Abstract
Objective
The Gynecologic Oncology Group (GOG) performed a detailed analysis of p53 overexpression in previously-untreated women with invasive early or advanced stage epithelial ovarian cancer (EOC).
Methods
Women were eligible for the study if they provided a tumor block for translational research and participated in either GOG-157, a randomized phase III trial of three versus (vs.) six cycles of paclitaxel+carboplatin in high-risk, early stage EOC, or GOG-111, a randomized phase III trial of cyclophosphamide+cisplatin vs. paclitaxel+cisplatin in suboptimally-resected, advanced stage EOC. The N-terminal DO-7 p53 antibody was used to examine the expression of the major normal and mutant p53-isoforms. p53 overexpression was defined as ≥10% tumor cells exhibiting nuclear staining.
Results
p53 was overexpressed in 51% (73/143) and 66% (90/136) of cases in the GOG-157 and GOG-111 cohorts, respectively. In the GOG-157 cohort, p53 overexpression was not associated with any clinical characteristics or overall survival (OS) but was associated with worse progression-free survival (PFS) (logrank test: p=0.013; unadjusted Cox modeling: p=0.015). In the GOG-111 cohort, p53 overexpression was associated with GOG performance status (p=0.018) and grade (p=0.003), but not with age, stage, cell type or with tumor response and disease status after primary chemotherapy, PFS or OS. Adjusted Cox regression modeling demonstrated that p53 overexpression was not an independent prognostic factor for PFS or OS in either cohort.
Conclusions
p53 overexpression assessed by DO-7 immunostaining is common in early and advanced stage EOC, but has limited prognostic value in women treated with surgical staging and platinum-based combination chemotherapy.
Keywords: p53, overexpression, IHC, ovarian cancer, prognostic markers
INTRODUCTION
Ovarian cancer is the leading cause of cancer-related death among the gynecologic malignancies (1). It is estimated that 21,650 new cases of ovarian cancer will be diagnosed in the United States in 2008 and that 15,520 women will die from disease (1). Currently, surgical staging followed by platinum- and taxane-based chemotherapy is the standard of care. Despite impressive initial response rates, the five-year survival rate is 92%, 71%, and 30% for women with localized, regional, and distant disease (1). Unfortunately, 68% of ovarian cancers are diagnosed with distant disease (1). Investigators continue to study biomarkers implicated in cancer, malignant progression, metastasis and drug sensitivity with the goal of identifying women with refractory or resistant vs. sensitive disease for whom alternative therapies may be useful.
p53, or TP53, is a multifunctional tumor suppressor that is often altered in ovarian and other cancers (2–11). The p53 gene regulates transcription, DNA repair, cell cycle arrest, differentiation, senescence, genomic instability, apoptosis and survival as well as glucose metabolism, oxidative stress and angiogenesis (3–7). Normal cells generally have low levels of p53 protein due to its short half-life. Mutations in p53 often encode proteins that are resistant to degradation, and mutant p53 protein often accumulates in the nucleus of cancer cells. Overexpression of p53 can occur by mutation, altered transcription and translation or post-translational modifications (3–7), and can be detected using an immunohistochemical method. Currently, alterations in p53 are the most common defects identified in women with epithelial ovarian cancer (EOC). Despite the prevalence of these alterations, overexpression of p53 protein has been inconsistently associated with tumor stage, cell type, grade, progression-free survival (PFS), overall survival (OS), and tumor response, and the value of p53 as an independent prognostic factor for disease progression (DP) and death in women with invasive EOC remains unclear (8–42).
Given the inconsistencies in the literature, the Gynecologic Oncology Group (GOG) sought to evaluate the prognostic relevance of p53 overexpression in women with EOC who participated in one of two randomized phase III treatment protocols (43,44). Our results using the DO-7 antibody (7,45,46) will be discussed in context with the other immunohistochemical studies of p53 overexpression in invasive EOC and the current understanding of the p53 family with its distinct family members and isoforms that exhibit diverse and at times, opposing functions.
MATERIALS AND METHODS
Patients
To participate in this study, the women must have provided a formalin-fixed and paraffin-embedded (FFPE) tumor block and participated in GOG-157 or GOG-111. Women on GOG-157 had to have previously-untreated, histologically-confirmed, optimally-resected EOC with stage IA or IB disease that was either clear cell histology or grade 3 disease, or stage IC or II disease independent of histologic subtype and grade, and a GOG performance status below 4 (43). Women on GOG-111 had to have previously-untreated, histologically-confirmed EOC with stage III disease that was suboptimally-resected (>1 cm residual disease) or stage IV disease, and a GOG performance status below 3 (44). Women on both protocols were required to have adequate borrow marrow cell counts, renal function, and hepatic function as previously described (43,44) but could not have a borderline tumor with low malignant potential. All women provided written informed consent and participating institutions were required to obtain annual Institutional Review Board approval for GOG-157 or GOG-111 consistent with federal, state, and local requirements.
Post-Operative Cancer Treatment
Women on GOG-157 were randomized to receive intravenous (IV) carboplatin (AUC 7.5) and a 3-hour continuous IV infusion of 175 mg/m2 paclitaxel on day 1 every 3 weeks for 3 vs. 6 cycles (43). Women on GOG-111 were randomized to receive either 75 mg/m2 cisplatin IV and 750 mg/m2 cyclophosphamide IV on day 1 every 3 weeks for a total of 6 cycles, or a 24-hour continuous IV infusion of 135 mg/m2 paclitaxel and 75 mg/m2 cisplatin IV on day 2 every 3 weeks for a total of 6 cycles (44). Treatment at the time of DP was left to the discretion of the treating physician and patient.
Clinical End-Points
All women were followed quarterly for 2 years, semi-annually for the next 3 years, and then annually until death from completion of primary chemotherapy. PFS was calculated as the time in months from study enrollment to DP or death (failure), or to the date of last contact for women who were alive with no evidence of DP (censored). OS was calculated as the time from enrollment to death or to the date of last contact for those who were still alive. Tumor response was evaluated after every two cycles of treatment in women on GOG-111 with measurable disease and cases categorized as a complete response, partial response, progressive disease or stable disease as previously defined (44). Women on GOG-111 who were clinically-free of disease after primary chemotherapy or who had CA125 <100 U/ml and were entered with non-measurable disease were required to undergo a reassessment laparotomy. Disease status was classified as negative when reassessment laparotomy showed no evidence of disease, or positive when DP was documented during primary chemotherapy or the reassessment laparotomy.
Tumor Specimens and Immunohistochemical Detection of p53 Overexpression
Tumor was excised during the primary cytoreductive surgery and prior to initiation of primary chemotherapy. p53 overexpression was evaluated without knowledge of the clinical data using 5 micrometer thick unstained sections and an immunohistochemistry procedure with the DO-7 mouse monoclonal antibody (DAKO, Carpinteria, CA) and an ABC Vectastain detection kit (Vector Laboratories, Burlingame, CA) in archival FFPE primary tumor according to the manufacturer’s instructions. Stained slides were independently evaluated by three reviewers using light microscopy to determine the percentage of p53-positive tumor cells with nuclear staining and staining intensity. Background staining was evaluated using an isotype control antibody in place of the DO-7 antibody and was found to be negligible. Stromal cells were used as an internal negative control within each tumor specimen. Breast cancer tissues of known p53 status were used as external positive and negative controls for p53 staining using the DO-7 antibody (42). The percentage of p53-positive tumor cells was categorized as 1 = <10%, 2 = 10–49%, 3 = 50–74%, or 4 = ≥75% p53-positive tumor cells. The intensity of p53 staining was scored as negative (no brown staining), 1+ (light brown staining), 2+ (moderate brown staining), or 3+ (dark brown staining). Majority determinations were calculated for the percentage of p53-positive tumor cells and staining intensity. p53 overexpression was defined as ≥ 10% of tumor cells exhibited nuclear p53 staining as previously reported (21,22,24,28,33,34,37,40,41).
Statistical Methods
Biomarker and clinical data were analyzed using SAS® version 9.1 software (SAS Institute, Inc. Cary, NC). All tests were two-sided and the level of significance was set at 0.05. Associations between p53 overexpression and clinical characteristics, tumor response or disease status were evaluated using Fisher’s exact test. Kaplan-Meier method with the logrank test were used to estimate survival probabilities and compare survival distributions categorized by p53 overexpression. Cox modeling was used to examine the association between p53 overexpression and PFS or OS. Multivariate models adjusted for patient age at enrollment and were stratified by tumor stage, tumor grade, and treatment regimen (43,44); additionally the GOG-111 cohort (44) was stratified by histologic subtype (clear cell vs. mucinous vs. other histologic subtypes) and gross residual disease (measurable vs. non-measurable).
RESULTS
High-Risk, Early Stage, Epithelial Ovarian Cancer
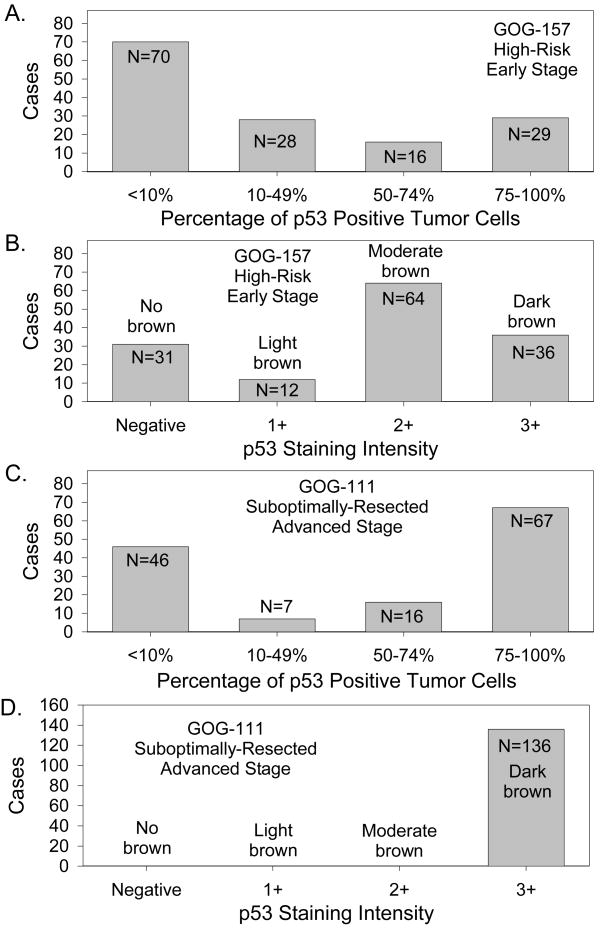
Of the 457 women enrolled on GOG-157, 143 (31%) provided archival FFPE primary tumor tissue for translational research. The patient characteristics for the 143 women in this cohort are summarized in Table 1 and are representative of that observed in the entire GOG-157 cohort (43). At the time of the final analyses, 92 women were alive with no evidence of disease, 13 were alive with DP, 25 had died due to DP, two had died due to treatment, 8 had died due to a reason other than DP or treatment, and three had died of unknown cause. Median follow-up for the 105 women in GOG-157 who were still alive at the time of the final analysis was 105 (range 14 to 137) months. Of the 70 cases (49%) with normal p53 expression; 31 were p53 negative and 39 exhibited p53 staining in <10% of the tumor cells. Of the 73 (51%) of women with p53 overexpression, 28 had limited overexpression (10–49% p53-positive tumor cells), 16 had moderate overexpression (50–74% p53-positive tumor cells) and 29 had extensive overexpression (≥75% p53-positive tumor cells) (Figure 1A). In terms of the intensity of p53 staining in the GOG-157 cohort, 12 displayed 1+ staining, 64 exhibited 2+ staining, and 36 stained 3+ (Figure 1B). Staining intensity was associated with percentage of p53-positive cells (p<0.001). The intracellular localization of the p53 staining was nuclear.
Table 1.
Clinical characteristics.
| Characteristics | GOG-157 Cohort
|
GOG-111 Cohort
|
Total
|
|||
|---|---|---|---|---|---|---|
| Cases | % | Cases | % | Cases | % | |
| Patient Age in Years, Median, (25th – 75th percentile) | 58 (47 – 64) | 60 (50 – 66) | 58 (49–65) | |||
| < 50 | 45 | 31.5 | 37 | 27.2 | 82 | 29.4 |
| 50–59 | 38 | 26.6 | 34 | 25.0 | 72 | 25.8 |
| 60–69 | 37 | 25.9 | 46 | 33.8 | 83 | 29.7 |
| ≥ 70 | 23 | 16.1 | 19 | 14.0 | 42 | 15.1 |
| Race/Ethnicity | ||||||
| Hispanic or Latino | 3 | 2.1 | 3 | 2.2 | 6 | 2.2 |
| Non-Hispanic Black | 4 | 2.8 | 9 | 6.6 | 13 | 4.7 |
| Non-Hispanic White | 132 | 92.3 | 121 | 89.0 | 253 | 90.7 |
| Asian | 4 | 2.8 | 2 | 1.5 | 6 | 2.2 |
| American Indian/Alaskan Native | 0 | 0 | 1 | 0.7 | 1 | 0.4 |
| GOG Performance Status | ||||||
| Asymptomatic (score = 0) | 73 | 51.4 | 42 | 30.9 | 115 | 41.4 |
| Symptomatic (score > 0) | 69 | 48.6 | 94 | 69.1 | 163 | 58.6 |
| Tumor Stage | ||||||
| I | 97 | 67.8 | 97 | 34.8 | ||
| II | 46 | 32.2 | 46 | 16.5 | ||
| III | 85 | 62.5 | 85 | 30.5 | ||
| IV | 51 | 37.5 | 51 | 18.3 | ||
| Histologic Cell Type | ||||||
| Serous | 31 | 21.7 | 97 | 71.3 | 128 | 45.9 |
| Endometrioid | 36 | 25.2 | 12 | 8.8 | 48 | 17.2 |
| Mucinous | 14 | 9.8 | 5 | 3.7 | 19 | 6.8 |
| Clear Cell | 42 | 29.4 | 3 | 2.2 | 45 | 16.1 |
| Other ** | 20 | 14.0 | 19 | 14.0 | 39 | 14.0 |
| Tumor Grade | ||||||
| 1-Well Differentiated | 20 | 14.3 | 6 | 4.4 | 26 | 9.4 |
| 2-Moderately Differentiated | 39 | 27.9 | 60 | 44.1 | 99 | 35.9 |
| 3-Poorly Differentiated | 81 | 57.9 | 70 | 51.5 | 151 | 54.7 |
| Residual Disease Status following Primary Surgery | ||||||
| None | 143 | 100 | 143 | 51.3 | ||
| Non-Measurable | 54 | 39.7 | 54 | 39.7 | ||
| Measurable | 82 | 60.3 | 81 | 60.3 | ||
| Primary Chemotherapy | ||||||
| Paclitaxel+Carboplatin (x3) | 74 | 51.7 | 0 | 0 | 74 | 26.5 |
| Paclitaxel+Carboplatin (x6) | 69 | 48.3 | 0 | 0 | 69 | 24.7 |
| Cyclophosphamide+Cisplatin (x6) | 0 | 0 | 69 | 50.7 | 69 | 24.7 |
| Paclitaxel+Cisplatin (x6) | 0 | 0 | 67 | 49.3 | 67 | 24.0 |
| Total | 143 | 136 | 279 | |||
High-risk, early stage EOC (GOG-157) or suboptimally-resected, advanced stage EOC (GOG-111).
Other histologic cell types include unspecified adenocarcinoma, mixed epithelial carcinoma, undifferentiated carcinoma, and transitional cell carcinoma.
Note: one patient was missing performance status; three patients did not have tumor grade.
Figure 1.
Immunohistochemical expression of p53 in high-risk, early stage EOC (A, B) and suboptimally-resected, advanced stage EOC (C, D) categorized as the percentage of p53 positive tumor cells (A, C) or p53 staining intensity (B, D).
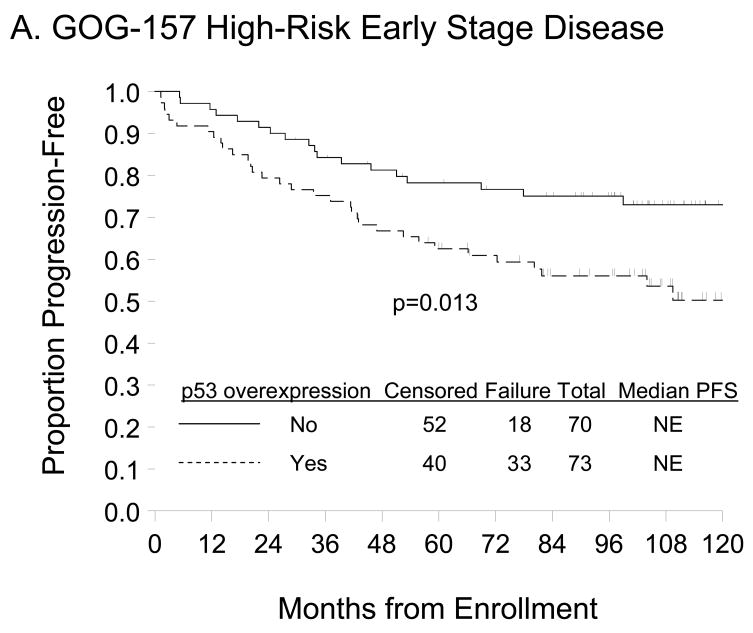
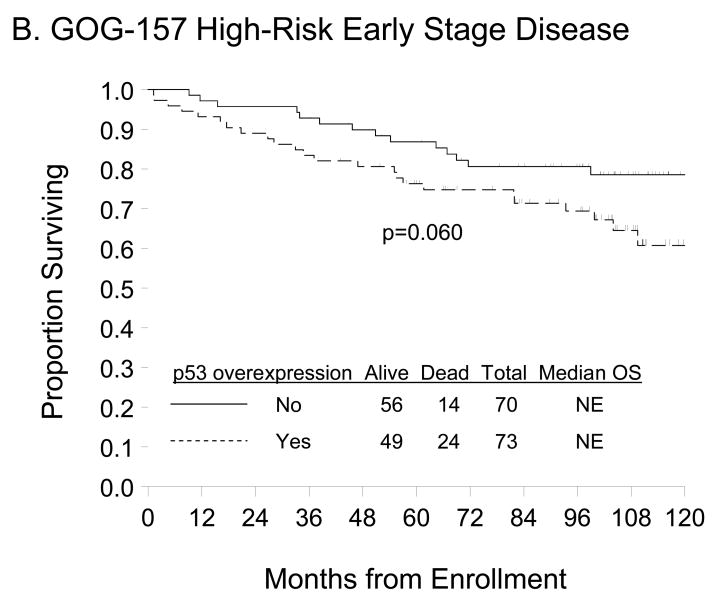
p53 overexpression (≥10% p53-positive tumor cells) was not associated with patient age at enrollment, race/ethnicity, performance status, tumor stage, grade or cell type (Table 2) but was associated with worse PFS (Figure 2A; p=0.013). Unadjusted Cox modeling demonstrated that women with p53 overexpression had a 2-fold higher risk of DP (95% confidence interval [CI]=1.15–3.63; p=0.015) compared with those expressing normal p53 expression (Table 3). A trend suggesting worse OS (Figure 2B, p=0.060) and an elevated risk of death (hazard ratio [HR]=1.86; 95% CI=0.96–3.61; p=0.064) was observed for women with relative to those without p53 overexpression but these associations did not achieve statistical significance. After adjusting for patient age and stratifying by FIGO stage, tumor grade, and primary treatment regimen, p53 overexpression was not an independent prognostic factor for PFS (HR=1.81, 95% CI=0.99–3.30; p=0.052) or OS (HR=1.79, 95% CI=0.90–3.59; p=0.100) in women with high-risk, early stage EOC treated with three or six cycles of carboplatin and paclitaxel (Table 3). There was no evidence of an association between p53 staining intensity and PFS or OS (data not shown).
Table 2.
Association between p53 overexpression† and clinical characteristics, tumor response or disease status.
| p53 overexpression† |
||||||
|---|---|---|---|---|---|---|
| GOG-157 Cohort
|
GOG-111 Cohort
|
|||||
| No | Yes | p-value* | No | Yes | p-value* | |
| Patient Age in years | 0.426 | 0.112 | ||||
| < 50 | 25 (55.6) | 20 (44.4) | 7 (18.9) | 30 (81.1) | ||
| 50–59 | 15 (39.5) | 23 (60.5) | 13 (38.2) | 21 (61.8) | ||
| 60–69 | 20 (54.1) | 17 (45.9) | 17 (37.0) | 29 (63.0) | ||
| 70–79 | 10 (43.5) | 13 (56.5) | 9 (47.4) | 10 (52.6) | ||
| Race/Ethnicity | 0.192 | 0.549 | ||||
| Hispanic or Latino | 2 (66.7) | 1 (33.3) | 1 (33.3) | 2 (66.7) | ||
| Non-Hispanic Black | 2 (50.0) | 2 (50.0) | 5 (55.6) | 4 (44.4) | ||
| Non-Hispanic White | 62 (47.0) | 70 (53.0) | 40 (33.1) | 81 (66.9) | ||
| Asian | 4 (100.0) | 0 (0.0) | 0 (0.0) | 2 (100.0) | ||
| American Indian/Alaskan | ||||||
| Native | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) | ||
| GOG Performance Status | 0.320 | 0.018 | ||||
| Asymptomatic (score = 0) | 39 (53.4) | 34 (46.6) | 8 (19.0) | 34 (81.0) | ||
| Symptomatic (score > 0) | 31 (44.9) | 38 (55.1) | 38 (40.4) | 56 (59.6) | ||
| Tumor Stage | 0.860 | 0.576 | ||||
| I | 48 (49.5) | 49 (50.5) | ||||
| II | 22 (47.8) | 24 (52.2) | ||||
| III | 27 (31.8) | 58 (68.2) | ||||
| IV | 19 (37.3) | 32 (62.7) | ||||
| Histologic Cell Type | 0.473 | 0.463 | ||||
| Serous | 11 (35.5) | 20 (64.5) | 32 (33.0) | 65 (67.0) | ||
| Endometrioid | 20 (55.6) | 16 (44.4) | 4 (33.3) | 8 (66.7) | ||
| Mucinous | 8 (57.1) | 6 (42.9) | 3 (60.0) | 2 (40.0) | ||
| Clear Cell | 22 (52.4) | 20 (47.6) | 2 (66.7) | 1 (33.3) | ||
| Other | 9 (45.0) | 11 (55.0) | 5 (26.3) | 14 (73.7) | ||
| Tumor Grade (excluding clear | 0.538 | 0.003 | ||||
| 1-Well Differentiated | 12 (60.0) | 8 (40.0) | 6 (100.0) | 0 (0.0) | ||
| 2-Moderately Differentiated | 21 (53.8) | 18 (46.2) | 18 (30.0) | 42 (70.0) | ||
| 3-Poorly Differentiated | 36 (44.4) | 45 (55.6) | 22 (31.4) | 48 (68.6) | ||
| Residual Disease Status following Primary Surgery | 0.140 | |||||
| None | 70 (49.0) | 73 (51.0) | ||||
| Non-Measurable | 14 (25.9) | 40 (74.1) | ||||
| Measurable | 32 (39.0) | 50 (61.0) | ||||
| Tumor Response | 0.621 | |||||
| Stable/Progressive Disease | 8 (25.0) | 24 (75.0) | ||||
| Complete/Partial Response | 16 (32.0) | 34 (68.0) | ||||
| Disease Status Assessment after Primary Chemotherapy | 1.000 | |||||
| No Evidence of Disease | 9 (20.9) | 34 (79.1)a | ||||
| Positive for Disease | 18 (22.0) | 64 (78.0) b | ||||
| Total | 70 (49.0) | 73 (51.0) | 46 (33.8) | 90 (66.2) | ||
High-risk, early stage EOC (GOG-157) or suboptimally-resected, advanced stage EOC (GOG-111).
Immunohistochemical expression of p53 was categorized as normal expression when <10% tumor cells exhibited p53 staining and overexpression when ≥10% tumor cells display p53 staining.
Fisher’s Exact Test
Includes one woman with microscopic evidence of disease and 19 women with macroscopic disease as assessed during the reassessment laparotomy and 14 women with clinically evidence of disease progression during treatment.
Includes ten women with microscopic evidence of disease and 28 women with macroscopic disease as assessed during the second look laparotomy and 26 women with clinically evidence of disease progression during treatment.
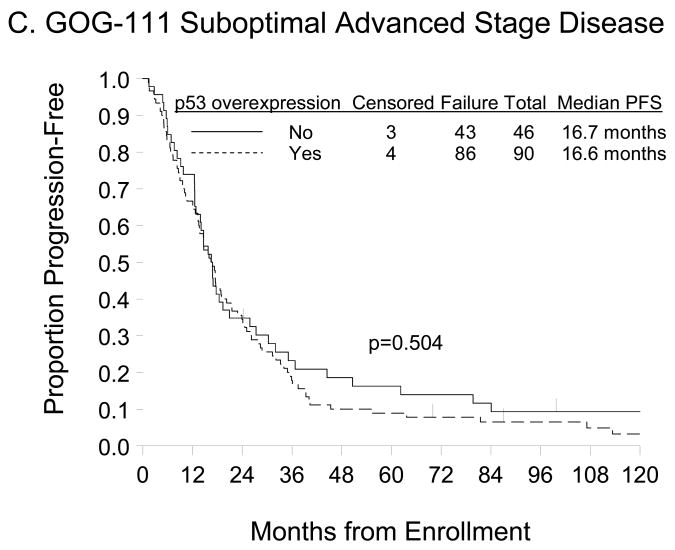
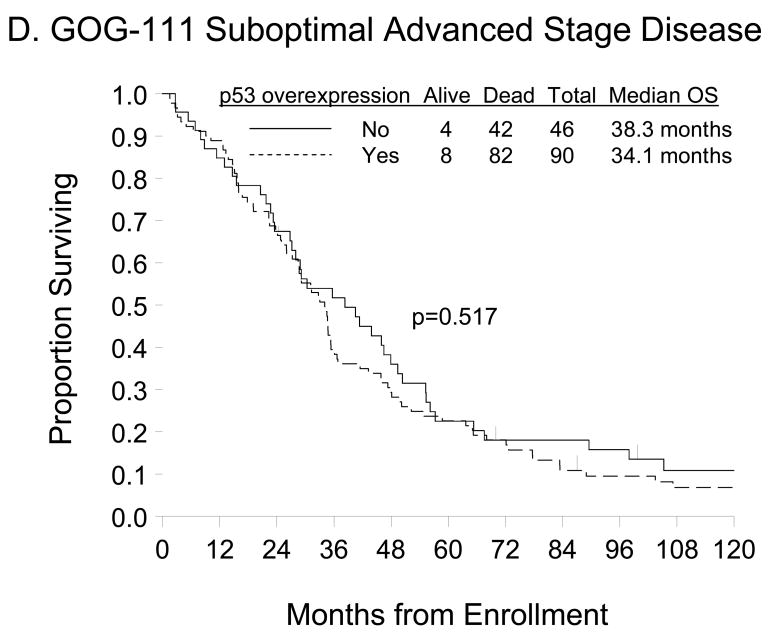
Figure 2.
Kaplan-Meier estimate of PFS (A, C) and OS (B, D) for women with normal p53 (<10% p53 positive tumor cells) or p53 overexpression (≥10% p53 positive tumor cells) in the GOG-157 cohort (A, B) or the GOG-111 cohort (C, D). Median PFS and OS provided in months from study enrollment. Logrank test was used to compare PFS and OS distributions by p53 overexpression.
Table 3.
Association between p53 overexpression† and PFS or OS.
| PFS
|
OS
|
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| GOG-157 Cohort | ||||||
| Unadjusted Model a | ||||||
| p53 overexpression† | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 2.04 | 1.15–3.63 | 0.015 | 1.86 | 0.96–3.61 | 0.064 |
| Multivariate Modela,b | ||||||
| p53 overexpression† | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.81 | 0.99–3.30 | 0.052 | 1.79 | 0.90–3.59 | 0.100 |
| GOG-111 Cohort | ||||||
| Unadjusted Model a | ||||||
| p53 overexpression† | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.13 | 0.78–1.64 | 0.505 | 1.13 | 0.78–1.64 | 0.517 |
| Multivariate Model a, c | ||||||
| p53 overexpression† | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 1.54 | 0.96–2.47 | 0.076 | 1.34 | 0.83–2.18 | 0.231 |
High-risk, early stage EOC (GOG-157) or suboptimally-resected, advanced stage EOC (GOG-111). Estimated hazard ratio (HR) and 95% confidence interval (95% CI).
Immunohistochemical expression of p53 was categorized as normal expression when <10% tumor cells exhibited p53 staining and overexpression when ≥10% tumor cells display p53 staining.
Cox regression analysis for modeling the relative risk of disease progression or death. The goodness of fit of the overall model was evaluated using the likelihood ratio test while the association between adduct level and outcome was assessed using the Wald test.
Models were adjusted for patient age at enrollment in years, and stratified by tumor stage (I vs. II), tumor grade (well differentiated vs. moderately differentiated vs. poorly differentiated), and treatment regimen (carboplatin + paclitaxel for three cycles [x3] vs. carboplatin + paclitaxel for six cycles [x6]). Histologic cell type was not included in adjusted Cox modeling performed for the primary analysis of the GOG-157 treatment arms (43) or in this translational research study.
Models were adjusted for patient age at enrollment in years, and stratified by tumor stage (III vs. IV), histologic subtype (clear cell or mucinous vs. other histologic subtypes), tumor grade (well differentiated vs. moderately differentiated vs. poorly differentiated),
Suboptimally-Resected, Advanced Stage, Epithelial Ovarian Cancer
Of the 410 women enrolled on GOG-111, 136 (33%) provided FFPE tumor tissue for this research study. The patient characteristics for the 136 women in this cohort are summarized in Table 1 and are representative of that observed in the entire GOG-111 cohort (44). At the time of the final analyses, seven women were alive with no evidence of disease; five were alive with DP, 120 had died due to DP, one had died due to treatment, two had died due to a reason other than DP or treatment, and the cause of death was unknown for one woman. Median follow-up for the 12 women in GOG-111 who were still alive at the time of the final analysis was 127 (range 15–194) months. Forty-six (34%) women displayed normal p53 expression (<10% positive tumor cells). Of the 90 (66%) women with p53 overexpression, seven had limited overexpression (10–49% p53-positive tumor cells), 16 had moderate overexpression (50–74% p53-postive tumor cells), and 67 had extensive overexpression (≥75% p53-positive tumor cells) (Figure 1C). The intensity of p53 staining in the advanced stage cases was always 3+ (Figure 1D) and the localization was nuclear.
Significant associations were observed between p53 overexpression and GOG performance status (p=0.018) and tumor grade (p=0.003), but not with patient age, race/ethnicity, tumor stage, histologic cell type or residual disease status after primary surgery (Table 2). Table 2 demonstrates that p53 overexpression was not associated with tumor response in the 82 women with measurable disease (p=0.621) or with disease status in the 125 women who either underwent reassessment laparotomy after completion (N=85) or experienced disease progression (N=40) during primary chemotherapy (p=1.000).
There was also no difference in the PFS (p=0.504) or OS (p=0.517) distributions for women categorized by p53 overexpression (Figures 2C and 2D, respectively). Unadjusted Cox regression analyses demonstrated that women with p53 overexpression did not have an increased risk of DP (HR=1.13; 95% CI=0.78–1.64; p=0.505) or death (HR=1.13; 95% CI=0.78–1.64; p=0.517) compared with those with normal p53 expression (Table 3). After adjusting for patient age and stratifying by FIGO stage, histologic subtype, tumor grade, gross residual disease, and primary treatment regimen, p53 overexpression was not an independent prognostic factor for PFS (HR=1.54, 95% CI=0.96–2.47; p=0.076) or OS (HR=1.34, 95% CI=0.83–2.18; p=0.231) in women with suboptimally-resected, advanced stage EOC treated with either cyclophosphamide+cisplatin or paclitaxel+cisplatin (Table 3).
DISCUSSION
Mutations in p53 are a common event in ovarian cancer (8–11,17,18,24,26,29,30,33,35,36,41) and have been shown to either be associated with OS (24,29,33) or response to platinum/paclitaxel based chemotherapy (26,30,33) or to not associated with OS (35,41). A strong correlation has been observed between a p53 mutation most notably a missense mutation and DO-7 expression of p53 protein (14,18,24,26,29,33). In the study reported herein, p53 overexpression was evaluated using the N-terminal DO-7 antibody in well-annotated FFPE tumor from 143 women with high-risk, early stage EOC and 136 women with suboptimally-resected, advanced stage EOC participated in a phase III treatment protocol and were treated in GOG institutions throughout the US (43,44). The fact that these specimens were from women recruited from multiple institutions who were uniformly-staged, treated, and managed, and were linked to detailed clinical data, including treatment information and long-term follow-up, is a major strength of this study. This study, however, did not included women with low-risk, early stage disease or women with optimally-resected, stage III disease, and did not examine p53 mutation status which are weaknesses.
The DO-7 antibody recognizes an epitope within the first 40 amino acids of p53 and reacts with wild type p53, p53-beta and p53-gamma, but not with the p53-isoforms which are missing the first 40 or 133 amino acids of full length p53 (7,45,46). Righetti and co-workers demonstrated that the DO-7 antibody was more reliable that the CM-1, DO-7, PAb1801, and PAb240 antibodies for detection of wild-type and mutant p53 in FFPE EOC (18). Given that a number of p53 cut-points have been reported in EOC but none have been validated, we defined p53 overexpression using the most commonly utilized cut-point (≥10%) in EOC (21,22,24,28,33,34,37,40,41) and demonstrated that p53 overexpression was observed in 51% of high-risk, early stage EOC and 66% of suboptimally-resected, advanced stage EOC. This is consistent with studies reporting a 40 to 75% prevalence rates in EOC (8,9,11–15,17–19,21,23–35,37–42) but is higher than the 16 to 35% levels reported by some groups (10,16,22,36). Using the 10% cut-point, p53 overexpression was associated with PFS but not OS and was not an independent prognostic factor for PFS or OS in the GOG-157 cohort. In the GOG-111 cohort, p53 overexpression was associated with performance status and tumor grade but not with tumor response, disease status assessment, risk of DP or risk of death, and was not an independent prognostic factor for PFS or OS.
Our findings are consistent with studies showing an association between p53 overexpression and PFS (16,22) or tumor grade (12,13,15,16,17,19,22–24,26,32,33,35,37,40,42) and with studies demonstrating that p53 overexpression was not associated with PFS (10,17,30,33,35,38,41,42), OS (8,10,11,17,24,25,29,30,32–39,41,42), tumor response (16,23,25,26,30,37,39–42), or disease status (12, 27), and that p53 was not an independent prognostic factor for PFS (16,17,34,35,42) or OS (12,14,16,17,19,22,24,29,32,33,35,39,40,42) in invasive EOC. However, these results contradict studies demonstrating that p53 overexpression was associated with OS (12,13,15,16,19,20,22,23,27,28,40), and/or tumor response (18,22), and that p53 was an independent prognostic factor for PFS (22) or OS (15,20,23,27,28,31) in invasive EOC. These inconsistencies can be explained at least in part by differences in sample size, stage of disease, type of primary chemotherapy, follow-up time and assessments, study design, type of tumor tissue, p53 antibody and/or the cut-point that was used to define p53 overexpression. For example, the different p53 antibodies (DO-7, DO-1, PAb1801, PAb240, Bp53-11, Bp53-12, CM-1) recognize distinct regions in p53 (45,46) and therefore bind to specific p53 isoforms (7) and selectively work in FFPE and/or frozen tumor (7,45).
Among the studies that examined OS as an end-point and used the N-terminal DO-7 antibody with different cut-points in FFPE or frozen tumor, most including ours showed that p53 overexpression was not associated with OS (14,24,30,32,33,34,39,42). In contrast, Ozalp and colleagues demonstrated that p53 overexpression categorized as negative or positive was associated with OS (27). Shahin and coworkers used a 5% cut point and reported that p53 overexpression was not associated with OS in women with early and advanced stage disease but was association with OS in a subset analysis in stage III or IV disease (29). The studies reported herein and by de Graeff (42) recruited women from multiple institutions and did not show an association between p53 overexpression and OS.
Since this study was initiated, our understanding of the p53 gene family has evolved. The nine p53 isoforms result from differential promoter utilization and alternative splicing, and contain distinct functional domains (7). In addition, p53 is a member of a family of transcriptional regulators that includes six p63-isoforms and 28 p73-isoforms, each with distinct domains for transcriptional regulation, protein-protein interactions, sequence-specific DNA binding, zinc-binding, nuclear localization, oligomerization and/or DNA repair (47). Some p63- and p73-isoforms are transcriptional activators while others are dominant negative transcriptional factors (47). p53 forms tetramers with itself and can bind to p63- and p73-isoforms. Alterations in p53 either by mutation, overexpression or indirect mechanisms can exert a diverse array of effects on cancer development and therapeutic responsiveness attributable to differential expression and oligomerization between normal and altered p53-, p63- and p73-isoforms, loss of normal p53 functions and/or gain of oncogenic p53 functions (3–7,47). Thus, given our current understanding of the p53 family, it is not surprising that studies evaluating p53 overexpression using immunohistochemical methods and the current p53 antibodies have yielded contradictory data. As a result, investigators are now proposing that high through-put platforms be used to provide a more accurate measure of p53 status and functional activity including the specific p53-dependent genes that exist in individual human cancers (48).
In conclusion, p53 overexpression, assessed by DO-7 immunostaining, was common in early and advanced stage EOC and was associated with PFS in high-risk early stage disease using the 10% cut-point, but this measure of p53 has limited independent clinical value in women with EOC treated with surgical staging and platinum-based combination chemotherapy. Alternative techniques for evaluating p53 status and functional activity (e.g., mass-spectroscopy, multiplex and gene expression array platforms) have yet to be fully evaluated in EOC and may have prognostic/predictive value in this disease setting.
Acknowledgments
The authors extend special thanks to Dr. Michael Birrer for his role as Study Chair for GOG-9404, coordinating the various aspects of this study of p53 in GOG-157 and GOG-111, and providing insightful comments and suggestions for this study and the manuscript. We also thank Anne Reardon for formatting this manuscript, and Suzanne Baskerville and Dr. Mark Brady for their efforts on GOG-157 and GOG-111. Finally, we would like to thank Dr. Heather Lankes and the GOG Publications Subcommittee for their critical review of and thoughtful suggestions for the manuscript.
This study was supported by National Cancer Institute grants of the Gynecologic Oncology Group Administrative Office (CA 27469), the Gynecologic Oncology Group Statistical and Data Center (CA 37517), and the Intramural Research Program of the National Cancer Institute of the National Institute of Health. The following Gynecologic Oncology Group (GOG) institutions participated in this study: University of Alabama at Birmingham, Duke University Medical Center, Abington Memorial Hospital, University of Rochester Medical Center, Walter Reed Army Medical Center, Wayne State University, University of Mississippi Medical Center, Colorado Gynecologic Oncology Group P.C., University of California at Los Angeles, University of Washington, University of Pennsylvania Cancer Center, Milton S. Hershey Medical Center, University of Cincinnati, University of North Carolina School of Medicine, University of Iowa Hospitals and Clinics, University of Texas Southwestern Medical Center of Dallas, Indiana University Medical Center, Georgetown University Hospital, Wake Forest University School of Medicine, Albany Medical College, University of California Medical Center at Irvine, Tuffs-New England Medical Center, Rush-Presbyterian-St. Luke’s Medical Center, SUNY Downstate Medical Center, University of Kentucky, The Cleveland Clinic Foundation, SUNY at Stony Brook, Washington University School of Medicine, Johns Hopkins Oncology Center, Eastern Pennsylvania Gyn/Onc Center, P.C., Cooper Hospital/University Medical Center, Columbus Cancer Council, University of Massachusetts Medical Center, Fox Chase Cancer Center, Medical University of South Carolina, Women’s Cancer Center, University of Oklahoma, University of Virginia, University of Chicago, Tacoma General Hospital, Thomas Jefferson University Hospital, Mayo Clinic, Case Western Reserve University, Tampa Bay Cancer Consortium, North Shore University Hospital, and Brookview Research Inc.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest with the exception of Dr. Ilona Linnoila who has ownership of General Electric stock worth less than $15,000.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Ward E, Murray R, Xu J, Thun M. Cancer statistics, 2007. CA - A Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Hainaut P, Hernandez T, Robinson A, et al. IARC Database of p53 gene mutations in human tumors and cell lines: updated compilation, revised formats and new visualization tools. Nucleic Acids Res. 1998;26:205–13. doi: 10.1093/nar/26.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petitjean A, Achatz MI, Borresen-Dale AL, Hainaut P, Olivier M. TP53 mutations in human cancers: functional selection and impact on cancer prognosis and outcomes. Oncogene. 2007;26:2157–65. doi: 10.1038/sj.onc.1210302. [DOI] [PubMed] [Google Scholar]
- 4.Soussi T. p53 alterations in human cancer: more questions than answers. Oncogene. 2007;26:2145–56. doi: 10.1038/sj.onc.1210280. [DOI] [PubMed] [Google Scholar]
- 5.May P, May E. Twenty years of p53 research: structural and functional aspects of the p53 protein. Oncogene. 1999;18:7621–36. doi: 10.1038/sj.onc.1203285. [DOI] [PubMed] [Google Scholar]
- 6.Oren M, Rotter V. Introduction: p53 - the first twenty years. Cell Mol Life Sci. 1999;55:9–11. doi: 10.1007/s000180050265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bourdon JC. p53 and its isoforms in cancer. Br J Cancer. 2007;97:277–82. doi: 10.1038/sj.bjc.6603886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marks J, Davidoff A, Kerns B, et al. Overexpression and mutation of p53 in epithelial ovarian cancer. Cancer Res. 1991;51:2979–84. [PubMed] [Google Scholar]
- 9.Kihana T, Tsuda H, Teshima S, Okada S, Matsuura S, Horohashi S. High incidence of p53 gene mutation in human ovarian cancer and its association with nuclear accumulation of p53 protein and tumor DNA aneuploidy. Jpn J Cancer Res. 1992;83:978–84. doi: 10.1111/j.1349-7006.1992.tb02010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohler MF, Kerns B-JM, Humphrey PA, Marks JR, Bast RC, Jr, Berchuck A. Mutation and overexpression of p53 in early-stage epithelial ovarian cancer. Obstet Gynecol. 1993;81:643–50. [PubMed] [Google Scholar]
- 11.Berchuck A, Kohler MF, Marks JR, Wiseman R, Boyd J, Bast RC., Jr The p53 tumor suppressor gene frequently is altered in gynecologic cancers. Am J Obstet Gynecol. 1994;170:246–52. doi: 10.1016/s0002-9378(94)70414-7. [DOI] [PubMed] [Google Scholar]
- 12.Hartmann LC, Podratz KC, Keeney GL, et al. Prognostic significance of p53 immunostaining in epithelial ovarian cancer. J Clin Oncol. 1994;12:64–9. doi: 10.1200/JCO.1994.12.1.64. [DOI] [PubMed] [Google Scholar]
- 13.Henriksen R, Stang P, Wilander E, Backstrom T, Tribukait B, Oberg K. p53 expression in epithelial ovarian neoplasms: relationship to clinical and pathological parameters, Ki-67 expression and flow cytometry. Gynecol Oncol. 1994;53:301–6. doi: 10.1006/gyno.1994.1138. [DOI] [PubMed] [Google Scholar]
- 14.Sheridan E, Silcocks P, Smith J, Hancock BW, Goyns MH. P53 mutation in a series of epithelial ovarian cancers from the U.K. and its prognostic significance. Eur J Cancer. 1994;30A:1701–4. doi: 10.1016/0959-8049(94)00325-y. [DOI] [PubMed] [Google Scholar]
- 15.Klemi P-J, Pylkkanen L, Kiilhoma P, Kurvinen K, Joensuu H. p53 protein detected by immunohistochemistry as a prognostic factor in patients with epithelial ovarian carcinoma. Cancer. 1995;76:1201–8. doi: 10.1002/1097-0142(19951001)76:7<1201::aid-cncr2820760716>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 16.van der Zee AGJ, Hollema H, Suurmeijer AJH, et al. Value of P-Glycoprotein, glutathione S-transferase pi, c-erb B-2, and p53 as prognostic factors in ovarian carcinomas. J Clin Oncol. 1995;13:70–8. doi: 10.1200/JCO.1995.13.1.70. [DOI] [PubMed] [Google Scholar]
- 17.Allan IA, Campbell MK, Milner BJ, et al. The significance of p53 mutation and over-expression in ovarian cancer prognosis. Int J Gynecol Cancer. 1996;6:483–90. [Google Scholar]
- 18.Righetti SC, Torre GD, Pilotti S, et al. A comparative study of p53 gene mutations, protein accumulation, and response to cisplatin-based chemotherapy in advanced ovarian carcinoma. Cancer Res. 1996;56:689–93. [PubMed] [Google Scholar]
- 19.Eltabbakh GH, Belinson JL, Kennedy AW, et al. p53 overexpression is not an independent prognostic factor for patients with primary ovarian epithelial cancer. Cancer. 1997;80:892–8. [PubMed] [Google Scholar]
- 20.Geisler J, Geisler H, Wiemann M, et al. Quantification of p53 in epithelial ovarian cancer. Gynecol Oncol. 1997;66:435–8. doi: 10.1006/gyno.1997.4799. [DOI] [PubMed] [Google Scholar]
- 21.Ioakim-Liossi A, Karakitsos P, Aroni K, et al. p53 protein expression and DNA ploidy in common epithelial tumors of the ovary. Acta Cytologica. 1997;41:1714–8. doi: 10.1159/000333174. [DOI] [PubMed] [Google Scholar]
- 22.Anttila MA, Ji H, Juhola MT, Saarikoski SV, Syrjanen KJ. The prognostic significance of p53 expression quantitated by computerized image analysis in epithelial ovarian cancer. Int J Gynecol Pathol. 1999;18:42–51. doi: 10.1097/00004347-199901000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Baekelandt M, Kristensen GB, Nesland JM, Trope CG, Holm R. Clinical significance of apoptosis-related factors p53, mdm2 and bcl-2 in advanced ovarian cancer. J Clin Oncol. 1999;17:2061–8. doi: 10.1200/JCO.1999.17.7.2061. [DOI] [PubMed] [Google Scholar]
- 24.Wen WH, Reles A, Runnebaum IB, et al. p53 mutations and expression in ovarian cancers: correlation with overall survival. Int J Gynecol Pathol. 1999;18:29–41. doi: 10.1097/00004347-199901000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Gadducci A, Cianic C, Cosio S, et al. p53 status is neither a predictive nor a prognostic variable in patients with advanced ovarian cancer treated with a paclitaxel-based regimen. Anticancer Res. 2000;20:4793–800. [PubMed] [Google Scholar]
- 26.Lavarino C, Pilotti S, Oggionni M, et al. p53 gene status and response to platinum/paclitaxel-based chemotherapy in advanced ovarian carcinoma. J Clin Oncol. 2000;18:3936–45. doi: 10.1200/JCO.2000.18.23.3936. [DOI] [PubMed] [Google Scholar]
- 27.Ozalp SS, Yalcin OT, Basaran N, Artan S, Kabukcuoglu S, Minsin TH. Prognostic significance of deletion and over-expression of the p53 gene in epithelial ovarian cancer. Eur J Gynaec Oncol. 2000;250:282–6. [PubMed] [Google Scholar]
- 28.Schildkraut JM, Halabi S, Bastos E, Marchbanks PA, McDonald JA, Berchuck A. Prognostic factors in early-onset epithelial ovarian cancer: a population-based study. Obstet Gynecol. 2000;95:119–27. doi: 10.1016/s0029-7844(99)00535-9. [DOI] [PubMed] [Google Scholar]
- 29.Shahin MS, Hughes JH, Sood AK, Butler RE. The prognostic significance of p53 tumor suppressor gene alterations in ovarian cancer. Cancer. 2000;89:2006–17. doi: 10.1002/1097-0142(20001101)89:9<2006::aid-cncr18>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- 30.Bar JK, Harlozinska A, Popiela A, Noga L. Expression and mutation of p53 in tumor effusion cells of patients with ovarian carcinoma: response to cisplatin-based chemotherapy. Tumor Biol. 2001;22:83–91. doi: 10.1159/000050601. [DOI] [PubMed] [Google Scholar]
- 31.Geisler HA, Geisler JP, Miller GA, Geisler MJ, Weimann MC, Zhou Z, Crabtree W. p21 and p53 in ovarian carcinoma. Their combined staining is more valuable than either alone. Cancer. 2001;92:781–6. doi: 10.1002/1097-0142(20010815)92:4<781::aid-cncr1383>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 32.Howells REJ, Holland T, Dhar KK, et al. Glutathione S-transferase GSTM1 and GSTT1 genotypes in ovarian cancer: association with p53 expression and survival. Int J Gynecol Cancer. 2001;11:107–12. doi: 10.1046/j.1525-1438.2001.011002107.x. [DOI] [PubMed] [Google Scholar]
- 33.Reles A, Wen WH, Schmider A, et al. Correlation of p53 mutations with resistance to platinum-based chemotherapy and shortened survival in ovarian cancer. Clin Cancer Res. 2001;7:2984–97. [PubMed] [Google Scholar]
- 34.Suzuki M, Ohwada M, Saga Y, Kohno T, Takei Y, Sato I. Micrometastatic p53 –positive cells in the lymph nodes of early stage epithelial ovarian cancer: prognostic significance. Onology. 2001;60:170–5. doi: 10.1159/000055315. [DOI] [PubMed] [Google Scholar]
- 35.Havrilesky L, Darcy KM, Hamdan H, Priore RL, Leo J, Bell J, Berchuck A. Prognostic significance of p53 mutation of p53 overexpression in advanced epithelial ovarian cancer: a gynecologic oncology group study. J Clin Oncol. 2003;21:3814–25. doi: 10.1200/JCO.2003.11.052. [DOI] [PubMed] [Google Scholar]
- 36.Okuda T, Otsuka J, Sekizawa A, et al. p53 mutations and overexpression affect prognosis of ovarian endometrioid cancer but not clear cell cancer. Gynecol Oncol. 2003;88:318–25. doi: 10.1016/s0090-8258(02)00149-x. [DOI] [PubMed] [Google Scholar]
- 37.Plisiecka-Halasa J, Karpinska G, Szymanska T, et al. p21WAF1, p27KIP1, TP53 and c-myc analysis in 204 ovarian carcinomas treated with platinum-based regimens. Annals Oncol. 2003;14:1078–85. doi: 10.1093/annonc/mdg299. [DOI] [PubMed] [Google Scholar]
- 38.Wisman GBA, Hollema H, Helder MN, et al. Telomerase in relation to expression of p53, c-myc and estrogen receptor in ovarian tumours. Int J Oncol. 2003;23:1451–9. doi: 10.3892/ijo.23.5.1451. [DOI] [PubMed] [Google Scholar]
- 39.Hashiguchi Y, Tsuda H, Inoue T, Nishimura S, Suzuki T, Kawamura N. Alteration of cell cycle regulators correlates with survival in epithelial ovarian cancer patients. Hum Pathol. 2004;35:165–75. doi: 10.1016/j.humpath.2003.07.018. [DOI] [PubMed] [Google Scholar]
- 40.Dogan E, Saygili U, Tuna B, et al. p53 and mdm2 as prognostic indicators in patients with epithelial ovarian cancer: am multivariate analysis. Gynecol Oncol. 2005;97:46–52. doi: 10.1016/j.ygyno.2004.12.053. [DOI] [PubMed] [Google Scholar]
- 41.Gadducci A, Di Cristofano C, Zavaglia M, et al. p53 gene status in patients with advanced serous epithelial ovarian cancer in relation to response to paclitaxel– plus platinum-based chemotherapy and long-term clinical outcome. Anticancer Res. 2006;26:687–94. [PubMed] [Google Scholar]
- 42.de Graeff P, Hall J, Crijns A, et al. Factors influencing p53 expression in ovarian cancer as a biomarker of clinical outcome in multicentre studies. Brit J Cancer. 2006;95:627–33. doi: 10.1038/sj.bjc.6603300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bell J, Brady M, Young R, et al. Randomized phase III trial of three versus six cycles of adjuvant carboplatin and paclitaxel in early stage epithelial ovarian carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol. 2006;102:432–9. doi: 10.1016/j.ygyno.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 44.McGuire W, Hoskins W, Brady M, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 45.Vojtesek B, Bartek J, Midgley CA, Lane DP. An immunochemical analysis of the human nuclear phosphoprotein p53. New monoclonal antibodies and epitope mapping using recombinant p53. J Immunol Methods. 1992;151:237–44. doi: 10.1016/0022-1759(92)90122-a. [DOI] [PubMed] [Google Scholar]
- 46.Stephen CW, Helminen P, Lane DP. Characterisation of epitopes on human p53 using phage-displayed peptide libraries: insights into antibody-peptide interactions. J Mol Biol. 1995;248:58–78. doi: 10.1006/jmbi.1995.0202. [DOI] [PubMed] [Google Scholar]
- 47.Deyoung MP, Ellisen LW. p63 and p73 in human cancer: defining the network. Oncogene. 2007;26:5169–83. doi: 10.1038/sj.onc.1210337. [DOI] [PubMed] [Google Scholar]
- 48.Foulkes WD. p53-Master and Commander. N Engl J Med. 2007;357:2539–41. doi: 10.1056/NEJMp0707422. [DOI] [PubMed] [Google Scholar]