Abstract
Retinoic acid plays a key role in the development and function of the immune system; however, the contribution of each of the three retinoic acid receptors (RARs) to the T cell immune response is not yet well understood. Of these receptors, both RARα and RARγ are expressed in T lymphocytes. While possible functional redundancy thus complicates understanding of the role of each receptor in T cells, emerging data suggest that RARα and RARγ function differently in thymocyte development and that RARγ is required for both primary and secondary CD8+ T cell immune responses.
Keywords: Retinoic acid receptor γ, CD8+ T lymphocytes
1. Introduction
The role of vitamin A in immunity has long been appreciated, with reports as early as the 1920s that vitamin A deficiency causes atrophy of lymphoid organs [1]. The importance of vitamin A in preventing infection has been recognized in numerous studies, and vitamin A supplementation is considered one of the major public health measures worldwide [2]. That vitamin A derivatives (referred to as retinoids) can modulate cell-mediated immunity was reported in the 1970s; however, the complex mechanisms by which retinoids regulate T cell development and function are only beginning to be elucidated [3]. This review will focus on the effects of retinoids, and in particular, retinoic acid receptor γ, on CD8+ T cells.
2. RAR and RXR families
Vitamin A (retinol) is intracellularly metabolized to form compounds known as retinoids. Derivatives of vitamin A, including all-trans-retinoic acid and 9-cis-retinoic acid, are recognized by members of two families of nuclear receptors: the retinoic acid receptors (RARs) and retinoic acid X receptors (RXRs) [4]. The three members of the RAR family, RARα, RARβ, and RARγ, are encoded by distinct genes, and bind both all-trans-retinoic acid and 9-cis-retinoic acid, while members of the RXR family bind only 9-cis-retinoic acid [5–9]. Each of the three RAR subtypes are present as multiple isoforms differing in the 5′ untranslated region and N terminal A region generated from differential promoter usage and alternative mRNA splicing [10–15].
Heterodimers formed between RARs and RXRs regulate transcription in a ligand-dependent manner. RAR/RXR heterodimers constitutively bind retinoic acid response elements (RAREs), and in the absence of ligand, recruit corepressor proteins to inhibit transcription of target genes [16–26]. Upon RAR ligand binding, corepressors are displaced, allowing heterodimers to instead recruit coactivators, resulting in transcriptional activation [24–26].
3. RAR expression in lymphocytes
During embryonic development, RARα is nearly ubiquitously expressed, while the expression patterns of RARβ and RARγ are much more limited [27, 28]. In adult mice and humans, RARγ expression is largely restricted to the skin, while RARβ is expressed in the cerebral cortex, prostate, and kidneys [29–33].
The expression of the various RAR isoforms has been examined in human and mouse lymphocytes and is summarized in Tables 1 and 2. RARα1 and RARγ1 are constitutively expressed in human T and B cells, while RARβ2 expression is only detected following treatment with all-trans-retinoic acid [34]. RARγ2 is expressed only at low levels in human lymphocytes, and its expression is not induced by all-trans-retinoic acid, while RARβ1 and RARβ3 are not expressed in T or B cells under any conditions [34]. Similarly, RARα and RARγ, but not RARβ, are expressed by T cell hybridomas as well as murine thymocytes and thymic stromal cells [35–37]. Expression of RARα begins in thymocytes by day 17 of embryonic development, while RARγ is not expressed, or is expressed only at very low levels prior to birth [38].
Table 1.
Expression of RARα, RARβ, and RARγ in thymocytes, peripheral T cells, and thymic stromal cells.
| RARα | RARβ | RARγ | |
|---|---|---|---|
| Fetal thymocytes | + | − | − |
| Postnatal thymocytes | + | − | + |
| Mature CD4 T cells | + | − | + |
| Mature CD8 T cells | + | − | + |
| Thymic stroma | + | + |
Table 2.
Relative expression levels of RARγ throughout T cell development.
| DP Thymocytes | CD4+ SP Thymocytes | CD8+ SP Thymocytes | Mature CD4+ T Cells | Mature CD8+ T Cells | |
|---|---|---|---|---|---|
| RARγ | + | ++ | ++++ | +++ | ++++ |
Postnatal RARγ expression is differentially regulated throughout T cell development. CD4+ CD8+ double positive (DP) thymocytes express low levels of RARγ [39]. While expression increases in both CD4+ and CD8+ single positive (SP) thymocytes, CD8+ SP thymocytes express 2–3 fold more RARγ than CD4+ SP thymocytes [39]. Expression continues to increase in mature CD4+ T cells; however, their expression level remains less than that of mature CD8+ T cells [39].
4. RARγ and T Cell Development
Vitamin A deficiency has long been known to cause thymic atrophy [1]. Further evidence for a role for retinoids in the thymus is provided by the observation that endogenous RAR ligands are generated from vitamin A in the thymus through a retinaldehyde dehydrogenase (RALDH)-dependent metabolic pathway [40]. Based on the results of both in vitro and in vivo studies, several potential functions for retinoic acid in thymocyte development have been proposed.
First, retinoic acid inhibits TCR-mediated thymocyte apoptosis in vitro [41]. In agreement with this finding, retinoic acid has been shown to inhibit negative selection of thymocytes. All-trans-retinoic acid or 9-cis-retinoic acid supplementation results in the development of increased numbers of SP thymocytes in fetal thymic organ culture [38]. This increase in mature thymocytes appears to be due to inhibition of negative selection, as all-trans-retinoic acid supplementation blocked superantigen-mediated deletion of CD4+ Vβ3+ thymocytes and partially prevented the SEB-mediated deletion of Vβ6+ and Vβ8+ thymocytes [38]. Similarly, retinoic acid inhibits negative selection of H-Y TCR transgenic thymocytes cultured with male thymic stromal cells, with 9-cis-retinoic acid showing a 10-fold more potent effect than all-trans-retinoic acid [42].
These effects appear to be mediated by an RARα/RXR-dependent pathway, and antagonized by an RARγ-dependent pathway. The greater potency of 9-cis-retinoic acid suggests that RXRs may be the relevant receptor in inhibiting thymocyte death; however, although addition of an RXR-specific agonist decreased the concentration of all-trans-retinoic acid required for inhibition of apoptosis, very high doses of this agonist were required to replicate the effects of 9-cis-retinoic acid [43]. A role for RARα was demonstrated by the ability of RARα-specific agonists to efficiently inhibit activation-induced thymocyte apoptosis both in vitro and in vivo [43]. Furthermore, addition of RARα-specific antagonists blocked the protective effects of retinoic acid [43]. RARγ-specific agonists, on the other hand, enhanced activation-induced thymocyte apoptosis, and RARγ-specific antagonists reduced the concentration of all-trans-retinoic acid necessary for inhibition of apoptosis [43]. Together, these results suggest that RARα is required for retinoic acid-mediated inhibition of apoptosis, and that RXR ligand binding counteracts antagonism from RARγ.
The ability of RARγ to cause thymocyte apoptosis has been documented both in vitro and in vivo. In contrast to observations in TCR-stimulated thymocytes, addition of either all-trans-retinoic acid or 9-cis-retinoic acid to unstimulated cultures of either whole thymocytes or sorted DP thymocytes results in increased apoptosis [36]. This increase in apoptosis appears to be mediated by RARγ, as RARγ-specific agonists, but not RARα-specific agonists were able to replicate the effects of retinoic acid [36]. The ability of an RARγ-mediated pathway to cause thymocyte apoptosis was confirmed in vivo, as treatment of mice with an RARγ-specific agonist resulted in rapid thymic involution largely due to loss of DP thymocytes [36].
The mechanism by which RARγ enhances apoptosis has not been examined in thymocytes. However, studies in T cell hybridomas suggest that RARγ ligation results in nur77-mediated upregulation of cell surface FasL, while RARα ligation inhibits FasL expression by blocking the ability of nur77 to bind DNA, and also inhibits activation-induced upregulation of the proapoptotic Bcl2 family member Bim [37, 44, 45].
The role of RARγ in thymocyte development has been further examined using genetic models allowing either overexpression or deletion of RARγ. Notably, no changes in thymic cellularity or apoptotic rates were reported in either model, possibly due to differences in concentration of exogenous ligand used in previous studies from physiologic levels present in the thymus [39, 46].
Mice expressing a human RARγ transgene in T cells display alterations in thymocyte development, with an increase in CD8 T cells in both the thymus and the periphery [46]. These results, along with the increased expression of RARγ observed in CD8+ SP thymocytes, suggest that RARγ may play a role in the CD4/CD8 lineage decision. However, T cell development is unaffected in the absence of RARγ. Mice lacking RARγ in hematopoietic cells have normal thymic cellularity, and the frequency of DN1-DN4, DP, and CD4+ and CD8+ SP thymocytes are similar to those in wild type mice [39]. Furthermore, normal numbers of mature CD4+ and CD8+ T cells were observed in the periphery [39]. While these data indicate that RARγ is not required for normal T cell development, it is possible that the presence of RARα may compensate for loss of RARγ in some functions. To better understand the function of each receptor in T cell development, analysis of RARα-deficient and RARα/RARγ double-deficient mice, along with in vivo administration of selective inhibitors of each RAR will be necessary.
5. RARγ and the CD8 T Cell Response
Early studies demonstrated that although high doses of retinoic acid cause a dramatic reduction in thymic and splenic cellularity, lower doses enhance cell-mediated immune responses [3]. In vivo treatment with low doses of retinoic acid resulted in a tenfold increase in cytotoxicity of splenocytes, but did not enhance mitogen-induced splenocyte proliferation [3]. While the functions of retinoic acid in cell-mediated immunity have not been completely elucidated, RARγ has been implicated in promoting the CD8+ T cell response.
Although the absence of RARγ has no effect on T cell development, CD4+ T cell polarization, or T-dependent humoral immune response, mice lacking RARγ in hematopoietic cells (referred to hereafter simply as RARγ –deficient) mount a defective CD8+ T cell response to L. monocytogenes infection [39]. Seven days following infection with OVA-expressing L. monocytogenes (rLmOVA), significantly fewer Ifnγ+ antigen-specific CD8+ T cells were present in RARγ-deficient mice than in wild type mice [39]. Additionally, splenocytes from rLmOVA-immunized RARγ-deficient mice displayed decreased cytotoxicity when compared to splenocytes from immunized wild type mice [39]. However, it is not clear whether the decreased cytotoxicity observed in total splenocytes is due simply to the decreased number of antigen-specific CD8+ T cells in these mice, or loss of cytotoxic function on a per cell basis in antigen-specific CD8+ T cells in the absence of RARγ.
The CD8+ memory response was also found to be impaired in the absence of RARγ. The numbers of both Ifnγ+ and antigen-specific CD8+ T cells present after rechallenge were significantly lower in mice lacking RARγ in hematopoietic cells than those in wild type mice [39]. These effects were specific to CD8+ T cells, as the numbers of Ifnγ+ antigen-specific CD4+ T cells were similar to those in wild type mice both at the peak of the primary response and after secondary challenge [39].
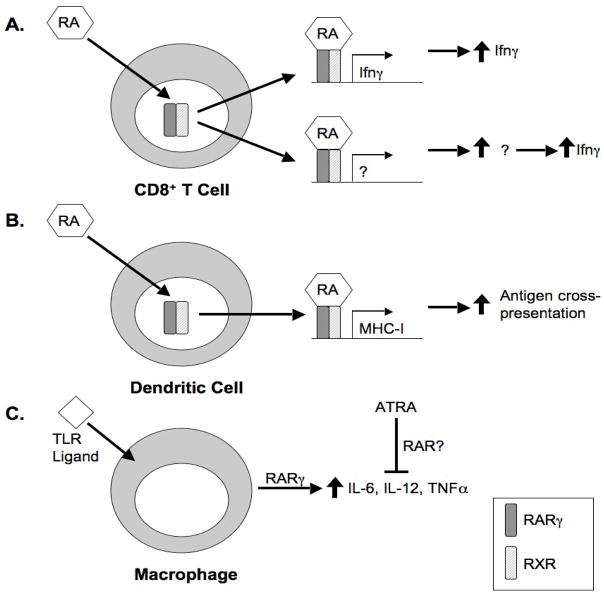
The cause of impaired effector and memory CD8+ T cell induction in the absence of RARγ remains unclear. It is possible that RARγ may play a role in inducing production of Ifnγ in CD8+ T cells (Fig. 1A). However, the role of retinoic acid generally, and RARγ specifically in Ifnγ expression is unknown. While supplementation with all-trans-retinoic acid enhances Ifnγ production in LPS-challenged rats, L. monocytogenes-infected rats receiving all-trans-retinoic acid supplementation produce less Ifnγ than unsupplemented rats [47, 48]. Furthermore, no RARγ binding sites were identified in the promoter region of the Ifnγ gene, suggesting that RARγ could only indirectly regulate transcription of Ifnγ [39]. Additionally, no defect in Ifnγ production was observed in RARγ-deficient Th1-polarized CD4+ T cells, indicating that any requirement for RARγ must be CD8+ T cell-specific [39].
Figure 1. Possible functions of RARγ in the CD8+ T cell response.
A. Ligation of RARγ may result in increased production of Ifnγ in CD8+ T cells. This may occur directly through RARγ-regulated transcription of Ifnγ (top); however, the lack of any identifiable RAR binding sites in the Ifnγ promoter region suggests that RARγ may instead upregulate an intermediate product that in turn mediates an increase in Ifnγ production (bottom).
B. RA-bound RARγ/RXR heterodimers may promote transcription of MHC class I through previously identified RAREs in the MHC class I promoter region. Increased MHC class I expression may facilitate antigen cross-presentation for CD8+ T cell activation.
C. RARγ is required for upregulation of inflammatory cytokines upon TLR stimulation of macrophages. RARγ-mediated cytokine production is antagonized by all-trans-retinoic acid; however, it is unclear which receptor or receptors are responsible for this antagonism. As serum levels of inflammatory cytokines are normal in the absence of RARγ, it is unclear whether RARγ-mediated production of these cytokines is necessary for an efficient CD8+ T cell response.
As RARγ was deleted in all hematopoietic cells, it is not clear whether the observed defects are intrinsic to CD8+ T cells, or due to changes in other cells of the immune system. For example, macrophages in RARγ-deficient mice produce lower levels of inflammatory cytokines after TLR stimulation (Fig. 1C) [39]. However, serum levels of inflammatory cytokines during early infection were normal in these mice, and S. typhimurium, GBS, and L. monocytogenes infections were cleared effectively, indicating that the decreased CD8+ T cell response is not likely caused by a defect in the innate immune response [39].
That CD8+ T cells develop normally in mice lacking RARγ in hematopoietic cells but show functional defects in the periphery could be indicative of defects in hematopoietic-derived antigen presenting cells that are not present in non-hematopoietic antigen presenting cells, such as thymic epithelial cells. Reports that expression of MHC class I may be regulated by retinoic acid raise the possibility that CD8+ T cells may fail to respond to infection due to a defect in antigen presentation in the periphery of RARγ-deficient mice (Fig. 1B).
A novel RARE has been identified in the downstream responsive element of the mouse H2Kb gene and found to be highly conserved in MHC class I genes [49]. The outer repeat sequences are present and unmutated in all 16 classical MHC class I genes in mice, and the intact RARE is also conserved in other mammalian species [49].
9-cis-retinoic acid was found to activate transcription of a reporter containing this RARE [49]. Furthermore, RAR/RXR heterodimers were shown to both bind this RARE, and to activate reporter transcription in the presence of ligand [49]. While RARβ2/RXRγ, RARα1/RXRγ, RARα1/RXRα, and RARβ2/RXRα heterodimers were most effective, all heterodimers were able to activate transcription [49].
However, whether MHC class I expression is regulated by retinoic acid in vivo is unknown. Additionally, although in vitro studies show that the three RAR subtypes are differentially expressed in human cord blood monocytes, monocyte-derived dendritic cells, and macrophages, the expression patterns of RARs in antigen presenting cells in vivo has not been examined [50]. As the ability to mount an effective CD8+ T cell response to L. monocytogenes is critically dependent on cross-presentation of antigens by CD11c+ dendritic cells, studies of the roles of RARs in dendritic cell function may prove particularly informative [51].
Thus, while it is clear that the CD8+ T cell response is impaired in mice lacking RARγ throughout the hematopoietic system, further experiments are necessary to determine which cell populations require RARγ and which gene products are regulated by RARγ during both primary and secondary immune responses.
6. RARγ and T lymphocyte gut homing
In addition to appropriate T cell activation, an effective CD8+ T cell response requires homing of T cells to sites of infection. The roles of retinoic acid in imprinting tissue tropism have been best studied in the context of CD8+ T cell gut homing; however, the mechanisms by which retinoic acid may mediate T cell homing to gut-associated lymphoid tissue (GALT) are not entirely clear.
The ability of antigen-experienced T cells to home to the gut is mediated in part by α4β7 integrin and CCR9 [52, 53]. The ligands for these receptors, MAdCAM-1 and the chemokine CCL25, are expressed by high endothelial venules in mucosa, and by epithelial cells of the small intestine, respectively [54–57].
MadCAM-1, which preferentially interacts with α4β7 over α4β1 integrin, has been shown to play a role in lymphocyte gut homing [54, 55]. That MadCAM-1 mediates lymphocyte extravasation into mucosal tissue was first demonstrated by experiments showing that treatment of frozen mesenteric lymph nodes and Peyer’s patches, but not peripheral lymph nodes, with MadCAM-1-blocking antibodies prevented lymphocytes isolated from mesenteric lymph nodes from binding HEVs [56]. This function was confirmed in vivo, as treatment of mice with anti-MadCAM-1 antibody completely inhibited lymphocyte homing to Peyer’s patches, partially inhibited homing to mesenteric lymph nodes, but did not affect homing to peripheral lymph nodes [56]. That MadCAM-1-dependent gut homing is mediated by its interaction with α4β7 integrin was demonstrated by studies showing that pretreatment of lymphocytes with anti-α4β7 Fab fragments prior to adoptive transfer inhibits migration to Peyer’s patches [58]. In contrast, blocking of the α4β1 integrin-specific ligands VCAM-1 and fibronectin had no effect on lymphocyte homing to Peyer’s patches, further suggesting that gut homing depends specifically on α4β7 integrin [58].
CCR9 is expressed by most naïve CD8+ T cells, but only half of recently activated CD8+ T cells, and a small population of previously activated CD8+ T cells [53]. Immunization results in sustained upregulation of CCR9 in antigen-specific CD8+ T cells in mesenteric but not peripheral lymph nodes, and 40–50% of intestinal epithelial lymphocytes (IELs) and lamina propria lymphocytes (LPLs) were found to be CCR9+ [53]. That CCR9 expression is required for migration of antigen-specific CD8+ T cells to the gut has been demonstrated by in vivo neutralization of CCL25. Treatment with a neutralizing anti-CCL25 antibody prior to immunization results in a significant reduction in the number of antigen-specific CD8+ T cells in the small intestinal epithelium, but does not affect the frequency of antigen-specific CD8+ T cells in spleen, mesenteric, or peripheral lymph nodes [53].
The expression and function of CCR9 in CD4+ T cells is less clear. Some studies have shown a small population of CCR9+ CD4+ T cells, while others do not detect CCR9 expression in CD4+ T cells [52, 53]. As in CD8+ T cells, CCR9 is selectively upregulated in antigen-specific CD4+ T cells activated in mesenteric lymph nodes; however, CCR9-deficient antigen-specific CD4+ T cells are able to migrate to the lamina propria, though less efficiently than wild type cells [52].
The gut tropism of CD8+ T cells activated in GALT appears to be a function of the antigen presenting cells present in these tissues, as dendritic cells from Peyer’s patches and mesenteric lymph nodes, but not peripheral lymph nodes or spleen induce upregulation of CCR9 and α4β7 integrin [59, 60]. In vitro experiments have shown that dendritic cells from Peyer’s patches and mesenteric lymph nodes, but not spleen, convert all-trans-retinol to all-trans-retinoic acid, and the presence of either all-trans-retinoic acid or Am80, which preferentially activates RARα, results in enhanced expression of α4β7 integrin and CCR9 in T cells [61, 62]. Further, treatment with the RAR antagonist LE135 suppresses the ability of Peyer’s patches and mesenteric lymph node dendritic cells to upregulate α4β7 integrin and CCR9 in T cells, while the RXR pan-antagonist PA452 has no effect, suggesting that RARs, but not RXRs are required for T cell gut tropism [61]. LE135 has been used as an RARβ-specific antagonist; however, in some studies, it has been shown to also act on RARα [63, 64]. As T cells do not express RARβ, these data suggest that RARα activation plays a role in induction of α4β7 integrin and CCR9 expression.
The function of RARγ in T cell gut tropism was examined in mice lacking RARγ in hematopoietic cells. Upon anti-CD3 stimulation in the presence or absence of all-trans-retinoic acid, RARγ-deficient CD4+ and CD8+ T cells upregulated α4β7 to levels similar to those in control T cells.[39] In addition, similar frequencies of TCRαβ+, TCRαδ+, CD4+ CD8−, CD4− CD8α+, and CD4+CD8+ IELs were observed in wild type and RARγ-deficient mice, suggesting that all-trans-retinoic acid-dependent T cell gut tropism does not require RARγ [39]. However, as mature T cells express both RARα and RARγ, it is possible that RARα is able to compensate for the loss of RARγ. To investigate this possibility, it will be necessary to compare the ability of T cells to home to the gut in RARα/RARγ double deficient mice to that in RARα and RARγ single deficient mice.
8. Summary
Understanding of the role of RARγ in CD8+ T cell function is complicated by the expression of both RARα and RARγ in developing and mature T cells. A role for RARγ in the CD8+ T cell response has been demonstrated in vivo; however, it is unclear whether RARγ is required by T cells or by antigen presenting cells. In vitro experiments have shown that RARγ can mediate thymocyte apoptosis; however, no pro-apoptotic function has been observed in vivo. Further, whether RARγ influences the CD4+ vs. CD8+ lineage decision in the thymus remains unclear. Finally, although no role for RARγ has been demonstrated in CD8+ T cell gut homing, it is possible that RARα may compensate for the loss of RARγ. In order to fully elucidate the functions of RARγ, future studies will need to address possible redundancy between members of the RAR family as well as determine which cell populations truly require RARγ expression.
Abbreviations
- RAR
retinoic acid receptor
- RXR
retinoic acid X receptor
- RARE
retinoic acid response element
- DP
CD4+ CD8+ double positive
- SP
single positive
- RALDH
retinaldehyde dehydrogenase
- DN
double negative
- rLmOVA
OVA-expressing L. monocytogenes
- IEL
intestinal epithelial lymphocyte
- LPL
lamina propria lymphocyte
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wolbach SB, Howe PR. Tissue changes following deprivation of fat-soluble A vitamin. J Exp Med. 1925;42:753–77. doi: 10.1084/jem.42.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Semba RD. The role of vitamin A and related retinoids in immune function. Nutr Rev. 1998;56:S38–48. doi: 10.1111/j.1753-4887.1998.tb01643.x. [DOI] [PubMed] [Google Scholar]
- 3.Dennert G, Lotan R. Effects of retinoic acid on the immune system: stimulation of T killer cell induction. Eur J Immunol. 1978;8:23–9. doi: 10.1002/eji.1830080106. [DOI] [PubMed] [Google Scholar]
- 4.Leid M, Kastner P, Chambon P. Multiplicity generates diversity in the retinoic acid signalling pathways. Trends Biochem Sci. 1992;17:427–33. doi: 10.1016/0968-0004(92)90014-z. [DOI] [PubMed] [Google Scholar]
- 5.Mattei MG, Riviere M, Krust A, Ingvarsson S, Vennstrom B, Islam MQ, et al. Chromosomal assignment of retinoic acid receptor (RAR) genes in the human, mouse, and rat genomes. Genomics. 1991;10:1061–9. doi: 10.1016/0888-7543(91)90199-o. [DOI] [PubMed] [Google Scholar]
- 6.Allenby G, Janocha R, Kazmer S, Speck J, Grippo JF, Levin AA. Binding of 9-cis-retinoic acid and all-trans-retinoic acid to retinoic acid receptors alpha, beta, and gamma. Retinoic acid receptor gamma binds all-trans-retinoic acid preferentially over 9-cis-retinoic acid. J Biol Chem. 1994;269:16689–95. [PubMed] [Google Scholar]
- 7.Levin AA, Sturzenbecker LJ, Kazmer S, Bosakowski T, Huselton C, Allenby G, et al. 9-cis retinoic acid stereoisomer binds and activates the nuclear receptor RXR alpha. Nature. 1992;355:359–61. doi: 10.1038/355359a0. [DOI] [PubMed] [Google Scholar]
- 8.Allenby G, Bocquel MT, Saunders M, Kazmer S, Speck J, Rosenberger M, et al. Retinoic acid receptors and retinoid X receptors: interactions with endogenous retinoic acids. Proc Natl Acad Sci U S A. 1993;90:30–4. doi: 10.1073/pnas.90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blumberg B, Mangelsdorf DJ, Dyck JA, Bittner DA, Evans RM, De Robertis EM. Multiple retinoid-responsive receptors in a single cell: families of retinoid “X” receptors and retinoic acid receptors in the Xenopus egg. Proc Natl Acad Sci U S A. 1992;89:2321–5. doi: 10.1073/pnas.89.6.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagpal S, Saunders M, Kastner P, Durand B, Nakshatri H, Chambon P. Promoter context- and response element-dependent specificity of the transcriptional activation and modulating functions of retinoic acid receptors. Cell. 1992;70:1007–19. doi: 10.1016/0092-8674(92)90250-g. [DOI] [PubMed] [Google Scholar]
- 11.Lehmann JM, Zhang XK, Pfahl M. RAR gamma 2 expression is regulated through a retinoic acid response element embedded in Sp1 sites. Mol Cell Biol. 1992;12:2976–85. doi: 10.1128/mcb.12.7.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leroy P, Nakshatri H, Chambon P. Mouse retinoic acid receptor alpha 2 isoform is transcribed from a promoter that contains a retinoic acid response element. Proc Natl Acad Sci U S A. 1991;88:10138–42. doi: 10.1073/pnas.88.22.10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kastner P, Krust A, Mendelsohn C, Garnier JM, Zelent A, Leroy P, et al. Murine isoforms of retinoic acid receptor gamma with specific patterns of expression. Proc Natl Acad Sci U S A. 1990;87:2700–4. doi: 10.1073/pnas.87.7.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giguere V, Shago M, Zirngibl R, Tate P, Rossant J, Varmuza S. Identification of a novel isoform of the retinoic acid receptor gamma expressed in the mouse embryo. Mol Cell Biol. 1990;10:2335–40. doi: 10.1128/mcb.10.5.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zelent A, Mendelsohn C, Kastner P, Krust A, Garnier JM, Ruffenach F, et al. Differentially expressed isoforms of the mouse retinoic acid receptor beta generated by usage of two promoters and alternative splicing. Embo J. 1991;10:71–81. doi: 10.1002/j.1460-2075.1991.tb07922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kastner P, Mark M, Ghyselinck N, Krezel W, Dupe V, Grondona JM, et al. Genetic evidence that the retinoid signal is transduced by heterodimeric RXR/RAR functional units during mouse development. Development. 1997;124:313–26. doi: 10.1242/dev.124.2.313. [DOI] [PubMed] [Google Scholar]
- 17.Leid M, Kastner P, Lyons R, Nakshatri H, Saunders M, Zacharewski T, et al. Purification, cloning, and RXR identity of the HeLa cell factor with which RAR or TR heterodimerizes to bind target sequences efficiently. Cell. 1992;68:377–95. doi: 10.1016/0092-8674(92)90478-u. [DOI] [PubMed] [Google Scholar]
- 18.Yu VC, Delsert C, Andersen B, Holloway JM, Devary OV, Naar AM, et al. RXR beta: a coregulator that enhances binding of retinoic acid, thyroid hormone, and vitamin D receptors to their cognate response elements. Cell. 1991;67:1251–66. doi: 10.1016/0092-8674(91)90301-e. [DOI] [PubMed] [Google Scholar]
- 19.Mader S, Chen JY, Chen Z, White J, Chambon P, Gronemeyer H. The patterns of binding of RAR, RXR and TR homo- and heterodimers to direct repeats are dictated by the binding specificites of the DNA binding domains. Embo J. 1993;12:5029–41. doi: 10.1002/j.1460-2075.1993.tb06196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mader S, Leroy P, Chen JY, Chambon P. Multiple parameters control the selectivity of nuclear receptors for their response elements. Selectivity and promiscuity in response element recognition by retinoic acid receptors and retinoid X receptors. J Biol Chem. 1993;268:591–600. [PubMed] [Google Scholar]
- 21.Kliewer SA, Umesono K, Mangelsdorf DJ, Evans RM. Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature. 1992;355:446–9. doi: 10.1038/355446a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang XK, Hoffmann B, Tran PB, Graupner G, Pfahl M. Retinoid X receptor is an auxiliary protein for thyroid hormone and retinoic acid receptors. Nature. 1992;355:441–6. doi: 10.1038/355441a0. [DOI] [PubMed] [Google Scholar]
- 23.Marks MS, Hallenbeck PL, Nagata T, Segars JH, Appella E, Nikodem VM, et al. H-2RIIBP (RXR beta) heterodimerization provides a mechanism for combinatorial diversity in the regulation of retinoic acid and thyroid hormone responsive genes. Embo J. 1992;11:1419–35. doi: 10.1002/j.1460-2075.1992.tb05187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurokawa R, DiRenzo J, Boehm M, Sugarman J, Gloss B, Rosenfeld MG, et al. Regulation of retinoid signalling by receptor polarity and allosteric control of ligand binding. Nature. 1994;371:528–31. doi: 10.1038/371528a0. [DOI] [PubMed] [Google Scholar]
- 25.Kurokawa R, Soderstrom M, Horlein A, Halachmi S, Brown M, Rosenfeld MG, et al. Polarity-specific activities of retinoic acid receptors determined by a co-repressor. Nature. 1995;377:451–4. doi: 10.1038/377451a0. [DOI] [PubMed] [Google Scholar]
- 26.Westin S, Kurokawa R, Nolte RT, Wisely GB, McInerney EM, Rose DW, et al. Interactions controlling the assembly of nuclear-receptor heterodimers and co-activators. Nature. 1998;395:199–202. doi: 10.1038/26040. [DOI] [PubMed] [Google Scholar]
- 27.Dolle P, Ruberte E, Leroy P, Morriss-Kay G, Chambon P. Retinoic acid receptors and cellular retinoid binding proteins. I A systematic study of their differential pattern of transcription during mouse organogenesis. Development. 1990;110:1133–51. doi: 10.1242/dev.110.4.1133. [DOI] [PubMed] [Google Scholar]
- 28.Mollard R, Viville S, Ward SJ, Decimo D, Chambon P, Dolle P. Tissue-specific expression of retinoic acid receptor isoform transcripts in the mouse embryo. Mech Dev. 2000;94:223–32. doi: 10.1016/s0925-4773(00)00303-8. [DOI] [PubMed] [Google Scholar]
- 29.Zelent A, Krust A, Petkovich M, Kastner P, Chambon P. Cloning of murine alpha and beta retinoic acid receptors and a novel receptor gamma predominantly expressed in skin. Nature. 1989;339:714–7. doi: 10.1038/339714a0. [DOI] [PubMed] [Google Scholar]
- 30.Krust A, Kastner P, Petkovich M, Zelent A, Chambon P. A third human retinoic acid receptor, hRAR-gamma. Proc Natl Acad Sci U S A. 1989;86:5310–4. doi: 10.1073/pnas.86.14.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benbrook D, Lernhardt E, Pfahl M. A new retinoic acid receptor identified from a hepatocellular carcinoma. Nature. 1988;333:669–72. doi: 10.1038/333669a0. [DOI] [PubMed] [Google Scholar]
- 32.Brand N, Petkovich M, Krust A, Chambon P, de The H, Marchio A, et al. Identification of a second human retinoic acid receptor. Nature. 1988;332:850–3. doi: 10.1038/332850a0. [DOI] [PubMed] [Google Scholar]
- 33.Petkovich M, Brand NJ, Krust A, Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987;330:444–50. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- 34.Ballow M, Wang X, Xiang S, Allen C. Expression and regulation of nuclear retinoic acid receptors in human lymphoid cells. J Clin Immunol. 2003;23:46–54. doi: 10.1023/a:1021900331580. [DOI] [PubMed] [Google Scholar]
- 35.Meco D, Scarpa S, Napolitano M, Maroder M, Bellavia D, De Maria R, et al. Modulation of fibronectin and thymic stromal cell-dependent thymocyte maturation by retinoic acid. J Immunol. 1994;153:73–83. [PubMed] [Google Scholar]
- 36.Szondy Z, Reichert U, Bernardon JM, Michel S, Toth R, Ancian P, et al. Induction of apoptosis by retinoids and retinoic acid receptor gamma-selective compounds in mouse thymocytes through a novel apoptosis pathway. Mol Pharmacol. 1997;51:972–82. doi: 10.1124/mol.51.6.972. [DOI] [PubMed] [Google Scholar]
- 37.Toth R, Szegezdi E, Reichert U, Bernardon JM, Michel S, Ancian P, et al. Activation-induced apoptosis and cell surface expression of Fas (CD95) ligand are reciprocally regulated by retinoic acid receptor alpha and gamma and involve nur77 in T cells. Eur J Immunol. 2001;31:1382–91. doi: 10.1002/1521-4141(200105)31:5<1382::AID-IMMU1382>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 38.Yagi J, Uchida T, Kuroda K, Uchiyama T. Influence of retinoic acid on the differentiation pathway of T cells in the thymus. Cell Immunol. 1997;181:153–62. doi: 10.1006/cimm.1997.1203. [DOI] [PubMed] [Google Scholar]
- 39.Dzhagalov I, Chambon P, He YW. Regulation of CD8+ T lymphocyte effector function and macrophage inflammatory cytokine production by retinoic acid receptor gamma. J Immunol. 2007;178:2113–21. doi: 10.4049/jimmunol.178.4.2113. [DOI] [PubMed] [Google Scholar]
- 40.Kiss I, Ruhl R, Szegezdi E, Fritzsche B, Toth B, Pongracz J, et al. Retinoid receptor-activating ligands are produced within the mouse thymus during postnatal development. Eur J Immunol. 2008;38:147–55. doi: 10.1002/eji.200737342. [DOI] [PubMed] [Google Scholar]
- 41.Iwata M, Mukai M, Nakai Y, Iseki R. Retinoic acids inhibit activation-induced apoptosis in T cell hybridomas and thymocytes. J Immunol. 1992;149:3302–8. [PubMed] [Google Scholar]
- 42.Yang Y, Vacchio MS, Ashwell JD. 9-cis-retinoic acid inhibits activation-driven T-cell apoptosis: implications for retinoid X receptor involvement in thymocyte development. Proc Natl Acad Sci U S A. 1993;90:6170–4. doi: 10.1073/pnas.90.13.6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szondy Z, Reichert U, Bernardon JM, Michel S, Toth R, Karaszi E, et al. Inhibition of activation-induced apoptosis of thymocytes by all-trans- and 9-cis-retinoic acid is mediated via retinoic acid receptor alpha. Biochem J. 1998;331(Pt 3):767–74. doi: 10.1042/bj3310767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Toth B, Ludanyi K, Kiss I, Reichert U, Michel S, Fesus L, et al. Retinoids induce Fas(CD95) ligand cell surface expression via RARgamma and nur77 in T cells. Eur J Immunol. 2004;34:827–36. doi: 10.1002/eji.200324760. [DOI] [PubMed] [Google Scholar]
- 45.Szegezdi E, Kiss I, Simon A, Blasko B, Reichert U, Michel S, et al. Ligation of retinoic acid receptor alpha regulates negative selection of thymocytes by inhibiting both DNA binding of nur77 and synthesis of bim. J Immunol. 2003;170:3577–84. doi: 10.4049/jimmunol.170.7.3577. [DOI] [PubMed] [Google Scholar]
- 46.Pohl J, LaFace D, Sands JF. Transcription of retinoic acid receptor genes in transgenic mice increases CD8 T-cell subset. Mol Biol Rep. 1993;17:135–42. doi: 10.1007/BF00996221. [DOI] [PubMed] [Google Scholar]
- 47.Devaux Y, Grosjean S, Seguin C, David C, Dousset B, Zannad F, et al. Retinoic acid and host-pathogen interactions: effects on inducible nitric oxide synthase in vivo. Am J Physiol Endocrinol Metab. 2000;279:E1045–53. doi: 10.1152/ajpendo.2000.279.5.E1045. [DOI] [PubMed] [Google Scholar]
- 48.Seguin-Devaux C, Hanriot D, Dailloux M, Latger-Cannard V, Zannad F, Mertes PM, et al. Retinoic acid amplifies the host immune response to LPS through increased T lymphocytes number and LPS binding protein expression. Mol Cell Endocrinol. 2005;245:67–76. doi: 10.1016/j.mce.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 49.Jansa P, Forejt J. A novel type of retinoic acid response element in the second intron of the mouse H2Kb gene is activated by the RAR/RXR heterodimer. Nucleic Acids Res. 1996;24:694–701. doi: 10.1093/nar/24.4.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fritsche J, Stonehouse TJ, Katz DR, Andreesen R, Kreutz M. Expression of retinoid receptors during human monocyte differentiation in vitro. Biochem Biophys Res Commun. 2000;270:17–22. doi: 10.1006/bbrc.2000.2371. [DOI] [PubMed] [Google Scholar]
- 51.Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, et al. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–20. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stenstad H, Ericsson A, Johansson-Lindbom B, Svensson M, Marsal J, Mack M, et al. Gut-associated lymphoid tissue-primed CD4+ T cells display CCR9-dependent and -independent homing to the small intestine. Blood. 2006;107:3447–54. doi: 10.1182/blood-2005-07-2860. [DOI] [PubMed] [Google Scholar]
- 53.Svensson M, Marsal J, Ericsson A, Carramolino L, Broden T, Marquez G, et al. CCL25 mediates the localization of recently activated CD8alphabeta(+) lymphocytes to the small-intestinal mucosa. J Clin Invest. 2002;110:1113–21. doi: 10.1172/JCI15988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berlin C, Berg EL, Briskin MJ, Andrew DP, Kilshaw PJ, Holzmann B, et al. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185–95. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 55.Briskin MJ, McEvoy LM, Butcher EC. MAdCAM-1 has homology to immunoglobulin and mucin-like adhesion receptors and to IgA1. Nature. 1993;363:461–4. doi: 10.1038/363461a0. [DOI] [PubMed] [Google Scholar]
- 56.Streeter PR, Berg EL, Rouse BT, Bargatze RF, Butcher EC. A tissue-specific endothelial cell molecule involved in lymphocyte homing. Nature. 1988;331:41–6. doi: 10.1038/331041a0. [DOI] [PubMed] [Google Scholar]
- 57.Wurbel MA, Philippe JM, Nguyen C, Victorero G, Freeman T, Wooding P, et al. The chemokine TECK is expressed by thymic and intestinal epithelial cells and attracts double- and single-positive thymocytes expressing the TECK receptor CCR9. Eur J Immunol. 2000;30:262–71. doi: 10.1002/1521-4141(200001)30:1<262::AID-IMMU262>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 58.Hamann A, Andrew DP, Jablonski-Westrich D, Holzmann B, Butcher EC. Role of alpha 4-integrins in lymphocyte homing to mucosal tissues in vivo. J Immunol. 1994;152:3282–93. [PubMed] [Google Scholar]
- 59.Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, et al. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 60.Johansson-Lindbom B, Svensson M, Wurbel MA, Malissen B, Marquez G, Agace W. Selective generation of gut tropic T cells in gut-associated lymphoid tissue (GALT): requirement for GALT dendritic cells and adjuvant. J Exp Med. 2003;198:963–9. doi: 10.1084/jem.20031244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–38. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 62.Delescluse C, Cavey MT, Martin B, Bernard BA, Reichert U, Maignan J, et al. Selective high affinity retinoic acid receptor alpha or beta-gamma ligands. Mol Pharmacol. 1991;40:556–62. [PubMed] [Google Scholar]
- 63.Li Y, Hashimoto Y, Agadir A, Kagechika H, Zhang X. Identification of a novel class of retinoic acid receptor beta-selective retinoid antagonists and their inhibitory effects on AP-1 activity and retinoic acid-induced apoptosis in human breast cancer cells. J Biol Chem. 1999;274:15360–6. doi: 10.1074/jbc.274.22.15360. [DOI] [PubMed] [Google Scholar]
- 64.Umemiya H, Fukasawa H, Ebisawa M, Eyrolles L, Kawachi E, Eisenmann G, et al. Regulation of retinoidal actions by diazepinylbenzoic acids. Retinoid synergists which activate the RXR-RAR heterodimers. J Med Chem. 1997;40:4222–34. doi: 10.1021/jm9704309. [DOI] [PubMed] [Google Scholar]