Abstract
Therapeutic approaches to the treatment of Alzheimer's disease are focused primarily on the Aß peptide which aggregates to form amyloid deposits in the brain. The amyloid hypothesis states that amyloid is the precipitating factor that results in the other pathologies of Alzheimer's, namely neurofibrillary tangles and neurodegeneration, as well as the clinical dementia. One such therapy that has attracted significant attention is anti-Aß immunotherapy. First described in 1999, immunotherapy uses anti-Aß antibodies to lower brain amyloid levels. Active immunization, in which Aß is combined with an adjuvant to stimulate an immune response producing antibodies and passive immunization, in which antibodies are directly injected, were shown to lower brain amyloid levels and improve cognition in multiple transgenic mouse models. Mechanisms of action were studied in these mice and revealed a complex set of mechanisms that depended on the type of antibody used. When active immunization advanced to clinical trials a subset of patients developed meningoencephalitis; an event not predicted in mouse studies. However, it was suspected that a T-cell response due to the type of adjuvant used was the cause of the meningoencephalitis and studies in mice indicated alternative methods of vaccination. Passive immunization has also advanced to phase III clinical trials on the basis of successful transgenic mouse studies. Reports from the active immunization clinical trial indicated that, indeed, amyloid levels in brain were reduced. While APP transgenic mouse models are useful in studying amyloid pathology these mice do not generate significant tau pathology or neuron loss. Continued development of new mouse models that do generate all of these pathologies will be critical in more accurately testing therapeutics and predicting the clinical outcome of such therapeutics.
Alzheimer's disease and the amyloid hypothesis
Alzheimer's disease (AD) is a neurodegenerative disorder leading to a dementia with progressive loss of brain function. The primary risk factor for AD is age, with onset typically in the 70s−90s. The mean life expectancy is anywhere from 7 to 15 years after the initial diagnosis, however, rates of progression vary significantly between patients. While diagnosis of AD may be made through a battery of cognitive tests, a definite diagnosis can only be made at autopsy by microscopic examination of the brain tissue. According to the NIA-Reagan criteria a diagnosis of AD requires the presence of amyloid deposits, neurofibrillary tangles and neurodegeneration as well as dementia [1]. Amyloid plaques are insoluble, extracellular accumulations of amyloid-beta (Aβ) peptides. Neurofibrillary tangles are intraneuronal accumulations of hyperphosphorylated, aggregated tau protein (a microtubule binding protein) that redistributes to the neuronal soma. There are many accompanying pathologies in AD including cerebral amyloid angiopathy (accumulation of amyloid in the cerebrovasculature) and neuroinflammation (microglial and astrocytic reactivity to the abnormal proteins in the Alzheimer brain). These likely play a significant role in the disease progression.
The amyloid hypothesis of AD is based upon the pathologic characteristics and the genetics of the disease. Early onset-familial Alzheimer's disease (FAD) is a rare, genetic form of the disease. To date, all genes known to cause FAD are involved in the production of Aß, and therefore amyloid. These genes are the amyloid precursor protein (APP) gene, and the presenilin 1 (PS1) and presenilin 2 (PS2) genes. APP is a single membrane-spanning protein whose exact physiological function is unknown. However, data suggest that APP may be involved in synapse formation and stability, cell adhesion, memory and even possibly may act as a G-protein coupled receptor (reviewed by [2]). APP can be cleaved by 3 enzymes; α, ß and γ secretase. Cleavage by ß and γ produces the Aß peptide; the length of which is determined by the γ-secretase cleavage. Under normal conditions an α cleavage is the dominant cleavage, which produces non-amyloidogenic fragments (reviewed by [3]). The presenilins are highly conserved proteins with 8 transmembrane domains and are now known to be part of the γ-secretase complex. Both PS1 and PS2 are physiologically cleaved forming 2 polypeptides that may function in the control of apoptosis. It is also known that genetic deletion of presenilins is lethal due to alteration of Notch processing and signaling (reviewed by [4]).
Very simply, the amyloid cascade hypothesis states that deposition of Aß in the brain is the precipitating factor that then results in tau hyperphosphorylation, aggregation and, ultimately, neurofibrillary tangles. Amyloid deposition and tau pathology are then thought to both contribute to neuronal degeneration, which results in the cognitive decline in AD [5]. In support of the amyloid hypothesis, all FAD mutations either increase total Aß production (via APP mutations) or shift Aß production to the more fibrillogenic Aß1−42 species (via PS mutations) (reviewed by [6]). Also supporting this hypothesis is the pathology of Down's syndrome. Down's syndrome is caused by a triplication of chromosome 21. This chromosome carries the APP gene, therefore, APP is triplicated along with a number of other important genes. It is well known that Down's syndrome patients develop AD. By 40 years of age 25% of Down's patients develop clinical AD and by 60 years of age 65% develop AD. At autopsy all Down's patients have significant amyloid deposition in their brains [7]. Similarly, there are families carrying duplication of the APP locus which results in autosomal dominant early-onset Alzheimer's disease with CAA [8-10].
Overview of transgenic mice
Mouse models of Alzheimer's disease (AD) are primarily focused around the familial mutations in APP or the PS1 and PS2 genes. Table 1 summarizes the transgenic mouse models that will be discussed in this review. The PDAPP transgenic mouse was the first reported APP transgenic mouse to develop amyloid deposits similar to those found in the brains of Alzheimer's disease (AD) patients. This mouse carries the V717F APP mutation that in humans is associated with early onset AD. An 18-fold overexpression of mutated human APP is observed, and amyloid deposits are first detected at approximately 6 months of age. The PDAPP mice also develop mild cerebral amyloid angiopathy (CAA) by 18 months [11]. A similar mouse, the Tg2576 mouse carries the Swedish mutation in APP, the K670M/ M671L. The overexpression of mutant human APP in the Tg2576 mouse is 5-fold, and the rate of amyloid deposition is moderate with amyloid deposits first detected at 6 months of age. CAA is sparse and is most commonly observed by 18 months of age [12]. The majority of studies examining immunotherapy have used either the PDAPP or Tg2576 mice.
Table 1.
Summary of transgenic mice discussed in this review. + scale indicates severity of given pathology.
| Transgenic mouse model | Mutation(s) | Expression level | Rate of amyloid deposition | Tau Pathology | Neuron loss | CAA severity | Primary Ref |
|---|---|---|---|---|---|---|---|
| PDAPP | Human APP V717F | 18-fold | Moderate | +/− | − | + | (Games et al., 1995 Nature 373, 523−527) |
| Tg2576 | Human APP K670M/M671L | 5-fold | Moderate | +/− | − | + | (Hsiao et al., 1996 Science 274, 99−102) |
| APP23 | Human APP K670N/M671L | 7-fold | Moderate | +/− | + | ++ | (Sturchler-Pierrat et al., 1997 Proc Natl Acad Sci USA 94, 13287−13292) |
| TgCRND8 | Human APP V717F and K670M/M671L | 5-fold | Rapid | +/− | − | ++ | (Chishti et al., 2001 J Biol Chem 276, 21562−21570) |
| APP:V717I | Human APP V717I | 10-fold | Moderate | +/− | − | ++ | (Moechars et al., 1999 J Biol Chem 274, 6483−6492) |
| APPSwDI | Human APP K670N/M671L, E693Q, D694N | 0.5-fold | Rapid | +/− | − | +++ | (Davis et al., 2004 J Biol Chem 279, 20296−20306) |
| APP+PS1 Tg2576 × M146L | Human APP KM670/671NL; PS1 M146L | 5-fold (APP) | Rapid | +/− | − | + | (Holcomb et al., 1998 Nat Med 4, 97−100) |
| 3×Tg | Human APP KM670/671NL; PS1 M146V. tau P301L | 4-fold (APP) | Moderate | ++ | (unknown) | (Oddo et al., 2003 Neuron 39, 409−421) |
Other commonly used APP transgenic mice include the APP23 mouse which carries the K670N, M671N mutations in human APP, and has higher level of expression than the Tg2576 mouse [13] and the TgCRND8 mouse, which carries both the V717F and the K670M, M671N mutations in APP [14]. Because of the mutations that affect both ß secretes and γ secretase activities, the TgCRND8 mouse has a very aggressive rate of amyloid deposition. Finally, the APP:V717I mouse carries an APP mutation at the same site as the PDAPP mouse, and it's pathology closely resembles that of the PDAPP [15]. However, it should be noted that all of these mouse models are most useful as models of amyloid deposition, as opposed to models of AD, since they do not develop significant tau pathology or neuron loss (the APP23 mouse has been shown to have some cortical neuron loss; [16]). A model of vascular amyloid pathology is the APPSwDI mouse, which carries the K670M/N671L, E693Q and the D694N APP mutations, corresponding to the Swedish, Dutch and Iowa mutations. This mouse has a rapid rate of amyloid deposition with a high percentage of the amyloid associated with the vasculature [17]. This mouse was later shown to have a deficient clearance of Aß across the blood-brain barrier [18]. The mutations in presenilin alone do not result in amyloid deposition [19]. However, when the mouse carrying the M146L mutation in PS1 [19] was crossed with the Tg2576 APP transgenic mouse [12] it was found that the rate of amyloid deposition was significantly accelerated, and Aß production favored the more fibrillogenic Aß1−42 species [20]. The APP/PS1 transgenic mouse also do not develop significant tau pathology or neuron loss.
More recent advances in Alzheimer's disease mouse models involve the addition of tau pathology. Mutations in the tau protein have been found in humans diagnosed with fronto-temporal dementia (FTD). The first transgenic mouse model incorporating a FTD human tau mutation was the JNPL3 mouse, which carries the human tau P301L mutation. The P301L mouse shows hyperphosphorylated tau at disease associated sites, redistribution of tau to the dendrites and soma of neurons, and formation of neurofibrillary tangle-like structures within neurons [21]. This mouse has provided useful information on the role of mutated human tau in neurofibrillary tangle formation and in neuronal pathology. In 2003 Oddo et al presented a triple transgenic (3×Tg) mouse that carried the Swedish mutation in human APP (same APP mutations as Tg2576), the M146L mutation in human PS1 and the P301L mutation in human tau. This mouse develops significant intra-neuronal Aß and parenchymal amyloid deposits, as well as intraneuronal hyperphosphorylated tau aggregates [22]. Neuron counts from this 3×Tg mouse by stereological methods show no significant loss of neurons, even in brain regions with high levels of tau and amyloid pathology [23].
Most recently, several novel approaches to the generation of transgenic mice modeling AD have been significantly more successful in demonstrating progression of disease beyond amyloid deposition. Capsoni et al (2000) showed that a mouse expressing recombinant antibodies neutralizing nerve growth factor (NGF), develops both amyloid plaques and tau pathology [24]. This represents a significant advance in transgenic mouse modeling as the amyloid and tau pathologies occur with normal mouse proteins. Also, we have recently shown in two different APP transgenic mice (the Tg2576; APPSw and the APPSwDI mice) that genetic deletion of nitric oxide synthase 2 (NOS2) results in progression of amyloid pathology to include normal mouse tau pathology and significant neuron loss [25;26] (NOS2 deletion is reviewed in this issue by Colton et al).
The beginning of anti-Aß immunotherapy
In 1999 an astounding publication by Schenk et al of Elan pharmaceuticals suggested that immunization against Aß peptide could be used as a potential therapeutic for AD. In Schenk's study PDAPP mice were either immunized with fibrillar Aß1−42 in an immune adjuvant prior to the onset of pathology or at an age when significant amyloid pathology was present. Mice that were immunized prior to the onset of pathology and continued to be immunized monthly had low levels of detectable amyloid. Furthermore, in those mice that were immunized at an age when significant amyloid pathology was already present, impressive reductions in amyloid deposition were noted [27]. Following this publication, two additional reports demonstrated that Aß vaccination in the APP+PS1 [28] or TgCRND8 [29] transgenic mice improved performance in learning and memory tasks. The same Aß immunization protocol used by Schenk et al was utilized in both studies, and behavioral deficits in the radial-arm water maze task [28] and the Morris water maze task [29] were significantly reduced by vaccination. Together, these initial data indicated that reduction of amyloid deposition could be achieved by immunization and was sufficient alone to improve learning and memory in mice.
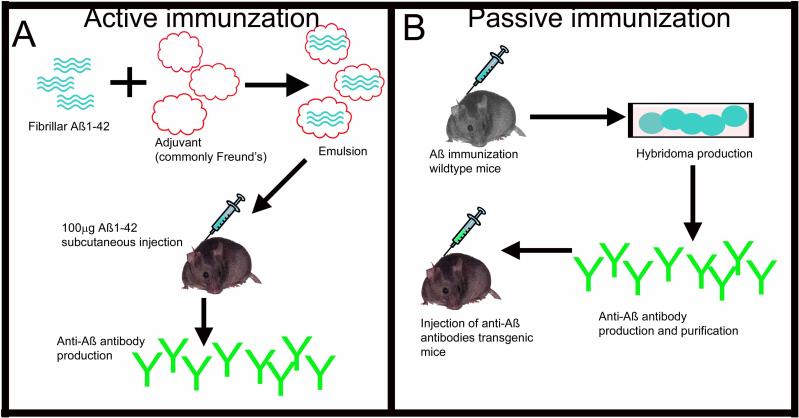
The early vaccination protocols in mice used an active immunization approach. Active immunization uses an immunogen, in this case Aß, combined with an adjuvant to stimulate an immune response, in this case Freund's adjuvant. The animal / patient's immune system generates anti-Aß antibodies that are then thought to result in reductions in amyloid deposition (Fig. 1A). Another approach of anti-Aß immunotherapy was suggested that would allow greater control of dose, and also allow a mechanism to withdraw treatment should any adverse events become apparent. This approach was passive immunization. Passive immunization involves the in vitro production of monoclonal anti-Aß antibodies, and then direct infusion of these antibodies into the patient / mouse (Fig. 1B).
Figure 1.
The two types of immunotherapy for Alzheimer's disease. Panel A depicts active immunization. Fibrillar Aß1−42 is combined with an adjuvant by emulsification. This mixture is then injected into the mouse. The mouse produces anti-Aß antibodies in response to the vaccination. Panel B depicts passive immunization. In this case, mice are immunized with Aß as with active immunization. Hybridomas are then produced and selected for the correct antibody. This antibody is then harvested, purified and administered to another mouse for treatment.
Active immunization
Administration of an antigen plus an adjuvant is the classical approach to an active immunization. An adjuvant is a solution that is designed to stimulate an immune response in order to generate antibodies to the immunogen. Many times, an adjuvant consists of bacteria fragments. For example, the adjuvant used in many of the mouse studies was Freund's adjuvant. Freund's consists of inactivated and dried mycobacteria, usually mycobacterium tuberculosis. The goal of this process is to generate a significant antibody response against the antigen via activation of B cell antibody production, and increased T cell activation. However, unregulated T cell responses, or the wrong type of T cell response (type 1 vs. type 2 for example), is detrimental and can result in an inappropriate immune response [30]. Active immunization against Aβ advanced to clinical trials in Alzheimer patients based on the initial transgenic mouse data showing reduced amyloid deposition and cognitive decline. The trial was called AN1792 and involved up to 5 immunizations over a 36 week period. The clinical trial was suspended due to an occurrence of subacute meningoencephalitis (inflammation of the brain and meninges) in approximately 6% of patients [31]. It is important to note that meningoencephalitis had not been observed in mouse studies using the amyloid deposition models available at that time. Following this disrupted clinical trial, subsequent active immunization studies have focused on 1) testing various adjuvants to overcome an inappropriate t-cell response, and 2) alternate immunization protocols (summarized in table 2).
Table 2.
Summary of alternate approaches to active vaccination protocols.
| Component | Modification | Effect |
|---|---|---|
| Adjuvant | IL-4 and GM-CSF | Aß + IL-4 + GM-CSF in TgCRND8 mice generated significant antibody titers and reduces brain Aß. DaSilva et al, Neurobiol Dis 2006 23:433−444. |
| Alum | Induces a beneficial Th1-type response and significant antibody titers. Cribbs et al, Int Immunol 2003 15:505−514. | |
| |
LT(R192G) |
No evidence of Th2 response. Maier et al, Vaccine 2005 23:5149−5159. Significant antibody titers and reduces brain Aß in PDAPP transgenic mice. Lemere et al, Neurobiol Aging 2002 23:991−100 |
| Immunogen | Dendrimeric Aß1−15 | Significant antibody titers and reduces brain Aß in J20 transgenic mice. Seabrook et al, J Neuroinflamm 2006 3:14. |
| Aß1−11 | Significant antibody titers, improved cognition and reduces brain Aß. Movsesyan N et al, PLoS ONE 2008 3:e2124. | |
| |
Aß3−6 |
Significant antibody titers and reduces brain Aß in APPV:717F mice. Frenkel et al, Vaccine 2003 21:1060−1065. |
| Mode of Administration |
Intranasal |
Useful for boosting, significant antibody titers and reduces brain Aß. Lemere et al, Ann NY Acad Sci 2000 920:328−331. |
| Mode | Peptide | Dendrimeric Aß1−15 (see above), Aß1−42 produces significant antibody titer and brain Aß reductions. Schenk et al, Nature 1999 400:173−177. |
| DNA | Plasmid containing 3 × Aß1−11, PADRE epitope and CCL22 chemokine improve cognition and reduce brain Aß in 3×Tg mice. Movseyan et al, PLoS ONE 2008 3:e2124. Amplicon vector containing Aß and IL-4 packaged in HSV amplicon produces significant antibody titers and lowers brain Aß. Frazer et al, Mol Ther 2008:845−853. | |
| Phage | Filamentous phage displaying Aß3−6 produce significant antibody titers and reduces brain Aß. Frenkel et al, Vaccine 2003 21:1060−1065. |
Speculation on the reasons for failure of the initial clinical trial on active vaccination focused on the type of adjuvant used to promote the antibody response. The adjuvant used in the AN1792 trial was QS21, which is a Th1 type adjuvant. This type of adjuvant can initiate the production of pro-inflammatory cytokines such as interferon-γ (IFNγ) [32] . QS21 likely stimulated a T-cell reaction leading to development of meningoencephalitis. Multiple studies using transgenic mouse models explored alternatives to the QS21 adjuvant that would stimulate a Th2 response, as opposed to a Th1 response. For example, when alum, an aluminum salt based Th2-based adjuvant, was used to stimulate a B cell humoral response to Aß1−42 in Tg2576 mice, significant reductions in amyloid levels were observed. The effect on amyloid levels, however, was only seen in mice treated from 11− 24 months of age. Tg2576 mice immunized with Aß1−42 from 19 to 24 months of age showed no significant changes [33]. Another elegant study compared the humoral and cellular immune responses produced by 2 different adjuvants used in subcutaneous Aß vaccination. In this study monophosphoryl lipid A (MPL)/trehalose dicorynomycolate (TDM) was compared to Escherichia coli heat-labile enterotoxin LT (R192G). MPL/TDM generated a much greater antibody titer than LT(R192G), and was accompanied by a moderate splenocyte proliferation and interferon-gamma (IFNγ) production indicating a cellular response [34].
A critical study separately tested complete Freund's adjuvant (CFA), alum, TiterMax Gold (TMG) and QS21 as adjuvants for Aß1−42 vaccination. QS21 and CFA induced significantly high antibody titers. Titers were intermediate with alum and lowest with TMG. Alum primarily produced a Th2 biased response (IgG1 antibody production) whereas the other adjuvants produced a Th1 biased response (IgG2a production). Because Th1-type responses have been implicated in autoimmune disease, adjuvants that induce Th-1 responses are likely to be less useful in Aß vaccination protocols. Also, both QS21 and CFA stimulated a significant T-cell response as indicated by IFNγ production [32]. Cribbs et al have further mapped the T-cell epitope of Aß to the 6−28 sequence, while the dominant B cell epitope was found within the 1−15 region [32]. Consequently, vaccines can be designed that preferentially use this identified B-cell epitope over those regions of the Aß peptide that initiate adverse T-cell responses. Together, these data provide valuable information for the design of future clinical trials, including improved antigen and adjuvant approaches. It is still uncertain whether, indeed, the adjuvant in the AN1792 trial did cause meningoencephalitis. If a T-cell response was the cause of the meningoencephalitis these mouse studies provide valuable information regarding the importance of adjuvant selection for design of future clinical trials.
Another approach to increase the safety of active immunization is to examine alternate approaches to administration of the vaccination as well as more sophisticated methods of stimulating anti-Aß antibody production. Lemere and colleagues from Harvard University were the first to suggest the use of intranasal administration of Aß vaccination as an alternative to the subcutaneous route. They showed that weekly intranasal immunizations using Aß1−40 alone could stimulate antibody responses in PDAPP transgenic mice sufficient for reduction in brain Aß levels (50−60% after 7 months of treatment) [35]. Importantly, it was shown that there was no detectable T- cell response by measuring IFNγ levels. It was later shown that addition of the LT (R192G) adjuvant produced 16-fold higher antibody titers than with no adjuvant at all [36].
Solomon and colleagues of Tel-Aviv University have studied the use of filamentous phage displaying 4 immunogenic residues of Aß. Filamentous phage stimulate a humoral immune response for antibody production. The phage can therefore be used to display short immunogenic determinants fused to their surface resulting in antibody production against these short fragments, in this case the EFRH residues of Aß. Solomon et al showed in 1997 that N-terminal anti-Aß antibodies bound to preformed amyloid fibrils and caused a disaggregation and neutralization of their neurotoxicity [37]. The EFRH residues of Aß, corresponding to positions 3−6 of Aß, were found to be the epitope of the anti-aggregating properties of Aß [38;39]. A filamentous phage displaying the EFRH peptide, the sequence required for disaggregation of amyloid, was used as antigen for immunization of wildtype [40] and APP:V717I transgenic mice [41]. In wildtype mice, the EFRH phage was found to generate significant antibody titers. In APP:V717I transgenic mice the EFRH phage was shown to reduce amyloid plaques levels by approximately 50% [41]. A similar approach to direct antibody production to the N-terminal of Aß is to use dendrimeric Aß1−15 (therefore excluding the T cell epitope of Aß in the Aß15−42 region). Aß1−15 alone was insufficient for antibody production [42]. However, when 16 copies of Aß1−15 were assembled on a branching tree (dAß1−15) and used as the immunogen with LT (R192G) as the adjuvant, significant antibody, titers were produced. In J20 transgenic mice (carrying the same mutations as the PDAPP mouse), a single Aß40/42 immunization followed by dAß1−15 boosts produced robust antibody titers, and significantly lowered amyloid plaque levels in the brain by approximately 60% [43]. Because the B cell epitope is located in the N-terminal of Aß and the T cell epitope is located in the middle of Aß, generation of N-terminal antibodies may avoid an inappropriate T cell response resulting in adverse events.
Approaches to active immunization have recently focused on avoiding the use of classical adjuvants, and instead employ techniques that direct the stimulated immune response to antibody production. Both IL-4 and GM-CSF are cytokines that drive dendritic cell differentiation and direct a Th2-type response. DaSilva et al (2006) used these cytokines in combination to elicit a Th2-type response as a replacement for a traditional adjuvant in TgCRND8 mice. This combination elicited high antibody titers and significantly reduced amyloid deposition [44]. Bowers et al (2005) directly stimulated an immune response by using herpes simplex virus (HSV)-amplicon mediated Aß vaccination. HSV amplicon vectors have been shown to elicit a vigorous transgene product-specific immune response in vivo [45]. In this case, an amplicon vector coding for Aß, with and without a tetanus toxin Fragment C (TxFC) adjuvant, was packaged in an HSV amplicon. The HSV amplicon was then used in a monthly vaccination protocol for 3 months in Tg2576 mice. Antibody titers were elevated and no significant difference was seen with the TxFC adjuvant alone. HSV-Aß resulted in a significant T-cell response and some T-cell infiltration into the brain parenchyma. However, only moderate amyloid reductions were observed with this protocol [46]. More recently, the authors improved this approach by including IL-4 with Aß in the expression vector. The rationale for this approach was that IL-4 is a Th2 cytokine that promotes a Th2-biased immune response. When administered to the 3×tg mice HSV-Aß-IL4 initiated a Th2 response, produced antibody titers, and reduced Aß levels to undetectable levels [47].
The most recent approach to active vaccination has been to engineer a molecule that will stimulate an irrelevant T-cell response (i.e. not directed to Aß) along with Aß1−11. A non-self promiscuous epitope termed PADRE (pan HLA DR-binding epitope) was combined with Aß1−11 using a DNA vaccine approach. This vaccination study utilized a plasmid containing three copies of the gene encoding the B cell epitope Aβ1−11, and the gene for the synthetic T cell peptide, PADRE. Also, expression of the macrophage derived chemokine (MDC/CCL22) was included, which has been shown to both stimulate Th2-type responses and suppress Th1-type responses. The construct produced pMDC-3Aß1−11-PADRE fusion protein in CHO cells in vitro. In vivo, a significant humoral response was detected with a highly polarized Th2-type response. Critically, cognitive performance of 3×Tg mice was improved and amyloid deposition was significantly reduced using the DNA vaccine protocols [48].
Passive immunization
Passive immunization describes the direct injection of antibodies, bypassing the requirement for an immune response to generate the antibodies. The benefits of this immunization technique include targeting of specific epitopes of the Aß molecule, controlling the amount of antibody administered, and the ability to rapidly withdraw treatment if adverse events are discovered. The major disadvantage of this method is the expense required to produce monoclonal antibodies. Bard et al (2000) were the first group to describe the use of passive immunization in mice [49]. Their study used several different monoclonal anti-Aß antibodies including 3D6 (IgG2b Aß1−15), 10D5 (IgG1 Aß3−7), or 16C11 (IgG1 Aß33−42) that targeted various Aß epitopes, and represented different IgG isotypes. The antibodies were injected intraperitoneally in 11−12 month old PDAPP mice weekly for 6 months. Amyloid deposits were significantly reduced in mice treated with 3D6 (IgG2b Aß1−15), or 10D5(IgG1 Aß3−7), whereas 16C11 (IgG1 Aß33−42) had no effect. The authors later mapped isotype and epitope specificities in PDAPP mice using antibodies that represented various epitopes and plaque binding abilities, as well as different IgG isotypes. The conclusion from this series of studies was that IgG2a antibodies recognizing the N-terminal of Aß (3D6 -IgG2b Aß1−15 or 10D5 -IgG1 Aß3−7), were most effective at reducing brain amyloid [50]. IgG2a has the highest affinity for the Fcγ receptor in mouse, and therefore, the authors suggest that Fcγ receptor mediated clearance by microglia is a requirement for amyloid removal. The use of N-terminal antibodies, however, has been associated with adverse events. In 2002 Pfeifer et al showed that passive immunization of aged APP23 mice with an IgG1 N-terminal Aß antibody caused a significant increase in the occurrence of CAA-associated microhemorrhage as well as acute hematomas [51].
Epitope requirements for Aß remains controversial. For example, an IgG1 antibody directed toward the mid-domain of Aß (Aß13−28) was shown by DeMattos et al (2001) to significantly reduce brain amyloid deposits in 4 month old PDAPP mice treated every other week for 5 months. Interestingly, this antibody did not bind in vivo plaques but does bind soluble, monomeric Aß with high affinity. Serum Aß levels increased rapidly, and significantly, following a single intravenous injection of the antibody (called m266). The authors, therefore, suggested that the m266 antibody worked to reduce brain amyloid via a peripheral clearance as opposed to a central phagocytic mechanism [52]. Later studies by this group showed that passive immunization the m266 mid-domain antibody did not cause microhemorrhage. This is in contrast to an N-terminal antibody that caused a significant increase in CAA-associated microhemorrhage occurrence in PDAPP transgenic mice [53].
C-terminal antibodies were also examined in aged Tg2576 transgenic mice. The 2286 antibody is an IgG1 that recognizes Aß28−40. This antibody was administered intraperitoneally to Tg2576 mice with a staggered start such that mice received weekly injections for 1, 2 or 3 months and were all sacrificed at 22 months of age. Unlike the C-terminal antibody included in the 2003 Bard et al study, this antibody was found to bind amyloid plaques in vivo after systemic administration. A transient but significant microglial reaction was observed but resolved by the 3 month treatment time-point. Significant amyloid reductions were observed following 2 months of treatment and cognitive improvement was also observed [54]. This same C-terminal antibody was later shown to significantly increase CAA levels and CAA-associated microhemorrhage [55]. An IgG2b C-terminal antibody (2H6) was shown to have the same effects as the 2286 antibody. A deglycosylated version of 2H6 was also examined and showed significant reductions in brain amyloid deposits, despite the absence of significant microglial reactivity. Normally, IgG molecules are heavily glycosylated on their Fc portion. This glycosylation is important for high affinity binding by Fcγ receptors, so therefore, deglycosylation of anti-Aβ antibodies should significantly impair microglial Fcγ-receptor mediated amyloid clearance. Importantly, this deglycosylated antibody attenuated the increase in CAA and CAA-associated microhemorrhage observed with the intact 2H6 antibody [56].
Recently, antibody therapy has been focused on oligomeric forms of Aß which are small, soluble assemblies of Aß (dimers, trimers, tetramers etc.) that have been shown, both in vitro and in vivo, to be neurotoxic at relatively low concentrations (reviewed by [57]. NAB61 is a monoclonal antibody that recognizes a complex conformational epitope in the N-terminal of Aß oligomers. Passive immunization of Tg2576 mice with NAB61 did not alter brain amyloid deposition or APP processing, but did, in fact, improve learning and memory [58]. Also, another antibody (NU-6) has been developed that is specific for Aß oligomers and binds amyloid plaques in human Alzheimer's tissue. This antibody has been shown to neutralize Aß oligomers in vitro and future studies will examine this antibody as a potential passive immunotherapeutic [59].
Uncovering the mechanisms of action in transgenic mouse models
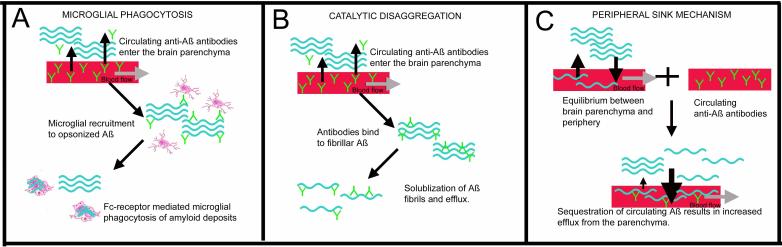
There have been three proposed mechanisms of action of immunotherapy. These are summarized in figure 2 and table 3.
Figure 2.
The three major proposed mechanisms of action for immunotherapy in amyloid reduction. Panel A shows the mechanism of microglial phagocytosis. In this case, amyloid fibers (shown in blue) are opsonized by antibodies (shown in green) entering the brain from the bloodstream. Microglia then recognize the opsonized Aß and phagocytose the amyloid via the Fcγ receptor. Panel B shows the mechanism of catalytic disaggregation. In this case amyloid fibers are bound by antibodies which then disrupt the tertiary structure of the amyloid deposit. This results in solublization of the Aß and efflux out of the brain. Panel C shows the peripheral sink mechanism. In this case monomeric soluble Aß circulating in the bloodstream is bound by the circulating antibodies. This sequestration of circulating Aß produces a shift in the concentration gradient of Aß between the brain and the blood causing an efflux of Aß out of the brain.
Table 3.
Mechanisms of action for immunotherapy and brief description of some of the studies providing evidence for the given mechanism.
| Mechanism | Method | Findings | Ref. |
|---|---|---|---|
| Microglial mediated phagocytosis. | Human microglia cultures incubated with antibody-opsonized Aß. | Enhanced uptake of Aß by microglial cells when antibody was present. | (Lue & Walker, 2002, J Neurosci Res 70,599−610) |
| Intracranial injection of anti-Aß antibodies or F(ab′)2 fragments in Tg2576 mice. | Inhibition of microglial activation due to IgG also inhibited removal of compact plaques. F(ab′)2 fragments did not result in significant compact plaque reductions. | (Wilcock et al., 2003 J Neurosci 23,3745−3751) | |
| |
Passive immunization and of PDAPP mice and ex vivo analysis of phagocytosis. |
Those antibodies that initiated with most robust Aß reductions in PDAPP mice were also the antibodies that demonstrated the most robust phagocytosis on brain slices. |
(Bard et al., 2003 Proc Natl Acad Sci USA 100,2023−2028) |
| Catalytic disaggregation. | Addition of N-terminal anti-Aß antibodies to in vitro fibrillar Aß. | Disaggregation of Aß fibrils after incubation with N-terminal antibodies that also reduced their toxicity in PC12 cells. | (Solomon et al., 1997 Proc Natl Acad Sci USA 94,4109−4112) |
| Intracranial administration of F(ab)′ fragments of antibodies. | F(ab′) fragments injected intracranially reduce amyloid deposition. | (Bacskai et al., 2002 J Neurosci 22,7873−7878) | |
| |
Inhibition of microglial activation by dexamethasone following intracranial anti-Aß antibody injection. |
Diffuse (non-fibrillar) amyloid deposits were cleared despite complete inhibition of microglial activation. |
(Wilcock et al, 2004, Neurobiol Dis 15,11−20) |
| Peripheral sink | Systemic injection of anti-Aß antibodies. | Systemic injection of anti-Aß antibodies into PDAPP mice resulted in a significant increase in plasma Aß levels and decrease in brain Aß. | (DeMattos et al., 2001 Proc Natl Acad Sci USA98,8850−8855) |
| Administration of anti-Aß antibodies to mice with deficient blood brain barrier Aß clearance (APPSwDI) | Systemic injection of anti-Aß antibodies into a mouse with impaired blood brain barrier Aß transport APPSwDI mice does not cause increased plasma Aß or decreased brain Aß. | (Vasilevko et al., 2007 J Neurosci 27,13376−13383) |
Mechanism 1
Microglial mediated removal
Microglial mediated phagocytosis was first suggested by Schenk et al (1999) in their original description of Aß vaccination. The authors suggested that microglial Fc-receptor phagocytosis may be responsible for removal of existing plaque. Microglia have been shown to phagocytose Aß both in vitro and in vivo through several different mechanisms involving opsonization through the complement cascade [60], or the scavenger receptor [61]. Indeed, across a series of active immunization studies it was shown that the reduction of compact, Congophilic amyloid deposits correlated with the degree of microglial activation [62]. Bard's study using passive immunization methods demonstrated that only antibodies of an isotype with high affinity for Fcγ-receptors (IgG2a; [63]) were able to lower brain amyloid deposits [49]. These data further supported the hypothesis that microglia are responsible for the clearance of Aß. Direct imaging of amyloid deposits in living mice using multiphoton microscopy [64] was used to examine the effects of antibody application to the brains of PDAPP mice. Three days following direct application of anti-Aß antibodies to the brain, there was significant removal of amyloid deposits accompanied by activation of microglia surrounding the remaining deposits. Direct injection of anti-Aß antibodies into the brains of Tg2576 mice resulted in rapid removal of diffuse amyloid deposits (by 24hr), followed by removal of compact deposits (between 2 and 3 days) accompanied by a transient microglial reaction (peaked at 3 days) [65].
Conflicting data suggest that effective clearance of Aß by anti-Aß antibodies can be obtained in the absence of Fc receptors. Das et al (2003) showed that active immunization of APP (Tg2576) transgenic mice crossed with Fc receptor knockout mice demonstrated the same amount of Aß reductions as immunized, age-matched APP transgenic mice [66]. As shown by live imaging using multiphoton microscopy F(ab’)2 fragments are capable of reducing amyloid deposition as effectively as the complete IgG molecule when applied directly to the brain [67]. F(ab’)2 fragments made from an anti-Aß28−40 IgG also lowered amyloid deposits when injected intracranially into Tg2576 mice [68]. Inhibition of microglial activation after intracranial injection of antibody using dexamethasone had no effect on removal of diffuse amyloid deposits. However, there was no apparent reduction in compact, thioflavine-S positive, amyloid deposits indicating that microglial activation facilitates the removal of compact amyloid deposits [68].
Mechanism 2
Catalytic disaggregation
Catalytic disaggregation describes the interaction between an antibody and an amyloid deposit whereby the binding of the antibody disrupts the tertiary structure of the plaque resulting in disaggregation. Solomon et al (1997) showed that monoclonal anti-Aß antibodies were capable of inhibiting amyloid plaque formation in vitro [37]. Later, Frenkel et al showed that anti-Aß antibodies are capable of disaggregating Aß plaques and neutralizing their neurotoxicity [38]. This indicated that antibody binding to Aß causes the Aß aggregates to dissolve forming monomeric Aß. Studies in which F(ab’)2 fragments were applied directly to the brain [67;68] and reduce Aß indicated a non-Fc mediated clearance mechanism, possibly via a direct interaction with amyloid deposits. Also, the reduction in diffuse amyloid deposits by intracranially administered anti-Aß antibodies despite complete inhibition of microglial activation suggested a direct interaction between the antibody and amyloid [68].
Mechanism 3
Peripheral sink
Studies involving passive immunization have suggested that the primary mechanism for Aβ clearance is peripheral, and is not due to the antibodies entering the CNS. Instead, the Aß antibodies act to reduce circulating Aß levels. The peripheral sink mechanism is derived from studies using anti-Aß antibodies that were specifically designed to not bind to amyloid plaques in the brain (the m266 antibody). When these antibodies were administered by intraperitoneal injection in the PDAPP mouse, a rapid 1,000-fold increase in circulating plasma Aß levels was observed. These data suggested that circulating Aß antibodies bind to plasma Aß and consequently transiently reduce the circulating levels of soluble Aß. In turn, this reduction promotes the removal of soluble Aß from the brain by mass action transfer across the blood brain barrier to the vasculature, hence the term peripheral sink [52]. Dodart et al further demonstrated that treatment of PDAPP mice with m266 antibody reversed memory deficits one day after injection, without a reduction in amyloid burden in the brain [69]. The authors suggested that this rapid reversal of cognitive deficits was due to removal of soluble Aβ from the CNS as opposed to reducing brain amyloid plaque burden. Cognitive improvement following passive immunization was also been shown in the Tg2576 mouse with an antibody recognizing Aß1−12, which also did not reduce brain Aß levels but did reverse memory deficits [70]. In a time-course study of weekly systemic anti-Aß antibody injection in Tg2576 mice circulating Aß levels in the serum were increased 100-fold following 1 month of treatment, and remained significantly elevated following 2 and 3 months of treatment [54].
Another study which supports the peripheral sink mechanism as being a primary mechanism of Aß removal used the APPSwDI transgenic mouse, which has been shown to have deficient clearance of Aß across the blood-brain barrier. Active vaccination of these mice produced significantly high antibody titers, however, no increase in plasma Aß was observed, and no change in brain Aß levels were apparent. The authors confirmed that there were equal amounts of IgG entering the brain by performing an IgG western blot on brain homogenates comparing immunized Tg2576 mice (that are known to have antibody entering the brain) with immunized APPSwDI mice [71].
In summary, evidence for each of the proposed mechanisms for amyloid removal is strong. It is likely that all proposed mechanisms occur as a result of immunotherapy. However, the dominant mechanism may be determined by several factors including blood brain barrier integrity, antibody epitope and isotype and individual disease characteristics.
Clinical trials
Based on the impressive data from transgenic mouse studies active Aß immunization progressed rapidly into a clinical trial called AN1792. Although the early response of the individuals was promising, the trial was suspended when 6% of patients in phase 2 of the study developed subacute meningoencephalitis; a debilitating, life threatening inflammation of the brain and meninges [31]. Many of these patients made at least a partial recovery following treatment with a corticosteroids. Over the course of several years after cessation of the trial, several autopsy reports were published on patients from the trial. These reports indicated that vaccination may have lowered amyloid levels in the brain and, therefore, interest in immunotherapy for treatment of Alzheimer's disease has remained intense.
Comparisons between data from mouse studies and data from the human clinical trials are provided in table 4. The initial reports following the human trial focused on the patients’ ability to generate anti-Aß antibodies in response to the vaccination. Indeed, some (but not all) patients generated significant antibody titers. Characterization of these antibodies showed that they cross reacted with amyloid deposits in brain (including CAA and diffuse amyloid), but did not cross react with full length APP [72]. Cognition was followed in a subset of patients from the AN1792 clinical trial in Zurich, Switzerland after suspension of the trial. Patients who generated anti-Aß antibodies that would cross-react with amyloid plaques in Alzheimer tissue showed a slowed cognitive decline when compared to those patients receiving placebo control [73]. These findings are reminiscent of the multiple reports of behavioral improvement following vaccination in various transgenic mouse models.
Table 4.
A table summarizing the effects of vaccination in the human clinical trial and in transgenic mice. Bold font indicates where human and mouse showed the same effects.
| Vaccination effects reported in human clinical trial | Vaccination effects reported in APP transgenic mice |
|---|---|
| Meningoencephalitis. 6% of patients in the phase II AN1792 active immunization trial (Orgogozo et al, Neurology 61:46−54). | No sickness behavior has been reported. |
| Reduced Aß plaque density (Nicoll et al, J Neuropathol Exp Neurol 65:1040−1048; Masliah et al, Neurology 64:129−131). | Reduced Aß plaque density (Schenk et al, Nature 400:173−178; Das et al, J Neurosci 25:8532−8538; Wilcock et al, J Neurosci 24:6144−2151). |
| Neurofibrillary tangles and tau pathology unaffected (Ferrer et al, Brain Pathol 14:11−20; Nicoll et al, Nat Med 9:448−452, Masliah et al, Neurology 64:129−131). | Some early tau species reduced (Billings et al, Neuron 45:675−688). |
| Microhemorrhage in association with CAA (Ferrer et al, Brain Pathol 14:11−20). | Microhemorrhage in association with CAA (Pfeifer et al, Science 298:1379; Wilcock et al, J Neuroinflamm 1:24; Racke et al, J Neurosci 25:629−636; Wilcock et al Neuroscience l44:950−960). |
| Persistence of CAA (Ferrer et al, Brain Pathol 14:11−20; Nicoll et al, Nat Med 9:448−452, Masliah et al, Neurology 64:129−131). | Persistence and increase in CAA (Pfeifer et al, Science 298:1379; Wilcock et al, J Neuroinflamm 1:24; Racke et al, J Neurosci 25:629−636; Wilcock et al Neuroscience l44:950−960). |
| Loss of myelin in cerebral white matter (Ferrer et al, Brain Pathol 14:11−20) – possibly complicated by encephalitis. | Not reported. |
| Slowed cognitive decline (Hock et al, Neuron 38:547−554). | Improved cognition (Morgan et al, Nature 408:982−985). |
The first post-mortem pathology report from a trial patient was published in 2003 [74]. This patient received 5 vaccinations (4 Aß+QS21 and 1 Aß+QS21+polysorbate-80) and 6 weeks following the final injection developed meningoencephalitis. The patient died from a pulmonary embolism 12 months after the last vaccination. Amyloid plaques were described as “patchy” in contrast to reasonably uniform plaques in an untreated AD control matched for Braak & Braak staging (V-VI). Quantification of amyloid plaque load in the patient compared to 7 untreated AD controls revealed 60−70% less amyloid plaque throughout the neocortex compared to a group of disease stage-matched untreated AD tissue. Some regions that typically showed high levels of amyloid, such as the inferior, middle and superior temporal gyri, were almost completely devoid of plaque. Other features of AD such as CAA, neurofibrillary tangles and neuropil threads were described as unchanged compared to untreated AD controls. Significant T-lymphocyte infiltration was noted, mostly CD4+ with few CD8+ T-cells.
A second pathology report in a trial patient that developed meningoencephalitis and later died echoed many of the same findings as Nicoll et al [75]. The patient received only 2 immunizations and nine months following the final injection showed symptoms of aseptic meningoencephalitis with both CD4+ and CD8+ t-cell infiltrates. The patient was treated but the encephalitis reactivated resulting in death. The brain showed extensive T cell infiltration consistent with the meningoencephalitis. CAA and cerebral microhemorrhages were also noted. Plaques were described as “collapsed” and were surrounded by activated microglial cells. Many areas showed significantly fewer amyloid plaques than untreated AD brains of the same Braak & Braak staging. Examination of tau pathology showed reduction / disappearance of neuritic tau pathology, however, there was still extensive tau pathology present. These reductions were in comparison to disease stage-matched AD tissue.
The first pathology report from a trial patient that did not develop encephalitis was published in 2005 [76]. The patient received 3 immunizations, developed significant antibody titers and did not develop any obvious adverse reaction. It should be noted, despite the absence of encephailits, B and T cell infiltrates were observed in the brain. The patient died one year after the final injection and cause of death was described as “failure to thrive”. The brain showed significant reductions in amyloid load, as detected by immunohistochemistry and biochemistry. Tangle score was generally lower than the average for untreated AD tissue. CAA score was the same as untreated controls. Astrogliosis was slightly lower and microgliosis was unchanged. Despite low antibody titers, another autopsy report also revealed significantly fewer amyloid deposits in the brain [77]. It was recently reported at the New York Academy of Sciences conference that, taken together, autopsy data indeed indicates that CAA levels are increased as well as an increased occurrence of microhemorrhage. However, 4 years after vaccination some patients showed resolution of even the CAA. Disappointingly, however, the patients failed to show a long term cognitive benefit of the vaccination [78].
Data from the AN1792 clinical trial suggest that immunotherapy is, indeed, effective in the removal (or prevention of accumulation) of amyloid from the human AD brain, however, tau pathology appeared to be relatively unaffected. It should be mentioned, however, that there remains the possibility that the non-specific inflammatory response to the adjuvant resulted in Aß reduction. In mice, stimulation of an inflammatory response by LPS certainly resulted in Aß reduction [79;80]. It is also unclear whether there was active clearance of amyloid or, simply, prevention of further amyloid accumulation. Further studies from the other patients involved in this trial will determine the exact effect of vaccination on other AD pathologies. Most recently, another setback for active immunization occurred when the trial ACC-001 failed. This trial used a 7 amino acid fragment of Aß from the N-terminal conjugated to a mutated diphtheria toxin protein called CRM 197. The vaccination was designed to avoid the T-cell response seen in the first trial. ACC-001 caused a vasculitis (inflammation of the blood vessels) resulting in skin lesions (see story at http://www.alzforum.org/new/detail.asp?id=1807) . The cause is currently unknown.
Several trials examining passive immunization are currently ongoing. While the antibodies used in mouse studies are somewhat equivalent to those in clinical trial, the antibodies must be humanized to avoid an immune response by the patient. Elan pharmaceuticals are in Phase IIb continuing to Phase III with their antibody called Bapineuzumab in trial AAB-001. While there has been no data published from this trial to date, the advancement to Phase IIb-III suggest that the antibody is well tolerated. Eli Lily pharmaceuticals are in Phase II with their monoclonal antibody 266 in trial LY2062430 Patients enrolled in the phase I trial received one or three doses of the antibody and showed increased plasma and CSF Aβ as predicted from the mouse studies. The key in these studies will be cognitive performance and avoidance of adverse effects predicted in mice; i.e. elevated CAA levels and subsequent microhemorrhage.
Conclusion
Discovery and development of immunotherapy as a treatment for Alzheimer's disease would not have been possible without the development of transgenic mouse models. The extensive mouse model data has supported the clinical trials in immunotherapy. Further, studies examining the mechanisms of action have allowed for advancement in development of types of immunotherapy. Adverse events have been predicted by mouse model studies (CAA and microhemorrhage effects, for example), while some adverse events were not apparent (meningoencephalitis) in mice. Also, all immunotherapy studies in mice have shown cognitive benefit, where tested. However, AN1792 trial failed to show any cognitive benefit over the longer term. It is important to note here that while transgenic mouse models are valuable to the study of Alzheimer's disease, they are generally incomplete. There are some significant differences between mouse and human, and one example is the immune response. It has been known for some time that human macrophages in vitro produce significantly less NO when stimulated under conditions that produce high NO levels in mouse macrophages in vitro [81;82]. The lack of neuron loss in APP transgenic mice suggests more subtle and reversible pathways leading to the cognitive deficits in these mice. It is likely that in human Alzheimer's neuron loss is the main contributor to the clinical dementia and, therefore, is an irreversible process once initiated. This would suggest that treatments such as immunotherapy may be most valuable in the early stages of disease to halt any further progression. Improvements on mouse models continue, and the generation of models demonstrating amyloid plaques, normal tau pathology and accompanying neuron loss will be critical in the future testing of potential therapeutics to assess not only the efficacy pathologically, but also the point at which therapeutic benefit is likely to occur. In conclusion, development of better transgenic mouse models will result in improved understanding of the disease process and therefore improved development of therapeutic interventions.
Reference List
- 1.Newell KL, Hyman BT, Growdon JH, Hedley-Whyte ET. Application of the National Institute on Aging (NIA)-Reagan Institute criteria for the neuropathological diagnosis of Alzheimer disease. J. Neuropathol. Exp. Neurol. 1999;58:1147–1155. doi: 10.1097/00005072-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Turner PR, O'Connor K, Tate WP, Abraham WC. Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory. Prog. Neurobiol. 2003;70:1–32. doi: 10.1016/s0301-0082(03)00089-3. [DOI] [PubMed] [Google Scholar]
- 3.Nunan J, Small DH. Regulation of APP cleavage by alpha-, beta- and gamma-secretases. FEBS Lett. 2000;483:6–10. doi: 10.1016/s0014-5793(00)02076-7. [DOI] [PubMed] [Google Scholar]
- 4.Hutton M, Hardy J. The presenilins and Alzheimer's disease. Hum. Mol. Genet. 1997;6:1639–1646. doi: 10.1093/hmg/6.10.1639. [DOI] [PubMed] [Google Scholar]
- 5.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 6.Tanzi RE, Kovacs DM, Kim TW, Moir RD, Guenette SY, Wasco W. The gene defects responsible for familial Alzheimer's disease. Neurobiol. Dis. 1996;3:159–168. doi: 10.1006/nbdi.1996.0016. [DOI] [PubMed] [Google Scholar]
- 7.Lott IT, Head E. Alzheimer disease and Down syndrome: factors in pathogenesis. Neurobiol. Aging. 2005;26:383–389. doi: 10.1016/j.neurobiolaging.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Rovelet-Lecrux A, Hannequin D, Raux G, Le MN, Laquerriere A, Vital A, Dumanchin C, Feuillette S, Brice A, Vercelletto M, Dubas F, Frebourg T, Campion D. APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat. Genet. 2006;38:24–26. doi: 10.1038/ng1718. [DOI] [PubMed] [Google Scholar]
- 9.Rovelet-Lecrux A, Frebourg T, Tuominen H, Majamaa K, Campion D, Remes AM. APP locus duplication in a Finnish family with dementia and intracerebral haemorrhage. J. Neurol. Neurosurg. Psychiatry. 2007;78:1158–1159. doi: 10.1136/jnnp.2006.113514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sleegers K, Brouwers N, Gijselinck I, Theuns J, Goossens D, Wauters J, Del-Favero J, Cruts M, van Duijn CM, Van BC. APP duplication is sufficient to cause early onset Alzheimer's dementia with cerebral amyloid angiopathy. Brain. 2006;129:2977–2983. doi: 10.1093/brain/awl203. [DOI] [PubMed] [Google Scholar]
- 11.Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature. 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- 12.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 13.Sturchler-Pierrat C, Abramowski D, Duke M, Wiederhold KH, Mistl C, Rothacher S, Ledermann B, Burki K, Frey P, Paganetti PA, Waridel C, Calhoun ME, Jucker M, Probst A, Staufenbiel M, Sommer B. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc. Natl. Acad. Sci. U. S. A. 1997;94:13287–13292. doi: 10.1073/pnas.94.24.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chishti MA, Yang DS, Janus C, Phinney AL, Horne P, Pearson J, Strome R, Zuker N, Loukides J, French J, Turner S, Lozza G, Grilli M, Kunicki S, Morissette C, Paquette J, Gervais F, Bergeron C, Fraser PE, Carlson GA, George-Hyslop PS, Westaway D. Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J. Biol. Chem. 2001;276:21562–21570. doi: 10.1074/jbc.M100710200. [DOI] [PubMed] [Google Scholar]
- 15.Moechars D, Dewachter I, Lorent K, Reverse D, Baekelandt V, Naidu A, Tesseur I, Spittaels K, Haute CV, Checler F, Godaux E, Cordell B, Van LF. Early phenotypic changes in transgenic mice that overexpress different mutants of amyloid precursor protein in brain. J. Biol. Chem. 1999;274:6483–6492. doi: 10.1074/jbc.274.10.6483. [DOI] [PubMed] [Google Scholar]
- 16.Calhoun ME, Wiederhold KH, Abramowski D, Phinney AL, Probst A, Sturchler-Pierrat C, Staufenbiel M, Sommer B, Jucker M. Neuron loss in APP transgenic mice. Nature. 1998;395:755–756. doi: 10.1038/27351. [DOI] [PubMed] [Google Scholar]
- 17.Davis J, Xu F, Deane R, Romanov G, Previti ML, Zeigler K, Zlokovic BV, Van Nostrand WE. Early-onset and robust cerebral microvascular accumulation of amyloid beta-protein in transgenic mice expressing low levels of a vasculotropic Dutch/Iowa mutant form of amyloid beta-protein precursor. J. Biol. Chem. 2004;279:20296–20306. doi: 10.1074/jbc.M312946200. [DOI] [PubMed] [Google Scholar]
- 18.Davis J, Xu F, Miao J, Previti ML, Romanov G, Ziegler K, Van Nostrand WE. Deficient cerebral clearance of vasculotropic mutant Dutch/Iowa Double A beta in human A betaPP transgenic mice. Neurobiol. Aging. 2006;27:946–954. doi: 10.1016/j.neurobiolaging.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 19.Duff K, Eckman C, Zehr C, Yu X, Prada CM, Perez-Tur J, Hutton M, Buee L, Harigaya Y, Yager D, Morgan D, Gordon MN, Holcomb L, Refolo L, Zenk B, Hardy J, Younkin S. Increased amyloid-beta42(43) in brains of mice expressing mutant presenilin 1. Nature. 1996;383:710–713. doi: 10.1038/383710a0. [DOI] [PubMed] [Google Scholar]
- 20.Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, Jantzen P, Wright K, Saad I, Mueller R, Morgan D, Sanders S, Zehr C, O'Campo K, Hardy J, Prada CM, Eckman C, Younkin S, Hsiao K, Duff K. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat. Med. 1998;4:97–100. doi: 10.1038/nm0198-097. [DOI] [PubMed] [Google Scholar]
- 21.Lewis J, McGowan E, Rockwood J, Melrose H, Nacharaju P, van SM, Gwinn-Hardy K, Paul MM, Baker M, Yu X, Duff K, Hardy J, Corral A, Lin WL, Yen SH, Dickson DW, Davies P, Hutton M. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat. Genet. 2000;25:402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- 22.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, Laferla FM. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 23.Morrissette DA, LaFerla FM. Stereological quantification of neuronal cells in the 3XTg-AD mice.. SFN 2007 Annual Meeting.; 2007; Ref Type: Abstract. [Google Scholar]
- 24.Capsoni S, Ugolini G, Comparini A, Ruberti F, Berardi N, Cattaneo A. Alzheimer-like neurodegeneration in aged antinerve growth factor transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 2000;97:6826–6831. doi: 10.1073/pnas.97.12.6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colton CA, Vitek MP, Wink DA, Xu Q, Cantillana V, Previti ML, Van Nostrand WE, Weinberg JB, Dawson H. NO synthase 2 (NOS2) deletion promotes multiple pathologies in a mouse model of Alzheimer's disease. Proc. Natl. Acad. Sci. U. S. A. 2006;103:12867–12872. doi: 10.1073/pnas.0601075103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilcock DM, Lewis MR, Van Nostrand WE, Davis J, Previti ML, Gharkholonarehe N, Vitek MP, Colton CA. Progression of amyloid pathology to Alzheimer's disease pathology in an amyloid precursor protein transgenic mouse model by removal of nitric oxide synthase 2. J. Neurosci. 2008;28:1537–1545. doi: 10.1523/JNEUROSCI.5066-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 28.Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, Duff K, Jantzen P, DiCarlo G, Wilcock D, Connor K, Hatcher J, Hope C, Gordon M, Arendash GW. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer's disease. Nature. 2000;408:982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- 29.Janus C, Pearson J, McLaurin J, Mathews PM, Jiang Y, Schmidt SD, Chishti MA, Horne P, Heslin D, French J, Mount HT, Nixon RA, Mercken M, Bergeron C, Fraser PE, St George-Hyslop P, Westaway D. A beta peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer's disease. Nature. 2000;408:979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- 30.Schijns VE. Immunological concepts of vaccine adjuvant activity. Curr. Opin. Immunol. 2000;12:456–463. doi: 10.1016/s0952-7915(00)00120-5. [DOI] [PubMed] [Google Scholar]
- 31.Orgogozo JM, Gilman S, Dartigues JF, Laurent B, Puel M, Kirby LC, Jouanny P, Dubois B, Eisner L, Flitman S, Michel BF, Boada M, Frank A, Hock C. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003;61:46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- 32.Cribbs DH, Ghochikyan A, Vasilevko V, Tran M, Petrushina I, Sadzikava N, Babikyan D, Kesslak P, Kieber-Emmons T, Cotman CW, Agadjanyan MG. Adjuvant-dependent modulation of Th1 and Th2 responses to immunization with beta-amyloid. Int. Immunol. 2003;15:505–514. doi: 10.1093/intimm/dxg049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asuni AA, Boutajangout A, Scholtzova H, Knudsen E, Li YS, Quartermain D, Frangione B, Wisniewski T, Sigurdsson EM. Vaccination of Alzheimer's model mice with Abeta derivative in alum adjuvant reduces Abeta burden without microhemorrhages. Eur. J. Neurosci. 2006;24:2530–2542. doi: 10.1111/j.1460-9568.2006.05149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maier M, Seabrook TJ, Lemere CA. Modulation of the humoral and cellular immune response in Abeta immunotherapy by the adjuvants monophosphoryl lipid A (MPL), cholera toxin B subunit (CTB) and E. coli enterotoxin LT(R192G) Vaccine. 2005;23:5149–5159. doi: 10.1016/j.vaccine.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 35.Lemere CA, Maron R, Spooner ET, Grenfell TJ, Mori C, Desai R, Hancock WW, Weiner HL, Selkoe DJ. Nasal A beta treatment induces anti-A beta antibody production and decreases cerebral amyloid burden in PDAPP mice. Ann. N. Y. Acad. Sci. 2000;920:328–331. doi: 10.1111/j.1749-6632.2000.tb06943.x. [DOI] [PubMed] [Google Scholar]
- 36.Lemere CA, Spooner ET, Leverone JF, Mori C, Clements JD. Intranasal immunotherapy for the treatment of Alzheimer's disease: Escherichia coli LT and LT(R192G) as mucosal adjuvants. Neurobiol. Aging. 2002;23:991–1000. doi: 10.1016/s0197-4580(02)00127-6. [DOI] [PubMed] [Google Scholar]
- 37.Solomon B, Koppel R, Frankel D, Hanan-Aharon E. Disaggregation of Alzheimer beta-amyloid by site-directed mAb. Proc. Natl. Acad. Sci. U. S. A. 1997;94:4109–4112. doi: 10.1073/pnas.94.8.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frenkel D, Balass M, Solomon B. N-terminal EFRH sequence of Alzheimer's beta-amyloid peptide represents the epitope of its anti-aggregating antibodies. J. Neuroimmunol. 1998;88:85–90. doi: 10.1016/s0165-5728(98)00098-8. [DOI] [PubMed] [Google Scholar]
- 39.Frenkel D, Balass M, Katchalski-Katzir E, Solomon B. High affinity binding of monoclonal antibodies to the sequential epitope EFRH of beta-amyloid peptide is essential for modulation of fibrillar aggregation. J. Neuroimmunol. 1999;95:136–142. doi: 10.1016/s0165-5728(99)00003-x. [DOI] [PubMed] [Google Scholar]
- 40.Frenkel D, Katz O, Solomon B. Immunization against Alzheimer's beta - amyloid plaques via EFRH phage administration. Proc. Natl. Acad. Sci. U. S. A. 2000;97:11455–11459. doi: 10.1073/pnas.97.21.11455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frenkel D, Dewachter I, Van LF, Solomon B. Reduction of beta-amyloid plaques in brain of transgenic mouse model of Alzheimer's disease by EFRH-phage immunization. Vaccine. 2003;21:1060–1065. doi: 10.1016/s0264-410x(02)00609-6. [DOI] [PubMed] [Google Scholar]
- 42.Leverone JF, Spooner ET, Lehman HK, Clements JD, Lemere CA. Abeta1−15 is less immunogenic than Abeta1−40/42 for intranasal immunization of wild-type mice but may be effective for ”boosting”. Vaccine. 2003;21:2197–2206. doi: 10.1016/s0264-410x(02)00754-5. [DOI] [PubMed] [Google Scholar]
- 43.Seabrook TJ, Jiang L, Thomas K, Lemere CA. Boosting with intranasal dendrimeric Abeta1−15 but not Abeta1−15 peptide leads to an effective immune response following a single injection of Abeta1−40/42 in APP-tg mice. J. Neuroinflammation. 2006;3:14. doi: 10.1186/1742-2094-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DaSilva K, Brown ME, Westaway D, McLaurin J. Immunization with amyloid-beta using GM-CSF and IL-4 reduces amyloid burden and alters plaque morphology. Neurobiol. Dis. 2006;23:433–444. doi: 10.1016/j.nbd.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 45.Willis RA, Bowers WJ, Turner MJ, Fisher TL, bdul-Alim CS, Howard DF, Federoff HJ, Lord EM, Frelinger JG. Dendritic cells transduced with HSV-1 amplicons expressing prostate-specific antigen generate antitumor immunity in mice. Hum. Gene Ther. 2001;12:1867–1879. doi: 10.1089/104303401753153929. [DOI] [PubMed] [Google Scholar]
- 46.Bowers WJ, Mastrangelo MA, Stanley HA, Casey AE, Milo LJ, Jr., Federoff HJ. HSV amplicon-mediated Abeta vaccination in Tg2576 mice: differential antigen-specific immune responses. Neurobiol. Aging. 2005;26:393–407. doi: 10.1016/j.neurobiolaging.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 47.Frazer ME, Hughes JE, Mastrangelo MA, Tibbens JL, Federoff HJ, Bowers WJ. Reduced pathology and improved behavioral performance in Alzheimer's disease mice vaccinated with HSV amplicons expressing amyloid-beta and interleukin-4. Mol. Ther. 2008;16:845–853. doi: 10.1038/mt.2008.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Movsesyan N, Ghochikyan A, Mkrtichyan M, Petrushina I, Davtyan H, Olkhanud PB, Head E, Biragyn A, Cribbs DH, Agadjanyan MG. Reducing AD-like pathology in 3xTg-AD mouse model by DNA epitope vaccine - a novel immunotherapeutic strategy. PLoS. ONE. 2008;3:e2124. doi: 10.1371/journal.pone.0002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat. Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 50.Bard F, Barbour R, Cannon C, Carretto R, Fox M, Games D, Guido T, Hoenow K, Hu K, Johnson-Wood K, Khan K, Kholodenko D, Lee C, Lee M, Motter R, Nguyen M, Reed A, Schenk D, Tang P, Vasquez N, Seubert P, Yednock T. Epitope and isotype specificities of antibodies to beta -amyloid peptide for protection against Alzheimer's disease-like neuropathology. Proc. Natl. Acad. Sci. U. S. A. 2003;100:2023–2028. doi: 10.1073/pnas.0436286100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pfeifer M, Boncristiano S, Bondolfi L, Stalder A, Deller T, Staufenbiel M, Mathews PM, Jucker M. Cerebral hemorrhage after passive anti-Abeta immunotherapy. Science. 2002;298:1379. doi: 10.1126/science.1078259. [DOI] [PubMed] [Google Scholar]
- 52.DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer's disease. Proc. Natl. Acad. Sci. U. S. A. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Racke MM, Boone LI, Hepburn DL, Parsadainian M, Bryan MT, Ness DK, Piroozi KS, Jordan WH, Brown DD, Hoffman WP, Holtzman DM, Bales KR, Gitter BD, May PC, Paul SM, DeMattos RB. Exacerbation of cerebral amyloid angiopathy-associated microhemorrhage in amyloid precursor protein transgenic mice by immunotherapy is dependent on antibody recognition of deposited forms of amyloid beta. J. Neurosci. 2005;25:629–636. doi: 10.1523/JNEUROSCI.4337-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilcock DM, Rojiani A, Rosenthal A, Levkowitz G, Subbarao S, Alamed J, Wilson D, Wilson N, Freeman MJ, Gordon MN, Morgan D. Passive amyloid immunotherapy clears amyloid and transiently activates microglia in a transgenic mouse model of amyloid deposition. J. Neurosci. 2004;24:6144–6151. doi: 10.1523/JNEUROSCI.1090-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilcock DM, Rojiani A, Rosenthal A, Subbarao S, Freeman MJ, Gordon MN, Morgan D. Passive immunotherapy against Abeta in aged APP-transgenic mice reverses cognitive deficits and depletes parenchymal amyloid deposits in spite of increased vascular amyloid and microhemorrhage. J. Neuroinflammation. 2004;1:24. doi: 10.1186/1742-2094-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilcock DM, Alamed J, Gottschall PE, Grimm J, Rosenthal A, Pons J, Ronan V, Symmonds K, Gordon MN, Morgan D. Deglycosylated anti-amyloid-beta antibodies eliminate cognitive deficits and reduce parenchymal amyloid with minimal vascular consequences in aged amyloid precursor protein transgenic mice. J. Neurosci. 2006;26:5340–5346. doi: 10.1523/JNEUROSCI.0695-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walsh DM, Selkoe DJ. A beta oligomers - a decade of discovery. J. Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- 58.Lee EB, Leng LZ, Zhang B, Kwong L, Trojanowski JQ, Abel T, Lee VM. Targeting amyloid-beta peptide (Abeta) oligomers by passive immunization with a conformation-selective monoclonal antibody improves learning and memory in Abeta precursor protein (APP) transgenic mice. J. Biol. Chem. 2006;281:4292–4299. doi: 10.1074/jbc.M511018200. [DOI] [PubMed] [Google Scholar]
- 59.Lambert MP, Velasco PT, Chang L, Viola KL, Fernandez S, Lacor PN, Khuon D, Gong Y, Bigio EH, Shaw P, De Felice FG, Krafft GA, Klein WL. Monoclonal antibodies that target pathological assemblies of Abeta. J. Neurochem. 2007;100:23–35. doi: 10.1111/j.1471-4159.2006.04157.x. [DOI] [PubMed] [Google Scholar]
- 60.Rogers J, Lue LF, Walker DG, Yan SD, Stern D, Strohmeyer R, Kovelowski CJ. Elucidating molecular mechanisms of Alzheimer's disease in microglial cultures. Ernst. Schering. Res. Found. Workshop. 2002:25–44. doi: 10.1007/978-3-662-05073-6_3. [DOI] [PubMed] [Google Scholar]
- 61.Paresce DM, Ghosh RN, Maxfield FR. Microglial cells internalize aggregates of the Alzheimer's disease amyloid beta-protein via a scavenger receptor. Neuron. 1996;17:553–565. doi: 10.1016/s0896-6273(00)80187-7. [DOI] [PubMed] [Google Scholar]
- 62.Wilcock DM, Gordon MN, Ugen KE, Gottschall PE, DiCarlo G, Dickey C, Boyett KW, Jantzen PT, Connor KE, Melachrino J, Hardy J, Morgan D. Number of Abeta inoculations in APP+PS1 transgenic mice influences antibody titers, microglial activation, and congophilic plaque levels. DNA Cell Biol. 2001;20:731–736. doi: 10.1089/10445490152717596. [DOI] [PubMed] [Google Scholar]
- 63.Radaev S, Sun PD. Recognition of IgG by Fcgamma receptor. The role of Fc glycosylation and the binding of peptide inhibitors. J. Biol. Chem. 2001;276:16478–16483. doi: 10.1074/jbc.M100351200. [DOI] [PubMed] [Google Scholar]
- 64.Bacskai BJ, Kajdasz ST, Christie RH, Carter C, Games D, Seubert P, Schenk D, Hyman BT. Imaging of amyloid-beta deposits in brains of living mice permits direct observation of clearance of plaques with immunotherapy. Nat. Med. 2001;7:369–372. doi: 10.1038/85525. [DOI] [PubMed] [Google Scholar]
- 65.Wilcock DM, DiCarlo G, Henderson D, Jackson J, Clarke K, Ugen KE, Gordon MN, Morgan D. Intracranially administered anti-Abeta antibodies reduce beta-amyloid deposition by mechanisms both independent of and associated with microglial activation. J. Neurosci. 2003;23:3745–3751. doi: 10.1523/JNEUROSCI.23-09-03745.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Das P, Howard V, Loosbrock N, Dickson D, Murphy MP, Golde TE. Amyloid-beta immunization effectively reduces amyloid deposition in FcRgamma−/− knock-out mice. J. Neurosci. 2003;23:8532–8538. doi: 10.1523/JNEUROSCI.23-24-08532.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bacskai BJ, Kajdasz ST, McLellan ME, Games D, Seubert P, Schenk D, Hyman BT. Non-Fc-mediated mechanisms are involved in clearance of amyloid-beta in vivo by immunotherapy. J. Neurosci. 2002;22:7873–7878. doi: 10.1523/JNEUROSCI.22-18-07873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilcock DM, Munireddy SK, Rosenthal A, Ugen KE, Gordon MN, Morgan D. Microglial activation facilitates Abeta plaque removal following intracranial anti-Abeta antibody administration. Neurobiol. Dis. 2004;15:11–20. doi: 10.1016/j.nbd.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 69.Dodart JC, Bales KR, Gannon KS, Greene SJ, DeMattos RB, Mathis C, DeLong CA, Wu S, Wu X, Holtzman DM, Paul SM. Immunization reverses memory deficits without reducing brain Abeta burden in Alzheimer's disease model. Nat. Neurosci. 2002;5:452–457. doi: 10.1038/nn842. [DOI] [PubMed] [Google Scholar]
- 70.Kotilinek LA, Bacskai B, Westerman M, Kawarabayashi T, Younkin L, Hyman BT, Younkin S, Ashe KH. Reversible memory loss in a mouse transgenic model of Alzheimer's disease. J. Neurosci. 2002;22:6331–6335. doi: 10.1523/JNEUROSCI.22-15-06331.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vasilevko V, Xu F, Previti ML, Van Nostrand WE, Cribbs DH. Experimental investigation of antibody-mediated clearance mechanisms of amyloid-beta in CNS of Tg-SwDI transgenic mice. J. Neurosci. 2007;27:13376–13383. doi: 10.1523/JNEUROSCI.2788-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hock C, Konietzko U, Papassotiropoulos A, Wollmer A, Streffer J, von Rotz RC, Davey G, Moritz E, Nitsch RM. Generation of antibodies specific for beta-amyloid by vaccination of patients with Alzheimer disease. Nat. Med. 2002;8:1270–1275. doi: 10.1038/nm783. [DOI] [PubMed] [Google Scholar]
- 73.Hock C, Konietzko U, Streffer JR, Tracy J, Signorell A, Muller-Tillmanns B, Lemke U, Henke K, Moritz E, Garcia E, Wollmer MA, Umbricht D, de Quervain DJ, Hofmann M, Maddalena A, Papassotiropoulos A, Nitsch RM. Antibodies against beta-amyloid slow cognitive decline in Alzheimer's disease. Neuron. 2003;38:547–554. doi: 10.1016/s0896-6273(03)00294-0. [DOI] [PubMed] [Google Scholar]
- 74.Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat. Med. 2003;9:448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- 75.Ferrer I, Boada RM, Sanchez Guerra ML, Rey MJ, Costa-Jussa F. Neuropathology and pathogenesis of encephalitis following amyloid-beta immunization in Alzheimer's disease. Brain Pathol. 2004;14:11–20. doi: 10.1111/j.1750-3639.2004.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Masliah E, Hansen L, Adame A, Crews L, Bard F, Lee C, Seubert P, Games D, Kirby L, Schenk D. Abeta vaccination effects on plaque pathology in the absence of encephalitis in Alzheimer disease. Neurology. 2005;64:129–131. doi: 10.1212/01.WNL.0000148590.39911.DF. [DOI] [PubMed] [Google Scholar]
- 77.Nicoll JA, Barton E, Boche D, Neal JW, Ferrer I, Thompson P, Vlachouli C, Wilkinson D, Bayer A, Games D, Seubert P, Schenk D, Holmes C. Abeta species removal after abeta42 immunization. J. Neuropathol. Exp. Neurol. 2006;65:1040–1048. doi: 10.1097/01.jnen.0000240466.10758.ce. [DOI] [PubMed] [Google Scholar]
- 78.Holmes C, Bosche D, Wilkinson D, et al. Long term effects of Aß42 immunization in Alzheimer's disease: immune response, plaque removal and clinical function. Lancet . 2008 Ref Type: Abstract. [Google Scholar]
- 79.Herber DL, Roth LM, Wilson D, Wilson N, Mason JE, Morgan D, Gordon MN. Time-dependent reduction in Abeta levels after intracranial LPS administration in APP transgenic mice. Exp. Neurol. 2004;190:245–253. doi: 10.1016/j.expneurol.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 80.DiCarlo G, Wilcock D, Henderson D, Gordon M, Morgan D. Intrahippocampal LPS injections reduce Abeta load in APP+PS1 transgenic mice. Neurobiol. Aging. 2001;22:1007–1012. doi: 10.1016/s0197-4580(01)00292-5. [DOI] [PubMed] [Google Scholar]
- 81.Weinberg JB, Misukonis MA, Shami PJ, Mason SN, Sauls DL, Dittman WA, Wood ER, Smith GK, McDonald B, Bachus KE. Human mononuclear phagocyte inducible nitric oxide synthase (iNOS): analysis of iNOS mRNA, iNOS protein, biopterin, and nitric oxide production by blood monocytes and peritoneal macrophages. Blood. 1995;86:1184–1195. [PubMed] [Google Scholar]
- 82.Colton C, Wilt S, Gilbert D, Chernyshev O, Snell J, Dubois-Dalcq M. Species differences in the generation of reactive oxygen species by microglia. Mol. Chem. Neuropathol. 1996;28:15–20. doi: 10.1007/BF02815200. [DOI] [PubMed] [Google Scholar]