EBV expresses more than 80 antigens during lytic replication. In addition to reduced viral protein expression, the virus performs some latency functions with non-translated RNAs which cannot be detected by T cells looking for small peptides presented on MHC class I and II molecules. EBV reduces antigenic protein expression further, dependent on the differentiation stage of the latently infected B cell 4. While all 8 latent EBV antigens can be found in naïve B cells in tonsils, the primary site of EBV infection after transmission in saliva, germinal center B cells express only EBNA1, LMP1 and LMP2 (Figure 1) 5. Furthermore, infected peripheral blood memory B cells express no EBV antigens or EBNA1 during homeostatic proliferation 6. Therefore, memory B cells, harboring the EBV genome without any EBV protein expression, are probably the site of long-term EBV persistence 7, and invisible to the immune system.
Figure 1.
EBV associated lymphomas are thought to primarily develop from two latency states. All eight latent EBV antigens can be found expressed in naïve B cells of healthy virus carriers. Only substantial immune escape by immune suppression from dominant and subdominant CD4+ (EBNA1 and LMP specific, respectively) and CD8+ (EBNA3 and EBNA1, LMP1, respectively) T cell responses can lead to PTLD and HIV-associate diffuse large cell lymphoma. In contrast, the restricted latency pattern of Hodgkin’s lymphoma only needs to escape dominant and subdominant CD4+, and CD8+ T cell responses of intermediate and low dominance. Finally, Burkitt’s lymphoma, which probably also develops from germinal center B cells, shuts down latent EBV infection even further and prevents, due to its constitutively active c-myc activity, LMP expression. Burkitt’s lymphoma, therefore, has only to compromise dominant CD4+ and subdominant CD8+ T cell responses against EBNA1 to escape from immune control (red circles: CD4+ T cells, blue circles CD8+ T cells, grouped into dominant, intermediate and subdominant responses).
In addition to the reduced number of viral protein expression during latent infection, the copy number of the expressed EBV antigens and of the antigenic peptides processing for MHC presentation from these is also kept very low. EBNA1 prevents efficient translation by a glycine-alanine encoding repeats (GA domain) 8. The same GA repeat domain inhibits proteasomal processing for efficient MHC class I presentation of EBNA1 9,10. In addition, EBNA3C limits its protein copy expression to a low level, from which antigen processing and MHC class I presentation can display only less than one peptide per cell 11. All this evidence suggests that EBV down-regulates detection by limiting the expressed viral protein number as well as the copy number per viral protein as main immune escape mechanisms during latent infection.
Despite the sophistication of the antigen downregulation during latent EBV infection, every infected individual develops T and B cell responses to the latent EBV antigens, and these are thought to keep persistent EBV infection in check and avoid EBV associated malignancies in most persistently infected individuals. In the following we will however discuss environmental circumstances, like coinfections, and changes in the tumor microenvironment, that favor the development of EBV associated malignancies. A good understanding of these changes is obviously instrumental to develop specific treatments of these EBV associated tumors and to determine possible cancer prevention strategies.
2. Manipulation of EBV specific immune control by co-infections
A. HIV associated lymphomas
One environmental trigger for the emergence of EBV associated malignancies is the coinfection with the human immunodeficiency virus (HIV). Since HIV infection causes progressive immune suppression, leading to the acquired immunodeficiency syndrome (AIDS), different EBV associated lymphomas develop at different stages of HIV infection 12. Primarily HIV coinfection promotes the development of EBV associated non-Hodgkin’s lymphomas of three types. Firstly, immunoblastic or diffuse large cell lymphomas develop after considerable immunosuppression by HIV, and also give rise to the central nervous system (CNS) lymphomas in AIDS patients 13. These lymphomas carry all latent EBV antigens and are therefore probably compareable to post-transplant lymphoproliferative disease (PTLD), in that quite extensive immunosuppression allows for the escape of EBV from nearly all EBV latent antigen specific immune control mechanisms, and proliferation of B cells via the most aggressive EBV latency program (Figure 1). Secondly, EBV joins forces with another human γ-herpesvirus, Kaposi’s sarcoma herpesvirus (KSHV) in the development of primary effusion lymphoma (PEL), which is frequently coinfected by both herpesviruses 14. While PEL is a rare tumor in immunocompetent individuals it arises with increased incidence rates in HIV infected patients. Interestingly, PEL expresses EBNA1 as the only EBV protein, but gains resistance against apopotosis most likely via the expression of this protein as well as non-translated EBV RNAs 15,3,16. In addition, KSHV contributes to PEL proliferation probably through upregulation of the cellular oncogene c-myc 17. Thirdly, EBV associated small noncleaved lymphomas also develop in HIV infected individuals, and within this category 30–40% of AIDS associated Burkitt’s lymphomas are EBV positive 18. While c-myc is deregulated in PEL by KSHV infection, it is translocated into one of the immunoblobulin loci in Burkitt’s lymphoma and stimulates proliferation of Burkitt’s lymphoma cells in this way 19. In addition, most EBV associated Burkitt’s lymphomas express the same EBV latency program as PELs (Figure 1), contributing to apoptosis resistance of these tumors 15. The later two categories of AIDS associated EBV positive lymphomas, PEL and Burkitt’s lymphoma, develop earlier after HIV infection with Burkitt’s lymphoma being often one of the earliest manifestations of AIDS 18.
Accordingly, two independent studies have found that selective loss of EBV specific CD4+ T cell responses correlates with the development of EBV associated non-Hodgkin’s lymphomas in HIV infected individuals 20,21. Piriou and colleagues even described that CD4+ and CD8+ T cell responses against EBNA1, the only EBV protein expressed in Burkitt’s lymphoma and PEL, were severely compromised in HIV infected individuals that developed AIDS-associated EBV positive lymphomas, while T cell responses against the immediate early lytic EBV antigen BZLF1 were maintained in these patients 20. This indicated that the selective loss of one particular immune response against EBV predisposes AIDS patients for EBV associated lymphomas. In another study, it was noted that HIV patients who developed EBV associated primary CNS lymphomas had lost EBV specific IFN-γ responses by CD4+ T cells despite maintaining healthy absolute CD4+ T cell counts 21, again arguing that a selective loss of EBV specific CD4+ T cell immune control predisposes for the development of EBV associated lymphomas in AIDS patients. EBV specific CD4+ T cells might be especially important to prevent AIDS associated PEL and Burkitt’s lymphoma since EBNA1, the only EBV protein expressed in these tumors, inhibits its processing onto MHC class I 22, but is recognized by CD4+ T cells after intracellular processing via macroautophagy 23,24. Therefore, EBNA1 specific CD4+ T cells are capable to recognize Burkitt’s lymphoma cells 25,26, even so this tumor down-regulates antigen processing towards MHC class I presentation via c-myc overexpression, and therefore escapes CD8+ T cell immune surveillance 27,28,26,29,30. These studies suggest that already early during HIV infection, when absolute CD4+ T cell counts are still maintained at normal levels, selective EBV specific CD4+ T cell responses, primarily directed against EBNA1, get depleted, and susceptibility to EBV associated lymphomas, particularly PEL and Burkitt’s lymphoma increases. One could even speculate that antigen persistence due to EBV latency activates these EBV specific CD4+ T cell preferentially, and makes them vulnerable to HIV infection. Irrespective of the mechanism, this preferential depletion of EBV specific immune control might lead to EBV associated lymphomas in AIDS patients.
B. Endemic Burkitt’s lymphoma
In addition to HIV infection, EBV associated malignancies are also associated with malaria. Indeed, the tumor, in which EBV was originally visualized is the most common childhood tumor in Sub-Saharan Africa and occurs mainly in holoendemic malaria regions of Africa and Papua New Guinea, where individuals are repeatedly infected with Plasmodium falciparum. This B cell lymphoma, endemic in Africa, is nearly 100% EBV associated 31,32. Similar, to HIV associated Burkitt’s lymphoma, endemic Burkitt’s lymphoma is characterized by a c-myc translocation into one of the immunoglobulin loci and expresses EBNA1 as the only EBV protein together with non-translated viral RNAs 33.
Despite its discovery 50 years ago the etiology of Burkitt’s lymphoma is still unclear. Two alternate though not mutually exclusive explanations for a role of malaria in Burkitt’s lymphomagenesis are discussed. One is that P. falciparum stimulates the B cell compartment, resulting eventually in a c-myc translocation in an EBV infected B cell as a side effect of somatic hypermutation of activated B cells in the germinal center reaction (Figure 1). The alternate explanation is that malaria infection compromises EBV specific immune control, leading to immune escape of an EBV infected B cell including those in which a c-myc translocation has occurred. Evidence for stimulation of the EBV infected B cell compartment has indeed been found in children from endemic malaria regions. EBV reactivates from this reservoir probably after B cell receptor stimulation and lytic EBV replication is primarily found in plasma cells 34. Accordingly, circulating EBV was detected in malaria infected children 35,36,37, and elevated antibody titers against lytic EBV antigens are associated with endemic Burkitt’s lymphoma 38. Two main B cell activation pathways have been suggested that potentially give rise to c-myc translocation in EBV infected B cells. Firstly, P. falciparum antigens, like the merozoite surface proteins 39, trigger B cell responses, and it has been shown that activation induced cytidine deaminase (AID) expression in germinal centers, to which activated B cells home, is required for c-myc translocation and lymphoma development 40,41,42. In addition, to malaria antigen induced B cell activation for AID upregulation, EBV LMP1 mediated B cell activation has also been described to upregulate AID 43. Secondly, the P. falciparum erythrocyte membrane protein 1 (PfEMP1) is a polyclonal B cell activator and has been shown to trigger lytic EBV replication in infected B cells 44. Other pathogen pattern for B cell activation like toll-like receptor (TLR) ligands have been identified in malaria 45, but how this may increase the risk of EBV associated lymphomas has yet to be explored. Therefore, malaria might have the means to directly activate EBV infected B cells to develop into Burkitt’s lymphoma.
In addition, however, these cells might need to escape EBV specific immune control. Indeed, T cell mediated immune control of EBV infected B cells was found to be compromised in malaria infected individuals 46. Especially in children, in which both malaria and EBV specific immune control need to develop simultaneously, immune suppressive effects of P. falciparum might impair the efficient establishment of cell-mediated EBV specific immune control. Accordingly, higher EBV loads, indicative of diminished EBV specific immune control, have been detected in children of holoendemic malaria regions 36. This P. falciparum mediated immune suppression could originate from its ability to inhibit early IFN-γ production via PfEMP1 mediated inhibition 47, and the ability of large numbers of Plasmodium infected erythrocytes to inhibit activation of immune responses inducing dendritic cells (DCs) 48. Whatever the mechanism, impaired EBV specific immune control might contribute to Burkitt’s lymphoma development in children of holoendemic malaria regions. In characterizing specific deficiencies, a lower percentage of 5–9 year old children in holoendemic malaria regions recognized dominant CD8+ T cell epitopes from EBV than children from a neighbouring region with sporadic malaria transmission 49. However, in the responding children the magnitude of EBV specific CD8+ T cells was not significantly different from age matched controls. In contrast to this rather mild deregulation of EBV specific CD8+ T cell control, T cell responses against EBNA1, the only EBV protein expressed in the majority of Burkitt’s lymphomas, were significantly decreased in nearly all children with Burkitt’s lymphoma, while CD4+ T cell responses against malaria antigens and CD8+ T cell responses against other EBV antigens were intact (unpublished data). These results suggest that selective deficiencies in EBV specific immune control assist in the development of Burkitt’s lymphoma, while P. falciparum mediated activation of the B cell system generate a higher frequency of Burkitt’s lymphoma precursor cells.
In addition, to these EBV associated malignancies arising in the presence of immune compromising coinfection, some EBV associated tumors seem to condition their microenvironment to induce local or even selective systemic immunosuppression for their growth in otherwise immunocompetent individuals. The two best characterized examples are EBV associated Hodgkin’s lymphoma and nasopharyngeal carcinoma, which we will discuss next.
3. Tumor microenvironments conditioned by EBV positive tumors
A. Nasopharyngeal carcinoma
Nasopharyngeal carcinoma (NPC) is a frequent epithelial cell cancer with a high incidence rate in Southeast China, especially the Guangdong province and neighboring Hong Kong 50. Since epithelial infection by EBV can be demonstrated in vitro 51, but has not been convincingly documented in vivo, the etiology of this tumor is still quite mysterious. However, it has been clearly documented that EBV is present in 100% of NPCs, and establishes a latency pattern with EBNA1 and LMP1 or LMP2 protein expression 52. Early after the discovery of EBV association with NPC a deregulation of the EBV specific immune response with elevated IgA titers against the virus was noted 53. This indicated that the immune response at the site of tumor development was changed, and that the tumor might condition its microenvironment to facilitate growth. Indeed recent studies support the notion that local immune suppression rather that systemic deficiency in EBV specific immune control might contribute to NPC development 54,55. In these studies, EBV specific CD4+ and CD8+ T cell responses could be reactivated from peripheral blood of NPC patients 54,55. Even though LMP1 and LMP2 specific CD8+ T cells were enriched in tumor infiltrating lymphocytes, their cytotoxicity and cytokine secretion was impaired 55. This impairment could be due to the presence of CD4+CD25+FoxP3+ natural regulatory T cells in the tumor tissue, which could suppress EBV specific immune responses against NPC even after correct homing of effector T cells 56,55.
In addition to active T cell suppression at the tumor site, the efficiency with which NPC can present antigens to T cells might also be compromised. While earlier studies based on a limited number of NPC cell lines suggested that antigen processing for MHC class I presentation was intact in NPC cells 57,58, a more recent study on primary tumor tissues suggested that the MHC class I antigen processing machinery is down-regulated in the majority of tumors 59. Even though no functional deficiency of MHC class I antigen presentation could be tested in this later study, it makes it possible to speculate that in addition to active immune suppression at NPC tumor sites the recognition of tumor cells by CD8+ T cells is also impaired. Together these data suggest that NPC impairs EBV specific immune control locally, while allowing efficient systemic immune responses against this virus.
B. Hodgkin’s lymphoma
Hodgkin’s lymphoma is the most common EBV associated lymphoma in the US and Europe. Around 40% of Hodgkin’s lymphomas are EBV associated 14. Interestingly only a small subset of cells, the so-called Hodgkin-Reed-Sternberg (HRS) cells, are the EBV transformed tumor cells, primarily of B cell origin, in the tumor tissue 60. HRS cells harbor the restricted EBV antigen expression pattern found in germinal center B cells of healthy EBV carriers (Figure 1) 61. The majority of cells in the tumor tissue are infiltrating lymphocytes. This indicates that Hodgkin’s lymphoma has already managed to generate an immunosuppressive environment that allows the tumor cells to grow despite extensive homing of immune cells to the tumor site. A number of immune escape mechanisms have been proposed and those can be subdivided into immunosuppressive functions of the HRS cells themselves and of the infiltrating lymphocytes. HRS cells have been shown to produce immunosuppressive cytokines like IL-10, IL-13 and TGF-β 62,63,64. IL-13 has been demonstrated to enhance HRS cell proliferation in an autocrine fashion in addition to its immune suppressive functions, which probably are primarily mediated through the induction of IL-10 producing Th2 polarized cells 63,65. In addition to immunosuppressive cytokine production by HRS cells, galectin-1 secretion could also contribute to immune escape in Hodgkin’s lymphoma 66. Elevated galectin-1 levels have been reported in tumor biopsies and recombinant galectin-1 has been shown to inhibit EBV specific T cell proliferation and cytokine secretion. Therefore, HRS cells employ several mechanisms, which are able to immune suppress their microenvironment.
As a result of this, regulatory T (Treg) cell populations seem to be enriched in Hodgkin’s lymphoma tissues. Several regulatory CD4+ T cell populations have been described in Hodgkin’s lymphoma. Among the CD4+ Treg cells are IL-10 producing Tr1 cells and CD4+CD25+ natural Treg cells 67. These have been shown to suppress peripheral blood cell proliferation and cytokine secretion in an IL-10 and cell contact dependent fashion. In addition, LAG-3 positive CD4+ T cells have been recently described to be selectively enriched in EBV positive Hodgkin’s lymphoma biopsies 68. They seem to be induced by soluble factors secreted by HRS cells and suppress LMP specific T cell responses. Therefore, regulatory T cell populations may suppress EBV specific immune control locally, and this involves cell-contact as well as the immunosuppressive cytokines IL-10 and TGF-β 64.
In addition, to this local immune suppression, however, selective systemic impairment of EBV specific T cell responses might also contribute to Hodgkin’s lymphoma development. Along these lines, Hodgkin’s lymphoma patients have diminished EBNA1 specific CD4+ T cell responses, while they maintain CD8+ T cell responses against other latent and lytic EBV antigens (unpublished data). These findings suggest that immunotherapeutic approaches should be developed to correct the selective systemic and tumor microenvironment specific deficiencies in EBV specific immune control. Since the tumor cells do not seem to have defects in antigen processing for MHC class I presentation 69,70, interventions to correct the selective systemic loss of EBV specific T cell responses and to overcome the local immune suppression in the tumor tissue should be explored as treatments of this EBV associated malignancy and ideally such modalities could be used for prevention in high risk populations.
4. Vaccine approaches against EBV associated malignancies
Recent evidence suggests that suboptimal initial immune control of EBV, as evidenced by symptomatic seroconversion, predisposes for the development of EBV associated disease. Along these lines, the risk of developing Hodgkin’s lymphoma is fourfold higher with a median incubation time of 4 years after resolution of infectious mononucleosis (IM), which is symptomatic primary EBV infection with high viral titers and therefore massively expanded, primarily EBV specific T cells 71. Therefore, preventive vaccination to avoid uncontrolled virus replication and successive “scaring” of the immune system 72, could decrease the incidence of EBV associated malignancies. For the prevention of IM, vaccines to induce neutralizing antibodies and T cell responses are under development. For the stimulation of EBV specific humoral immune control, recombinant gp350, the major EBV surface glycoprotein, has been tested. It was able to elicit neutralizing antibodies in a phase I/II trial 73, but the vaccine’s efficacy in preventing IM remains unclear. Alternatively, a single CD8+ T cell epitope vaccine has been tested to elicit protective immunity against IM 74. While it was successful in eliciting peptide specific T cell responses in a phase I clinical trial, the number of vaccinated individuals was too low to allow conclusions about its efficacy. In addition to these preventive vaccines, therapeutic immunizations against EBV associated malignancies are also being pursued.
The most successful of these is passive immunization by adoptive transfer of EBV specific T cells. This approach was developed from the observation that donor leucocyte infusions could be used to treat PTLD 75. It was further refined by expanding EBV specific T cells from seropositive donors in co-cultures with irradiated autologous EBV transformed B cells (LCLs) and injecting these enriched cultures into PTLD patients 76. This therapy is to date the most commonly practiced passive immunotherapy involving T cells. Unfortunately, expanding this success story to NPC, Hodgkin’s and Burkitt’s lymphoma has proven difficult, in part due to the fact that LCL stimulation will primarily expand immunodominant T cell responses, specific for the latent EBNA3 antigens, which are not expressed in these tumors. In focusing in vitro T cell expansions to the EBNA1, LMP1 and LMP2 antigens, which are present in these malignancies, recombinant viruses encoding for these EBV products have been utilized to expand specific CD8+ T cells, which could protect against LMP positive tumor growth in mice in vivo 777879. However, these T cell lines, targeting a select subset of EBV antigens, are just now starting to be tested in patients. As an alternative to passive immunization by adoptive T cell transfer EBV antigen loaded DCs have been evaluated for inducing protective CD8+ T cell responses against NPC. Although LMP2 specific CD8+ T cells could be expanded after peptide pulsed DC injection in NPC patients, these responses were too weak or transient to achieve clinical effects 80. Thus, vaccine approaches that primarily target CD8+ T cells have not yielded sufficient therapeutical success against EBV associated lymphomas.
Learning from these trials and as a result of a better understanding of the crucial role for CD4+ T cells in assisting CD8+ T cell immunity 81, more recent vaccine formulations aim to incorporate both CD4+ and CD8+ T cell antigens. In addition to CD4+ T cell help for CD8+ T cell responses, CD4+ T cells can also target EBV transformed B cells directly 25,82,83,84, adding to their value as vaccine targets. As above with CD8+ T cell epitope pulsing, many of these immunization strategies target DCs, which have been shown to be more efficient than LCLs in expanding EBV specific T cells 85 and are capable of priming protective CD4+ and CD8+ T cell responses against EBV transformed B cells in vitro 86. CD4+ and CD8+ T cells, expanded with DCs, which had been infected with a recombinant adenovirus encoding LMP2, were able to kill NPC cells 87. Furthermore, recombinant modified vaccinia virus Ankara (MVA) has been used to express a fusion protein between the immunogenic C-terminal domain of EBNA1, a reliable CD4+ T cell target 23,88, and LMP2, which is frequently recognized by CD8+ T cells 89. DCs infected with this recombinant MVA were able to expand EBNA1 specific CD4+ and LMP2 specific CD8+ T cell from seropositive donors. These approaches open promising avenues to enhance or prime selective protective EBV specific immune responses, whose absence might predispose for the development of EBV associated malignancies or which have been suppressed by the tumor cells itself or their microenvironment.
5. Conclusions
There is now mounting evidence that in addition to growth transforming contributions of both EBV and mutations, EBV associated malignancies in otherwise immune competent individuals escape immune control by either immune compromising coinfection or conditioning of their microenvironment. Since these tumors, like NPC, Hodgkin’s and Burkitt’s lymphoma express only a limited group of EBV antigens, only a select group of the protective EBV specific T cell responses needs to be compromised to allow their emergence. The challenge of developing immunotherapies against these EBV associated malignancies lies now in formulating vaccines comprised of relevant CD4+ and CD8+ T cell EBV antigens expressed in these tumors with a potent adjuvant that will elicit protective EBV specific Th1 immunity 90,91 with a strong central memory component 92. The consensus at the moment seems to be that EBNA1 as a promising CD4+ T cell antigen, should be combined with LMP1 and LMP2 for CD8+ T cell stimulation in such a vaccine for both prevention of symptomatic EBV infection as well as immunotherapy against EBV associated malignancies.
Acknowledgments
Our research is supported by the Arnold and Mabel Beckman Foundation, the Alexandrine and Alexander Sinsheimer Foundation, the Burroughs Wellcome Fund, the Dana Foundation’s Neuroimmunology program, the Starr Foundation, the National Cancer Institute (R01CA108609 and R01CA101741), the National Institute of Allergy and Infectious diseases (RFP-NIH-NIAID-DAIDS-BAA-06-19), the Foundation for the National Institutes of Health (Grand Challenges in Global Health) (to C.M.), and an Institutional Clinical and Translational Science Award (to the Rockefeller University Hospital). A. M. is supported by NIH K08-AI51565 and NIAID-AI43906.
Nonstandard abbreviations
- AID
activation induced deaminase
- BART
BamHI A rightward transcript
- EBER
EBV-encoded RNAs
- EBNA
EBV nuclear antigen
- EBV
Epstein Barr virus
- HRS cells
Hodgkin-Reed-Sternberg cells
- IM
infectious mononucleosis
- KSHV
Kaposi’s sarcoma herpesvirus
- LCL
lymphoblastoid cell line
- LMP
latent membrane protein
- NPC
nasopharyngeal carcinoma
- MVA
modified vaccinia virus Ankara
- PEL
primary effusion lymphoma
- PfEMP1
P. falciparum erythrocyte membrane protein 1
- PTLD
post-transplant lymphoproliferative disease
- TLR
toll-like receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer. 2004;4(10):757–68. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- 2.Pfeffer S, Zavolan M, Grasser FA, Chien M, Russo JJ, Ju J, John B, Enright AJ, Marks D, Sander C, et al. Identification of virus-encoded microRNAs. Science. 2004;304(5671):734–6. doi: 10.1126/science.1096781. [DOI] [PubMed] [Google Scholar]
- 3.Nanbo A, Inoue K, Adachi-Takasawa K, Takada K. Epstein-Barr virus RNA confers resistance to interferon-alpha-induced apoptosis in Burkitt’s lymphoma. Embo J. 2002;21(5):954–65. doi: 10.1093/emboj/21.5.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thorley-Lawson DA, Gross A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med. 2004;350(13):1328–37. doi: 10.1056/NEJMra032015. [DOI] [PubMed] [Google Scholar]
- 5.Babcock JG, Hochberg D, Thorley-Lawson AD. The expression pattern of Epstein-Barr virus latent genes in vivo is dependent upon the differentiation stage of the infected B cell. Immunity. 2000;13(4):497–506. doi: 10.1016/s1074-7613(00)00049-2. [DOI] [PubMed] [Google Scholar]
- 6.Hochberg D, Middeldorp JM, Catalina M, Sullivan JL, Luzuriaga K, Thorley-Lawson DA. Demonstration of the Burkitt’s lymphoma Epstein-Barr virus phenotype in dividing latently infected memory cells in vivo. Proc Natl Acad Sci U S A. 2004;101(1):239–44. doi: 10.1073/pnas.2237267100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Babcock GJ, Decker LL, Volk M, Thorley-Lawson DA. EBV persistence in memory B cells in vivo. Immunity. 1998;9(3):395–404. doi: 10.1016/s1074-7613(00)80622-6. [DOI] [PubMed] [Google Scholar]
- 8.Yin Y, Manoury B, Fahraeus R. Self-inhibition of synthesis and antigen presentation by Epstein-Barr virus-encoded EBNA1. Science. 2003;301(5638):1371–4. doi: 10.1126/science.1088902. [DOI] [PubMed] [Google Scholar]
- 9.Levitskaya J, Coram M, Levitsky V, Imreh S, Steigerwald-Mullen PM, Klein G, Kurilla MG, Masucci MG. Inhibition of antigen processing by the internal repeat region of the Epstein-Barr virus nuclear antigen-1. Nature. 1995;375(6533):685–8. doi: 10.1038/375685a0. [DOI] [PubMed] [Google Scholar]
- 10.Levitskaya J, Sharipo A, Leonchiks A, Ciechanover A, Masucci MG. Inhibition of ubiquitin/proteasome-dependent protein degradation by the Gly-Ala repeat domain of the Epstein-Barr virus nuclear antigen 1. Proc Natl Acad Sci U S A. 1997;94(23):12616–21. doi: 10.1073/pnas.94.23.12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crotzer VL, Christian RE, Brooks JM, Shabanowitz J, Settlage RE, Marto JA, White FM, Rickinson AB, Hunt DF, Engelhard VH. Immunodominance among EBV-derived epitopes restricted by HLA-B27 does not correlate with epitope abundance in EBV-transformed B-lymphoblastoid cell lines. J Immunol. 2000;164(12):6120–9. doi: 10.4049/jimmunol.164.12.6120. [DOI] [PubMed] [Google Scholar]
- 12.Boshoff C, Weiss R. AIDS-related malignancies. Nature Reviews Immunology. 2002;2:373–382. doi: 10.1038/nrc797. [DOI] [PubMed] [Google Scholar]
- 13.Levine AM. Acquired immunodeficiency syndrome-related lymphoma. Blood. 1992;80(1):8–20. [PubMed] [Google Scholar]
- 14.Küppers R. B cells under influence: transformation of B cells by Epstein-Barr virus. Nat Rev Immunol. 2003;3(10):801–12. doi: 10.1038/nri1201. [DOI] [PubMed] [Google Scholar]
- 15.Kelly GL, Milner AE, Baldwin GS, Bell AI, Rickinson AB. Three restricted forms of Epstein-Barr virus latency counteracting apoptosis in c-myc-expressing Burkitt lymphoma cells. Proc Natl Acad Sci U S A. 2006;103(40):14935–40. doi: 10.1073/pnas.0509988103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammerschmidt W, Sugden B. Epstein-Barr virus sustains Burkitt’s lymphomas and Hodgkin’s disease. Trends Mol Med. 2004;10(7):331–6. doi: 10.1016/j.molmed.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Bubman D, Guasparri I, Cesarman E. Deregulation of c-Myc in primary effusion lymphoma by Kaposi’s sarcoma herpesvirus latency-associated nuclear antigen. Oncogene. 2007;26(34):4979–86. doi: 10.1038/sj.onc.1210299. [DOI] [PubMed] [Google Scholar]
- 18.Kelly GL, Rickinson AB. Burkitt lymphoma: revisiting the pathogenesis of a virus-associated malignancy. Hematology Am Soc Hematol Educ Program. 2007;2007:277–84. doi: 10.1182/asheducation-2007.1.277. [DOI] [PubMed] [Google Scholar]
- 19.Rappold GA, Hameister H, Cremer T, Adolph S, Henglein B, Freese UK, Lenoire GM, Bornkamm GW. c-myc and immunoglobulin kappa light chain constant genes are on the 8q+ chromosome of three Burkitt lymphoma lines with t(2;8) translocations. Embo J. 1984;3(12):2951–5. doi: 10.1002/j.1460-2075.1984.tb02239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piriou E, van Dort K, Nanlohy NM, van Oers MH, Miedema F, van Baarle D. Loss of EBNA1-specific memory CD4+ and CD8+ T cells in HIV-infected patients progressing to AIDS-related non-Hodgkin lymphoma. Blood. 2005;106(9):3166–74. doi: 10.1182/blood-2005-01-0432. [DOI] [PubMed] [Google Scholar]
- 21.Gasser O, Bihl FK, Wolbers M, Loggi E, Steffen I, Hirsch HH, Gunthard HF, Walker BD, Brander C, Battegay M, et al. HIV patients developing primary CNS lymphoma lack EBV-specific CD4+ T cell function irrespective of absolute CD4+ T cell counts. PLoS Med. 2007;4(3):e96. doi: 10.1371/journal.pmed.0040096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SP, Brooks JM, Al-Jarrah H, Thomas WA, Haigh TA, Taylor GS, Humme S, Schepers A, Hammerschmidt W, Yates JL, et al. CD8 T cell recognition of endogenously expressed Epstein-Barr virus nuclear antigen 1. J Exp Med. 2004;199:1409–1420. doi: 10.1084/jem.20040121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Münz C, Bickham KL, Subklewe M, Tsang ML, Chahroudi A, Kurilla MG, Zhang D, O’Donnell M, Steinman RM. Human CD4+ T lymphocytes consistently respond to the latent Epstein-Barr virus nuclear antigen EBNA1. J Exp Med. 2000;191(10):1649–60. doi: 10.1084/jem.191.10.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paludan C, Schmid D, Landthaler M, Vockerodt M, Kube D, Tuschl T, Münz C. Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science. 2005;307(5709):593–6. doi: 10.1126/science.1104904. [DOI] [PubMed] [Google Scholar]
- 25.Paludan C, Bickham K, Nikiforow S, Tsang ML, Goodman K, Hanekom WA, Fonteneau JF, Stevanovic S, Münz C. EBNA1 specific CD4+ Th1 cells kill Burkitt’s lymphoma cells. J Immunol. 2002;169:1593–1603. doi: 10.4049/jimmunol.169.3.1593. [DOI] [PubMed] [Google Scholar]
- 26.Khanna R, Burrows SR, Thomson SA, Moss DJ, Cresswell P, Poulsen LM, Cooper L. Class I processing-defective Burkitt’s lymphoma cells are recognized efficiently by CD4+ EBV-specific CTLs. J Immunol. 1997;158(8):3619–25. [PubMed] [Google Scholar]
- 27.Rooney CM, Rowe M, Wallace LE, Rickinson AB. Epstein-Barr virus-positive Burkitt’s lymphoma cells not recognized by virus-specific T-cell surveillance. Nature. 1985;317(6038):629–31. doi: 10.1038/317629a0. [DOI] [PubMed] [Google Scholar]
- 28.Torsteinsdottir S, Masucci MG, Ehlin-Henriksson B, Brautbar C, Ben Bassat H, Klein G, Klein E. Differentiation-dependent sensitivity of human B-cell-derived lines to major histocompatibility complex-restricted T-cell cytotoxicity. Proc Natl Acad Sci U S A. 1986;83(15):5620–4. doi: 10.1073/pnas.83.15.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Staege MS, Lee SP, Frisan T, Mautner J, Scholz S, Pajic A, Rickinson AB, Masucci MG, Polack A, Bornkamm GW. MYC overexpression imposes a nonimmunogenic phenotype on Epstein-Barr virus-infected B cells. Proc Natl Acad Sci U S A. 2002;99(7):4550–5. doi: 10.1073/pnas.072495599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gavioli R, Frisan T, Vertuani S, Bornkamm GW, Masucci MG. c-myc overexpression activates alternative pathways for intracellular proteolysis in lymphoma cells. Nat Cell Biol. 2001;3(3):283–8. doi: 10.1038/35060076. [DOI] [PubMed] [Google Scholar]
- 31.Burkitt D. A children’s cancer dependent on climatic factors. Nature. 1962;194:232–234. doi: 10.1038/194232a0. [DOI] [PubMed] [Google Scholar]
- 32.Epstein MA, Henle G, Achong BG, Barr YM. Morphological and biological studies on a virus in cultured lymphoblasts from Burkitt’s lymphoma. J Exp Med. 1964;121:761–770. doi: 10.1084/jem.121.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rochford R, Cannon MJ, Moormann AM. Endemic Burkitt’s lymphoma: a polymicrobial disease? Nat Rev Microbiol. 2005;3(2):182–7. doi: 10.1038/nrmicro1089. [DOI] [PubMed] [Google Scholar]
- 34.Laichalk LL, Thorley-Lawson DA. Terminal differentiation into plasma cells initiates the replicative cycle of Epstein-Barr virus in vivo. J Virol. 2005;79(2):1296–307. doi: 10.1128/JVI.79.2.1296-1307.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rasti N, Falk KI, Donati D, Gyan BA, Goka BQ, Troye-Blomberg M, Akanmori BD, Kurtzhals JA, Dodoo D, Consolini R, et al. Circulating Epstein-Barr virus in children living in malaria-endemic areas. Scand J Immunol. 2005;61(5):461–5. doi: 10.1111/j.1365-3083.2005.01589.x. [DOI] [PubMed] [Google Scholar]
- 36.Moormann AM, Chelimo K, Sumba OP, Lutzke ML, Ploutz-Snyder R, Newton D, Kazura J, Rochford R. Exposure to holoendemic malaria results in elevated Epstein-Barr virus loads in children. J Infect Dis. 2005;191(8):1233–8. doi: 10.1086/428910. [DOI] [PubMed] [Google Scholar]
- 37.Donati D, Espmark E, Kironde F, Mbidde EK, Kamya M, Lundkvist A, Wahlgren M, Bejarano MT, Falk KI. Clearance of circulating Epstein-Barr virus DNA in children with acute malaria after antimalaria treatment. J Infect Dis. 2006;193(7):971–7. doi: 10.1086/500839. [DOI] [PubMed] [Google Scholar]
- 38.Henle G, Henle W, Clifford P, Diehl V, Kafuko GW, Kirya BG, Klein G, Morrow RH, Munube GM, Pike P, et al. Antibodies to Epstein-Barr virus in Burkitt’s lymphoma and control groups. J Natl Cancer Inst. 1969;43(5):1147–57. [PubMed] [Google Scholar]
- 39.Akpogheneta OJ, Duah NO, Tetteh KK, Dunyo S, Lanar DE, Pinder M, Conway DJ. Duration of naturally acquired antibody responses to blood stage Plasmodium falciparum is age dependent and antigen specific. Infect Immun. 2008 doi: 10.1128/IAI.01333-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dorsett Y, Robbiani DF, Jankovic M, Reina-San-Martin B, Eisenreich TR, Nussenzweig MC. A role for AID in chromosome translocations between c-myc and the IgH variable region. J Exp Med. 2007;204(9):2225–32. doi: 10.1084/jem.20070884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pasqualucci L, Bhagat G, Jankovic M, Compagno M, Smith P, Muramatsu M, Honjo T, Morse HC, 3rd, Nussenzweig MC, Dalla-Favera R. AID is required for germinal center-derived lymphomagenesis. Nat Genet. 2008;40(1):108–12. doi: 10.1038/ng.2007.35. [DOI] [PubMed] [Google Scholar]
- 42.Ramiro AR, Jankovic M, Eisenreich T, Difilippantonio S, Chen-Kiang S, Muramatsu M, Honjo T, Nussenzweig A, Nussenzweig MC. AID is required for c-myc/IgH chromosome translocations in vivo. Cell. 2004;118(4):431–8. doi: 10.1016/j.cell.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 43.He B, Raab-Traub N, Casali P, Cerutti A. EBV-encoded latent membrane protein 1 cooperates with BAFF/BLyS and APRIL to induce T cell-independent Ig heavy chain class switching. J Immunol. 2003;171(10):5215–24. doi: 10.4049/jimmunol.171.10.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chene A, Donati D, Guerreiro-Cacais AO, Levitsky V, Chen Q, Falk KI, Orem J, Kironde F, Wahlgren M, Bejarano MT. A molecular link between malaria and Epstein-Barr virus reactivation. PLoS Pathog. 2007;3(6):e80. doi: 10.1371/journal.ppat.0030080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parroche P, Lauw FN, Goutagny N, Latz E, Monks BG, Visintin A, Halmen KA, Lamphier M, Olivier M, Bartholomeu DC, et al. Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to Toll-like receptor 9. Proc Natl Acad Sci U S A. 2007;104(6):1919–24. doi: 10.1073/pnas.0608745104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whittle HC, Brown J, Marsh K, Greenwood BM, Seidelin P, Tighe H, Wedderburn L. T-cell control of Epstein-Barr virus-infected B cells is lost during P. falciparum malaria. Nature. 1984;312(5993):449–50. doi: 10.1038/312449a0. [DOI] [PubMed] [Google Scholar]
- 47.D’Ombrain MC, Voss TS, Maier AG, Pearce JA, Hansen DS, Cowman AF, Schofield L. Plasmodium falciparum erythrocyte membrane protein-1 specifically suppresses early production of host interferon-gamma. Cell Host Microbe. 2007;2(2):130–8. doi: 10.1016/j.chom.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 48.Urban BC, Ferguson DJ, Pain A, Willcox N, Plebanski M, Austyn JM, Roberts DJ. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature. 1999;400(6739):73–7. doi: 10.1038/21900. [DOI] [PubMed] [Google Scholar]
- 49.Moormann AM, Chelimo K, Sumba PO, Tisch DJ, Rochford R, Kazura JW. Exposure to holoendemic malaria results in suppression of Epstein-Barr virus-specific T cell immunosurveillance in Kenyan children. J Infect Dis. 2007;195(6):799–808. doi: 10.1086/511984. [DOI] [PubMed] [Google Scholar]
- 50.Wei WI, Sham JS. Nasopharyngeal carcinoma. Lancet. 2005;365(9476):2041–54. doi: 10.1016/S0140-6736(05)66698-6. [DOI] [PubMed] [Google Scholar]
- 51.Borza CM, Hutt-Fletcher LM. Alternate replication in B cells and epithelial cells switches tropism of Epstein-Barr virus. Nat Med. 2002;8(6):594–9. doi: 10.1038/nm0602-594. [DOI] [PubMed] [Google Scholar]
- 52.Heussinger N, Buttner M, Ott G, Brachtel E, Pilch BZ, Kremmer E, Niedobitek G. Expression of the Epstein-Barr virus (EBV)-encoded latent membrane protein 2A (LMP2A) in EBV-associated nasopharyngeal carcinoma. J Pathol. 2004;203(2):696–9. doi: 10.1002/path.1569. [DOI] [PubMed] [Google Scholar]
- 53.Henle G, Henle W. Epstein-Barr virus-specific IgA serum antibodies as an outstanding feature of nasopharyngeal carcinoma. Int J Cancer. 1976;17(1):1–7. doi: 10.1002/ijc.2910170102. [DOI] [PubMed] [Google Scholar]
- 54.Lin X, Gudgeon NH, Hui EP, Jia H, Qun X, Taylor GS, Barnardo MC, Lin CK, Rickinson AB, Chan AT. CD4 and CD8 T cell responses to tumour-associated Epstein-Barr virus antigens in nasopharyngeal carcinoma patients. Cancer Immunol Immunother. 2007 doi: 10.1007/s00262-007-0427-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li J, Zeng XH, Mo HY, Rolen U, Gao YF, Zhang XS, Chen QY, Zhang L, Zeng MS, Li MZ, et al. Functional Inactivation of EBV-Specific T-Lymphocytes in Nasopharyngeal Carcinoma: Implications for Tumor Immunotherapy. PLoS ONE. 2007;2(11):e1122. doi: 10.1371/journal.pone.0001122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lau KM, Cheng SH, Lo KW, Lee SA, Woo JK, van Hasselt CA, Lee SP, Rickinson AB, Ng MH. Increase in circulating Foxp3+CD4+CD25high regulatory T cells in nasopharyngeal carcinoma patients. Br J Cancer. 2007;96(4):617–22. doi: 10.1038/sj.bjc.6603580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee SP, Chan AT, Cheung ST, Thomas WA, CroomCarter D, Dawson CW, Tsai CH, Leung SF, Johnson PJ, Huang DP. CTL control of EBV in nasopharyngeal carcinoma (NPC): EBV-specific CTL responses in the blood and tumors of NPC patients and the antigen- processing function of the tumor cells. J Immunol. 2000;165(1):573–82. doi: 10.4049/jimmunol.165.1.573. [DOI] [PubMed] [Google Scholar]
- 58.Khanna R, Busson P, Burrows SR, Raffoux C, Moss DJ, Nicholls JM, Cooper L. Molecular characterization of antigen-processing function in nasopharyngeal carcinoma (NPC): evidence for efficient presentation of Epstein-Barr virus cytotoxic T-cell epitopes by NPC cells. Cancer Res. 1998;58(2):310–4. [PubMed] [Google Scholar]
- 59.Ogino T, Moriai S, Ishida Y, Ishii H, Katayama A, Miyokawa N, Harabuchi Y, Ferrone S. Association of immunoescape mechanisms with Epstein-Barr virus infection in nasopharyngeal carcinoma. Int J Cancer. 2007;120(11):2401–10. doi: 10.1002/ijc.22334. [DOI] [PubMed] [Google Scholar]
- 60.Kutok JL, Wang F. Spectrum of Epstein-Barr virus-associated diseases. Annu Rev Pathol. 2006;1:375–404. doi: 10.1146/annurev.pathol.1.110304.100209. [DOI] [PubMed] [Google Scholar]
- 61.Babcock GJ, Thorley-Lawson DA. Tonsillar memory B cells, latently infected with Epstein-Barr virus, express the restricted pattern of latent genes previously found only in Epstein-Barr virus-associated tumors. Proc Natl Acad Sci U S A. 2000;97(22):12250–5. doi: 10.1073/pnas.200366597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Herbst H, Foss HD, Samol J, Araujo I, Klotzbach H, Krause H, Agathanggelou A, Niedobitek G, Stein H. Frequent expression of interleukin-10 by Epstein-Barr virus-harboring tumor cells of Hodgkin’s disease. Blood. 1996;87(7):2918–29. [PubMed] [Google Scholar]
- 63.Kapp U, Yeh WC, Patterson B, Elia AJ, Kagi D, Ho A, Hessel A, Tipsword M, Williams A, Mirtsos C, et al. Interleukin 13 is secreted by and stimulates the growth of Hodgkin and Reed-Sternberg cells. J Exp Med. 1999;189(12):1939–46. doi: 10.1084/jem.189.12.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hsu SM, Lin J, Xie SS, Hsu PL, Rich S. Abundant expression of transforming growth factor-beta 1 and -beta 2 by Hodgkin’s Reed-Sternberg cells and by reactive T lymphocytes in Hodgkin’s disease. Hum Pathol. 1993;24(3):249–55. doi: 10.1016/0046-8177(93)90034-e. [DOI] [PubMed] [Google Scholar]
- 65.McKenzie GJ, Emson CL, Bell SE, Anderson S, Fallon P, Zurawski G, Murray R, Grencis R, McKenzie AN. Impaired development of Th2 cells in IL-13-deficient mice. Immunity. 1998;9(3):423–32. doi: 10.1016/s1074-7613(00)80625-1. [DOI] [PubMed] [Google Scholar]
- 66.Gandhi MK, Moll G, Smith C, Dua U, Lambley E, Ramuz O, Gill D, Marlton P, Seymour JF, Khanna R. Galectin-1 mediated suppression of Epstein-Barr virus specific T-cell immunity in classic Hodgkin lymphoma. Blood. 2007;110(4):1326–9. doi: 10.1182/blood-2007-01-066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marshall NA, Christie LE, Munro LR, Culligan DJ, Johnston PW, Barker RN, Vickers MA. Immunosuppressive regulatory T cells are abundant in the reactive lymphocytes of Hodgkin lymphoma. Blood. 2004;103(5):1755–62. doi: 10.1182/blood-2003-07-2594. [DOI] [PubMed] [Google Scholar]
- 68.Gandhi MK, Lambley E, Duraiswamy J, Dua U, Smith C, Elliott S, Gill D, Marlton P, Seymour J, Khanna R. Expression of LAG-3 by tumor-infiltrating lymphocytes is coincident with the suppression of latent membrane antigen-specific CD8+ T-cell function in Hodgkin lymphoma patients. Blood. 2006;108(7):2280–9. doi: 10.1182/blood-2006-04-015164. [DOI] [PubMed] [Google Scholar]
- 69.Lee SP, Constandinou CM, Thomas WA, Croom-Carter D, Blake NW, Murray PG, Crocker J, Rickinson AB. Antigen presenting phenotype of Hodgkin Reed-Sternberg cells: analysis of the HLA class I processing pathway and the effects of interleukin-10 on Epstein-Barr virus-specific cytotoxic T-cell recognition. Blood. 1998;92(3):1020–30. [PubMed] [Google Scholar]
- 70.Murray PG, Constandinou CM, Crocker J, Young LS, Ambinder RF. Analysis of major histocompatibility complex class I, TAP expression, and LMP2 epitope sequence in Epstein-Barr virus-positive Hodgkin’s disease. Blood. 1998;92(7):2477–83. [PubMed] [Google Scholar]
- 71.Hjalgrim H, Askling J, Rostgaard K, Hamilton-Dutoit S, Frisch M, Zhang JS, Madsen M, Rosdahl N, Konradsen HB, Storm HH, et al. Characteristics of Hodgkin’s lymphoma after infectious mononucleosis. N Engl J Med. 2003;349(14):1324–32. doi: 10.1056/NEJMoa023141. [DOI] [PubMed] [Google Scholar]
- 72.Sauce D, Larsen M, Curnow SJ, Leese AM, Moss PA, Hislop AD, Salmon M, Rickinson AB. EBV-associated mononucleosis leads to long-term global deficit in T cell responsiveness to IL-15. Blood. 2006;108(1):11–8. doi: 10.1182/blood-2006-01-0144. [DOI] [PubMed] [Google Scholar]
- 73.Moutschen M, Leonard P, Sokal EM, Smets F, Haumont M, Mazzu P, Bollen A, Denamur F, Peeters P, Dubin G, et al. Phase I/II studies to evaluate safety and immunogenicity of a recombinant gp350 Epstein-Barr virus vaccine in healthy adults. Vaccine. 2007;25(24):4697–705. doi: 10.1016/j.vaccine.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 74.Elliott SL, Suhrbier A, Miles JJ, Lawrence G, Pye SJ, Le TT, Rosenstengel A, Nguyen T, Allworth A, Burrows SR, et al. Phase I trial of a CD8+ T-cell peptide epitope-based vaccine for infectious mononucleosis. J Virol. 2008;82(3):1448–57. doi: 10.1128/JVI.01409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Papadopoulos EB, Ladanyi M, Emanuel D, Mackinnon S, Boulad F, Carabasi MH, Castro-Malaspina H, Childs BH, Gillio AP, Small TN, et al. Infusions of donor leukocytes to treat Epstein-Barr virus-associated lymphoproliferative disorders after allogeneic bone marrow transplantation. N Engl J Med. 1994;330(17):1185–91. doi: 10.1056/NEJM199404283301703. [DOI] [PubMed] [Google Scholar]
- 76.Gottschalk S, Rooney CM, Heslop HE. Post-transplant lymphoproliferative disorders. Annu Rev Med. 2005;56:29–44. doi: 10.1146/annurev.med.56.082103.104727. [DOI] [PubMed] [Google Scholar]
- 77.Duraiswamy J, Bharadwaj M, Tellam J, Connolly G, Cooper L, Moss D, Thomson S, Yotnda P, Khanna R. Induction of therapeutic T-cell responses to subdominant tumor-associated viral oncogene after immunization with replication-incompetent polyepitope adenovirus vaccine. Cancer Res. 2004;64(4):1483–9. doi: 10.1158/0008-5472.can-03-2196. [DOI] [PubMed] [Google Scholar]
- 78.Duraiswamy J, Sherritt M, Thomson S, Tellam J, Cooper L, Connolly G, Bharadwaj M, Khanna R. Therapeutic LMP1 polyepitope vaccine for EBV-associated Hodgkin disease and nasopharyngeal carcinoma. Blood. 2003;101(8):3150–6. doi: 10.1182/blood-2002-10-3092. [DOI] [PubMed] [Google Scholar]
- 79.Smith C, Cooper L, Burgess M, Rist M, Webb N, Lambley E, Tellam J, Marlton P, Seymour JF, Gandhi M, et al. Functional reversion of antigen-specific CD8+ T cells from patients with Hodgkin lymphoma following in vitro stimulation with recombinant polyepitope. J Immunol. 2006;177(7):4897–906. doi: 10.4049/jimmunol.177.7.4897. [DOI] [PubMed] [Google Scholar]
- 80.Lin CL, Lo WF, Lee TH, Ren Y, Hwang SL, Cheng YF, Chen CL, Chang YS, Lee SP, Rickinson AB, et al. Immunization with Epstein-Barr Virus (EBV) peptide-pulsed dendritic cells induces functional CD8+ T-cell immunity and may lead to tumor regression in patients with EBV-positive nasopharyngeal carcinoma. Cancer Res. 2002;62(23):6952–8. [PubMed] [Google Scholar]
- 81.Bevan MJ. Helping the CD8+ T-cell response. Nat Rev Immunol. 2004;4(8):595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- 82.Nikiforow S, Bottomly K, Miller G, Münz C. Cytolytic CD4+-T-Cell Clones Reactive to EBNA1 Inhibit Epstein-Barr Virus-Induced B-Cell Proliferation. J Virol. 2003;77(22):12088–12104. doi: 10.1128/JVI.77.22.12088-12104.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Adhikary D, Behrends U, Moosmann A, Witter K, Bornkamm GW, Mautner J. Control of Epstein-Barr virus infection in vitro by T helper cells specific for virion glycoproteins. J Exp Med. 2006 doi: 10.1084/jem.20051287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Long HM, Haigh TA, Gudgeon NH, Leen AM, Tsang CW, Brooks J, Landais E, Houssaint E, Lee SP, Rickinson AB, et al. CD4+ T-cell responses to Epstein-Barr virus (EBV) latent-cycle antigens and the recognition of EBV-transformed lymphoblastoid cell lines. J Virol. 2005;79(8):4896–907. doi: 10.1128/JVI.79.8.4896-4907.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Subklewe M, Sebelin K, Block A, Meier A, Roukens A, Paludan C, Fonteneau JF, Steinman RM, Münz C. Dendritic Cells Expand Epstein Barr Virus Specific CD8+ T Cell Responses More Efficiently Than EBV Transformed B Cells. Hum Immunol. 2005;66(9):938–49. doi: 10.1016/j.humimm.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 86.Bickham K, Goodman K, Paludan C, Nikiforow S, Tsang ML, Steinman RM, Münz C. Dendritic cells initiate immune control of Epstein-Barr virus transformation of B lymphocytes in vitro. J Exp Med. 2003;198(11):1653–63. doi: 10.1084/jem.20030646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pan Y, Zhang J, Zhou L, Zuo J, Zeng Y. In vitro anti-tumor immune response induced by dendritic cells transfected with EBV-LMP2 recombinant adenovirus. Biochem Biophys Res Commun. 2006;347(3):551–7. doi: 10.1016/j.bbrc.2006.05.214. [DOI] [PubMed] [Google Scholar]
- 88.Leen A, Meij P, Redchenko I, Middeldorp J, Bloemena E, Rickinson A, Blake N. Differential immunogenicity of Epstein-Barr virus latent-cycle proteins for human CD4+ T-helper 1 responses. J Virol. 2001;75(18):8649–59. doi: 10.1128/JVI.75.18.8649-8659.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Taylor GS, Haigh TA, Gudgeon NH, Phelps RJ, Lee SP, Steven NM, Rickinson AB. Dual stimulation of Epstein-Barr Virus (EBV)-specific CD4+- and CD8+-T-cell responses by a chimeric antigen construct: potential therapeutic vaccine for EBV-positive nasopharyngeal carcinoma. J Virol. 2004;78(2):768–78. doi: 10.1128/JVI.78.2.768-778.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bickham K, Münz C, Tsang ML, Larsson M, Fonteneau JF, Bhardwaj N, Steinman R. EBNA1-specific CD4+ T cells in healthy carriers of Epstein-Barr virus are primarily Th1 in function. J Clin Invest. 2001;107(1):121–30. doi: 10.1172/JCI10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.MacArthur GJ, Wilson AD, Birchall MA, Morgan AJ. Primary CD4+ T-cell responses provide both helper and cytotoxic functions during Epstein-Barr virus infection and transformation of fetal cord blood B cells. J Virol. 2007;81(9):4766–75. doi: 10.1128/JVI.02608-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Heller KN, Upshaw J, Seyoum B, Zebroski H, Münz C. Distinct memory CD4+ T-cell subsets mediate immune recognition of Epstein Barr virus nuclear antigen 1 in healthy virus carriers. Blood. 2007;109(3):1138–46. doi: 10.1182/blood-2006-05-023663. [DOI] [PMC free article] [PubMed] [Google Scholar]