Abstract
Three papers in this issue of the European Journal of Immunology describe the use of cytokine vaccines to prevent autoimmune disease in experimental animals. The vaccines are based on interleukin 17 (IL-17), a cytokine that has recently been shown to play a central role in inflammation.
Keywords: Autoimmune disease, Cytokine, Interleukin 17, Vaccine, Immunotherapy
The T-cell cytokine network: Time for a paradigm change
The pioneering work of Mosmann and Coffman, in the mid-1980s, transformed our understanding of how T-helper (Th) cells control immunity [1]. They described two subsets of helper cells that control different arms of the immune system and counter-regulate each other through cytokine secretion. Th1 cells support cell mediated immunity and are essential for effective defence against intracellular bacteria and viruses. Th2 cells, on the other hand, support humoral immunity and are particularly important for dealing with helminth infections. A similar division of labour was adopted by immunologists to account for the role of Th1 and Th2 cells in autoimmunity and allergy. T-cell cloning techniques and cell transfer experiments provided convincing evidence that Th1 cells were responsible for organ-specific autoimmune diseases [2, 3]. The link between Th1 cells and organ specific autoimmunity was further supported by evidence that mice lacking the p40 chain of IL-12 were resistant to such diseases [4]. Th2 cells help B cells to secrete IgE antibodies, through secretion of IL-4, and thus play a central role in allergy. An individual’s susceptibility to either autoimmune disease or allergy could therefore be explained on the basis of their Th1-Th2 balance. Further development of the concept led to the idea that it would be possible to treat autoimmune and allergic diseases by changing the balance between Th1 and Th2 cells [5]. We now appreciate, however, that the Th1-Th2 paradigm is too simple and a number of additional CD4 T-cell subsets have been defined. These include a variety of T cells with regulatory properties (Treg) such as ‘natural’ Foxp3+ Treg, IL-10 secreting Treg (Tr1) and TGF-β secreting Th3 cells [6].
Questions have been raised about the apparent association between Th1 cells and organ specific autoimmunity. IFN-γ knockout mice suffer more severe or accelerated disease in various models of organ specific autoimmunity [7-9]. This suggests that IFN-γ may even play a protective role in these models and clearly dissociates Th1 cells from such organ specific diseases.
Discovery of IL-17-secreting Th cells
Mice lacking the IL-12 p40 subunit are resistant to the induction of organ specific autoimmune diseases such as EAE [10] and yet such diseases appear to be Th1 cell independent (see previous section). At first sight this is paradoxical since IL-12 supports the differentiation of Th1 cells. The p40 subunit is, however, shared with another cytokine, IL-23. Both cytokines have two subunits with IL-12 being made up of p40 and p35 whereas IL-23 contains p40 and p19. Mice deficient in the p19 subunit of IL-23 failed to generate a distinct subset of T-cells that secrete IL-17. Moreover these mice were resistant to EAE and collagen-induced arthritis [10,11]. It appears, after all, that Th1 cells are not essential for disease induction in these models but that a distinct IL-17-secreting Th-cell subset (Th17 cells) may fulfil this role.
Differentiation of Th17 cells
Th17 cells differentiate from naive precursors in vitro in the presence of TGF-β and IL-6 [12]. Furthermore, overexpression of TGF-β in vivo results in a significant increase in Th17 cells capable of causing encephalomyelitis [13]. Interestingly, IL-23 appears to be more important for the expansion and survival of this cell population rather than for their differentiation from naive precursors. The differentiation of Th17 cells is enhanced in the presence of IL-1 and TNF-α and recent studies have shown that IL-1 receptor type I deficient mice have reduced numbers of such cells [14]. In addition, recently published observations reveal that RORγkt is the transcription factor responsible for directing differentiation of Th17 cells [15], much as T-bet and GATA-3 are the differentiation factors for Th1 and Th2 cells [16].
Function of Th17 cells
IL-17 was originally described as a pro-inflammatory cytokine produced by activated CD4 cells [17]. It induces secretion of inflammatory mediators including IL-8, TNF, GM-CSF and CXCL1 from stromal endothelial cells and monocytes and hence promotes the mobilization of neutrophils (Fig. 1). Th17 cells are important for controlling the early response to injury and infection by recruiting neutrophils and thus limiting tissue necrosis and sepsis [4]. One can speculate that Th17 cells contribute to early, inflammatory responses while Th1 cells may have a more significant role in subsequent chronic inflammatory processes. With this in mind, it may be possible to rationalise previously conflicting data on the role of Th1 cells in autoimmune diseases such as EAE and collagen-induced arthritis. Both disease models are induced by injection of autoantigens in potent adjuvants such as complete Freund’s adjuvant (CFA). The immediate response to such potent inflammatory stimuli may well require Th17 cells, hence the need for IL-23, IL-1 and IL-6 in order to instigate such diseases. This does not exclude, however, a later role for Th1 cells, especially in the chronic phase of disease. This would explain why Th17 cells appear essential for autoimmune disease induction while polarized Th1 cell lines and clones can cause EAE following cell transfer. Nevertheless, the fact that Th17 cells play such an important role in the initiation of disease introduces IL-17 as a valid target for immune intervention.
Figure 1.
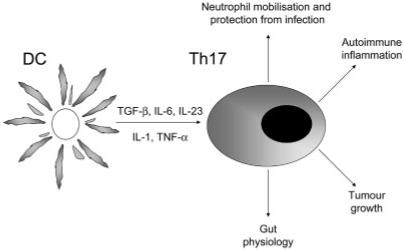
Differentiation of Th17 cells is supported by TGF-β, IL-6, TNF and IL-1 while IL-23 is required for their expansion and survival. Th17 cells secrete IL-17 and their major function is to enhance neutrophil mobilisation. IL-17 is required for effective immunity against specific bacterial and fungal infections in mice. Th17 cells contribute to autoimmune disease in certain models and can either increase or reduce immunity to cancer.
IL-17 as a vaccine for autoimmune diseases
Mice deficient in IL-17 are resistant to the induction of experimental autoimmune diseases such as collagen-induced arthritis [18]. Furthermore, treatment of mice with a neutralizing anti-IL-17 antibody suppresses autoimmune inflammation in the EAE model [11]. Three papers [19-21] in this issue of the European Journal of Immunology extend these previous findings and describe the use of IL-17 itself as a vaccine against autoimmunity. Sonderegger et al. [19] studied the role of IL-17 in experimental autoimmune myocarditis. This disease can be induced by injection of a peptide from cardiac myosin emulsified in CFA in Balb/c mice. Similar to previous studies in the EAE and collagen-induced arthritis models, the IL-12 p35 deficient mice developed myocarditis as severely as wild type mice whereas p40 deficient mice failed to show signs of disease. The role of IL-23 in experimental myocarditis was confirmed when it was shown that injection of anti-p19 antibody reduced the severity of disease. These results clearly add myocarditis to the list of autoimmune diseases, induced by injection of autoantigen in CFA, in which IL-17 plays a pivotal role. The next step was to test whether vaccination against IL-17 would interfere with disease induction. IL-17 was chemically coupled to virus-like particles (VLP) and injected without adjuvant three times over 28 days. Mice vaccinated with the VLP-IL-17 complex suffered significantly less heart inflammation than suitable controls. This correlated with a significant reduction in anti-myosin antibody titres in vaccinated mice. In a second paper, Röhn et al. [20] use the same VLP-IL-17 construct in models of arthritis and multiple sclerosis. Immunisation with VLP-IL-17 vaccine led to a lower incidence of disease and reduced severity in both collagen-induced arthritis and EAE. Finally, Uyttenhove and Van Snick [21] describe the use of IL-17 chemically coupled to ovalbumin as a vaccine for prevention of EAE. As in the two previous studies [20, 21], IL-17A was used for preparation of the vaccine. The antibodies induced by vaccination were specific for IL-17A and failed to bind any other isoform (IL-17B-F). Furthermore, a monoclonal antibody raised from a vaccinated mouse shared the same specificity. This study measured the stability of the antibody response induced by the vaccine and noted that the high levels of antibody declined slowly with a half-life of approximately five months. These results demonstrate that anti-cytokine vaccination can elicit long-term inhibition of IL-17 function.
Role of IL-17 in human pathology
As mentioned above, IL-17 plays an important role in neutrophil migration and inflammation and its involvement in experimental autoimmunity is indisputable. Two questions arise: does IL-17 play an equally important role in human disease and, secondly, will it be safe to target this cytokine for immunotherapy of human disease. Increased levels of IL-17 have been found in association with a range of human pathologies. The number of IL-17 mRNA expressing mononuclear cells was shown to increase in both blood and the cerebrospinal fluid in multiple sclerosis and this correlated with clinical disease [22]. Synovial fluids from patients with rheumatoid arthritis, but not with osteoarthritis, contain high levels of both IL-15 and IL-17 [23]. Importantly, IL-17 has been shown to contribute to osteoclastic bone resorption in rheumatoid arthritis patients [24]. IL-17 expression was also detectable in CD3+ T cells or CD68+ monocytes/macrophages in the inflamed mucosa of active ulcerative colitis and Crohn’s disease patients [25]. The average number of IL-17+ cells was significantly increased in both patient groups during active disease. Taken together these results show that IL-17 plays an important role in human inflammatory conditions and therefore this cytokine serves as a valid target for immune intervention.
Targeting IL-17 for immunotherapy
A number of questions arise from the studies conducted to date. IL-17 clearly plays a role in acute models of autoimmunity, induced by immunisation with autoantigen in CFA. Furthermore, treatment with IL-17 blocking agents has been shown to reduce symptoms after onset of disease [26]. As yet, however, there are no reported intervention studies in chronic-relapsing disease. An obvious concern when targeting any important cytokine is the effect this might have on immunity to infections and cancers. The IL-23-IL-17 axis plays a significant role in host defence. The IL-23-supported IL-17 response is vital for host defence against lung pathogens such as Klebsiella pneumoniae [26] and Citrobacter rodentium [27]. Moreover, recent data suggests that the mIL-17A/mIL-17AR system is required for normal fungal-host defence in vivo. Mice deficient for the IL-17A receptor display increased sensitivity to infection with Candida albicans [28]. It is important to stress, however, that humans and mice with defects in the IL-12-Th1 axis are more frequently affected by serious infections than those with genetic defects in IL-23 [29]. As such, it may be safer to target the IL-23-Th17 rather than the IL-12-Th1 axis in the hope that this would alleviate the symptoms of organ specific autoimmunity without causing serious infection.
IL-17 family members have displayed both pro- and anti-tumour activities. IL-17 has tumour-promoting angiogenic properties [30] and both IL-23 and IL-17 are found at higher levels in certain tumours [27]. Recent work has suggested that IL-12 and IL-23 antagonize each other in tumour surveillance. Thus, IL-12 promotes tumour infiltration by cytotoxic T cells whereas IL-23 may decrease infiltration of cytotoxic T cells while promoting angiogenesis [31] in some cancers. By contrast, IL-17 was shown to enhance the cytotoxic T cell response to murine haematopoietic tumours [32] and hence to limit their growth in vivo. It is difficult, therefore, to predict the effect that IL-17 inhibition will have on the susceptibility of an individual to different types of cancer. This may depend on the contribution of angiogenesis to progression of the particular tumour, the immunogenicity of the tumour and the immune status of the host.
A further concern in targeting IL-17 is the role that this molecule appears to play in gut physiology. IL-17 induces formation of tight junctions in human intestinal epithelial cells, a process that is likely to have evolved to help maintain gut barrier function in the face of mucosal immune responses [32]. This could explain why IL-17 is upregulated during inflammatory bowel disease and why neutralisation of IL-17 aggravates dextran sulphate-induced colitis in mice [33].
In conclusion, the three papers [19-21] published in this issue of the European Journal of Immunology support the central role of IL-17 in certain organ specific inflammatory autoimmune diseases. Furthermore, they demonstrate the ability to vaccinate against the cytokine and hence generate antibodies that provide long-term inhibition of IL-17 activity in vivo. Anti-cytokine vaccines will undoubtedly prove to be important tools for studying the role of specific cytokines in disease. At this stage, however, it is not clear whether long-term inhibition of IL-17 activity will be safe in man. More extensive human studies are required to define the precise role of IL-17 in inflammatory autoimmune diseases and asthma. If IL-17 is shown to play a central role in these diseases then cautious intervention would be warranted. It will be sensible, however, to intervene first with neutralizing antibodies. This will reveal whether or not neutralisation of IL-17 will increase the risk of infection and/or cancer. Only if the use of cytokine neutralising antibodies were to provide significant benefit over the associated risk of infection, would it be safe to consider the use of anti-IL-17 vaccines.
Abbreviation
- VLP
virus-like particle
References
- 1.Mosmann TR, Coffman RL. Two types of mouse helper T-cell clone. Implications for immune regulation. Immunol. Today. 1987;8:223–227. doi: 10.1016/0167-5699(87)90171-X. [DOI] [PubMed] [Google Scholar]
- 2.Khoruts A, Miller SD, Jenkins MK. Neuroantigen-specific Th2 cells are inefficient suppressors of experimental autoimmune encephalomyelitis induced by effector Th1 cells. J. Immunol. 1995;155:5011–5017. [PubMed] [Google Scholar]
- 3.Katz JD, Benoist C, Mathis D. T helper cell subsets in insulin-dependent diabetes. Science. 1995;268:1185–1188. doi: 10.1126/science.7761837. [DOI] [PubMed] [Google Scholar]
- 4.McKenzie BS, Kastelein RA, Cua DJ. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 2006;27:17–23. doi: 10.1016/j.it.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Liblau RS, Singer SM, McDevitt HO. Th1 and Th2 CD4+ T-cells in the pathogenesis of organ-specific autoimmune diseases. Immunol. Today. 1995;16:34–38. doi: 10.1016/0167-5699(95)80068-9. [DOI] [PubMed] [Google Scholar]
- 6.Wraith DC, Nicolson KS, Whitley NT. Regulatory CD4+ T cells and the control of autoimmune disease. Curr. Opin. Immunol. 2004;16:695–701. doi: 10.1016/j.coi.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Chu CQ, Wittmer S, Dalton DK. Failure to suppress the expansion of the activated CD4 T cell population in interferon gamma-deficient mice leads to exacerbation of experimental autoimmune encephalomyelitis. J. Exp. Med. 2000;192:123–128. doi: 10.1084/jem.192.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manoury-Schwartz B, Chiocchia G, Bessis N, Abehsira-Amar O, Batteux F, Muller S, Huang S, et al. High susceptibility to collagen-induced arthritis in mice lacking IFN-gamma receptors. J. Immunol. 1997;158:5501–5506. [PubMed] [Google Scholar]
- 9.Vermeire K, Heremans H, Vandeputte M, Huang S, Billiau A, Matthys P. Accelerated collagen-induced arthritis in IFN-gamma receptor-deficient mice. J. Immunol. 1997;158:5507–5513. [PubMed] [Google Scholar]
- 10.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 11.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 14.Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J. Exp. Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The Orphan Nuclear Receptor RORγt Directs the Differentiation Program of Proinflammatory IL-17+ T Helper Cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 16.O’Garra A, Arai N. The molecular basis of T helper 1 and T helper 2 cell differentiation. Trends Cell Biol. 2000;10:542–550. doi: 10.1016/s0962-8924(00)01856-0. [DOI] [PubMed] [Google Scholar]
- 17.Rouvier E, Luciani MF, Mattei MG, Denizot F, Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J. Immunol. 1993;150:5445–5456. [PubMed] [Google Scholar]
- 18.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J. Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 19.Sonderegger I, Röhn TA, Kurrer MO, Iezzi G, Zou Y, Kastelein RA, Bachmann MF, Kopf M. Neutralization of IL-17 by active vaccination inhibits IL-23-mediated autoimmune myocarditis. Eur. J. Immunol. 2006;36 doi: 10.1002/eji.200636484. DOI: 10.1002/eji.200636484. [DOI] [PubMed] [Google Scholar]
- 20.Röhn TA, Jennings GT, Hernandez M, Grest P, Beck M, Zou Y, Kopf M, Bachmann MF. Vaccination against IL 17 suppresses autoimmune arthritis and encephalomyelitis. Eur. J. Immunol. 2006;36 doi: 10.1002/eji.200636658. DOI: 10.1002/eji.200636658. [DOI] [PubMed] [Google Scholar]
- 21.Uyttenhove C, Van Snick J. Development of an anti-IL 17A auto-vaccine that prevents experimental auto-immune encephalomyelitis. Eur. J. Immunol. 2006;36 doi: 10.1002/eji.200636662. DOI: 10.1002/eji.200636662. [DOI] [PubMed] [Google Scholar]
- 22.Matusevicius D, Kivisakk P, He B, Kostulas N, Ozenci V, Fredrikson S, Link H. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult. Scler. 1999;5:101–104. doi: 10.1177/135245859900500206. [DOI] [PubMed] [Google Scholar]
- 23.Ziolkowska M, Koc A, Luszczykiewicz G, Ksiezopolska-Pietrzak K, Klimczak E, Chwalinska-Sadowska H, Maslinski W. High levels of IL-17 in rheumatoid arthritis patients: IL-15 triggers in vitro IL-17 production via cyclosporin A-sensitive mechanism. J. Immunol. 2000;164:2832–2838. doi: 10.4049/jimmunol.164.5.2832. [DOI] [PubMed] [Google Scholar]
- 24.Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, Saito S, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J. Clin. Invest. 1999;103:1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Happel KI, Zheng M, Young E, Quinton LJ, Lockhart E, Ramsay AJ, Shellito JE, et al. Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J. Immunol. 2003;170:4432–4436. doi: 10.4049/jimmunol.170.9.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 28.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J. Infect. Dis. 2004;190:624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 29.Bowman EP, Chackerian AA, Cua DJ. Rationale and safety of anti-interleukin-23 and anti-interleukin-17A therapy. Curr. Opin. Infect. Dis. 2006;19:245–252. doi: 10.1097/01.qco.0000224818.42729.67. [DOI] [PubMed] [Google Scholar]
- 30.Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, et al. Interleukin-17 promotes angiogenesis and tumour growth. Blood. 2003;101:2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 31.Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K, Basham B, et al. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- 32.Benchetrit F, Ciree A, Vives V, Warnier G, Gey A, Sautes-Fridman C, Fossiez F, et al. Interleukin-17 inhibits tumour cell growth by means of a T-cell-dependent mechanism. Blood. 2002;99:2114–2121. doi: 10.1182/blood.v99.6.2114. [DOI] [PubMed] [Google Scholar]
- 33.Ogawa A, Andoh A, Araki Y, Bamba T, Fujiyama Y. Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin. Immunol. 2004;110:55–62. doi: 10.1016/j.clim.2003.09.013. [DOI] [PubMed] [Google Scholar]


