Abstract
Occludin is phosphorylated on tyrosine residues during the oxidative stress-induced disruption of tight junction, and in vitro phosphorylation of occludin by c-Src attenuates its binding to ZO-1. In the present study mass spectrometric analyses of C-terminal domain of occludin identified Tyr-379 and Tyr-383 in chicken occludin as the phosphorylation sites, which are located in a highly conserved sequence of occludin, YETDYTT; Tyr-398 and Tyr-402 are the corresponding residues in human occludin. Deletion of YETDYTT motif abolished the c-Src-mediated phosphorylation of occludin and the regulation of ZO-1 binding. Y398A and Y402A mutations in human occludin also abolished the c-Src-mediated phosphorylation and regulation of ZO-1 binding. Y398D/Y402D mutation resulted in a dramatic reduction in ZO-1 binding even in the absence of c-Src. Similar to wild type occludin, its Y398A/Y402A mutant was localized at the plasma membrane and cell-cell contact sites in Rat-1 cells. However, Y398D/Y402D mutants of occludin failed to localize at the cell-cell contacts. Calcium-induced reassembly of Y398D/Y402D mutant occludin in Madin-Darby canine kidney cells was significantly delayed compared with that of wild type occludin or its T398A/T402A mutant. Furthermore, expression of Y398D/Y402D mutant of occludin sensitized MDCK cells for hydrogen peroxide-induced barrier disruption. This study reveals a unique motif in the occludin sequence that is involved in the regulation of ZO-1 binding by reversible phosphorylation of specific Tyr residues.
Epithelial tight junctions (TJs)2 form a selective barrier to the diffusion of toxins, allergens, and pathogens from the external environment into the tissues in the gastrointestinal tract, lung, liver, and kidney (1). Disruption of TJs is associated with the gastrointestinal diseases such as inflammatory bowel disease, celiac disease, infectious enterocolitis, and colon cancer (2–4) as well as in diseases of lung and kidney (5, 6). Numerous inflammatory mediators such as tumor necrosis factor α, interferon γ, and oxidative stress (7–12) are known to disrupt the epithelial TJs and the barrier function. Several studies have indicated that hydrogen peroxide disrupts the TJs in intestinal epithelium by a tyrosine kinase-dependent mechanism (11, 12).
Four types of integral proteins, occludin, claudins, junctional adhesion molecules, and tricellulin are associated with TJs. Occludin, claudins, and tricellulin are tetraspan proteins, and their extracellular domains interact with homotypic domains of the adjacent cells (1, 2, 13). The intracellular domains of these proteins interact with a variety of soluble proteins such as ZO-1, ZO-2, ZO-3, 7H6, cingulin, and symplekin (14–23); this protein complex interacts with the perijunctional actomyosin ring. The interactions among TJ proteins are essential for the assembly and the maintenance of TJs. Therefore, regulation of the interactions among TJ proteins may regulate the TJ integrity. A significant body of evidence indicates that numerous signaling molecules are associated with the TJs. Protein kinases and protein phosphatases such as protein kinase Cζ (PKCζ), PKCι/λ (24), c-Src (25), c-Yes (26, 27), mitogen-activated protein kinase (28), PP2A, and PP1 (29) interact with TJs, indicating that TJs are dynamically regulated by intracellular signal transduction involving protein phosphorylation. Additionally, other signaling molecules such as calcium (30), phosphatidylinositol 3-kinase (31), Rho (32), and Rac (33) are involved in the regulation of TJs.
Occludin, a ∼65-kDa protein, has been well characterized to be assembled into the TJs. Although occludin knock-out mice showed the formation of intact TJs in different epithelia (34), numerous studies have emphasized that it plays an important role in the regulation of TJ integrity. Occludin spans the membrane four times to form two extracellular loops and one intracellular loop, and the N-terminal and C-terminal domains hang into the intracellular compartment (35–37). In epithelium with intact TJs, occludin is highly phosphorylated on Ser and Thr residues (38), whereas Tyr phosphorylation is undetectable. However, the disruption of TJs in Caco-2 cell monolayers by oxidative stress and acetaldehyde leads to Tyr phosphorylation of occludin; the tyrosine kinase inhibitors attenuate the disruption of TJs (39, 40). Furthermore, a previous in vitro study demonstrated that Tyr phosphorylation of the C-terminal domain of occludin leads to the loss of its interaction with ZO-1 and ZO-3 (25).
In the present study we identified the Tyr residues in occludin that are phosphorylated by c-Src and determined their role in regulated interaction between occludin and ZO-1 and its assembly into the TJs. Results show that 1) Tyr-379 and Tyr-383 in chicken occludin and Tyr-398 and Tyr-402 in human occludin are the exclusive sites of phosphorylation by c-Src, and these Tyr residues are located in a highly conserved sequence of occludin, YET-DYTT, 2) deletion of YEDTYTT or point mutation of Tyr-398 and Tyr-402 in human occludin attenuates the phosphorylation-dependent regulation of ZO-1 binding, 3) Y398D/Y402D mutation of human occludin leads to loss of ZO-1 binding and prevents its translocation to the plasma membrane and cell-cell contact sites in Rat-1 cells, 4) Y398D/Y402D mutation of occludin delays its assembly into the intercellular junctions during the calcium-induced assembly of TJs, and 5) expression of Y398D/Y402D mutant occludin sensitizes cell monolayers for hydrogen peroxide-induced disruption of barrier function.
EXPERIMENTAL PROCEDURES
Chemicals
Cell culture reagents and supplies, G418, Lipofectamine-R, and Plus reagent were purchased from Invitrogen. FuGENE was purchased from Roche Diagnostics, and glutathione (GSH), leupeptin, aprotinin, pepstatin A, phenylmethylsulfonyl fluoride, protease inhibitor mixture, GSH-agarose, Triton X-100, and vanadate were purchased from Sigma. The QuikChange XL site-directed mutagenesis kit was from Stratagene, La Jolla, CA. Active c-Src (recombinant protein) was purchased from Upstate Biotechnology, Inc. (Lake Placid, NY). All other chemicals were of analytical grade and were purchased either from Sigma or Fisher.
Plasmids and Recombinant Proteins
cDNA for the C-terminal tail of chicken occludin (amino acids 358–504) was a kind gift from Dr. James Anderson (University of North Carolina, Chapel Hill, NC); this was used to amplify and insert into pGEX2T vector. The C-terminal tail of human occludin 378–522 was amplified from a full-length human occludin in pEGFP and then shuttled into pGEX2T vector. Site directed mutations were induced in both chicken and human occludin (C-terminal domain as well as full-length occludin). The sequences of the primers used for this are provided in Table 1. The mutations were confirmed by sequencing. pGEX2T constructs containing wild type occludin C-terminal domain (GST-cOcl-C and GST-hOcl-C) were transformed into BL21DE3 cells, and recombinant proteins were purified. The full-length human occludin (wild type and mutants) in pEGFP vector was used for transfection into Rat-1 and MDCK cells.
TABLE 1.
Sequences of primers used to generate various mutations Sequences in bold substitute tyrosine residues in the wild type occludin.
| Predicted mutant | Forward primer sequence 5′–3′ |
|---|---|
| Chicken occludin | |
| Δ378–385 | CCCGAGTTGGATGAGTCCGCCGTGGAGTCCAGTGAT |
| Y379A | TGGATGAGTCCCAGGCTGAGACCGACTAC |
| Y379F | TGGATGAGTCCCAGTTTGAGACCGACTAC |
| Y379D | TGGATGAGTCCCAGGATGAGACCGACTAC |
| Y383A | GTATGAGACCGACGCCACCACGGCCG |
| Y383F | GTATGAGACCGACTTCACCACGGCCG |
| Y383D | GTATGAGACCGACGACACCACGGCCG |
| Y379/383A | GGATGAGTCCCAGGCTGAGACCGACGCCACCACGGCCGTGG |
| Y379/383F | GGATGAGTCCCAGTTTGAGACCGACTTCACCACGGCCGTGG |
| Y379/383D | GGATGAGTCCCAGGATGAGACCGACGACACCACGGCCGTGG |
| Human occludin | |
| Y398A | GAGAACAGAGCAAGATCACGCTGAGACAGACTACACAACTGG |
| Y402A | GCAAGATCACTATGAGACAGACGCCACAACTGGCGGCGAGTCCTG |
| Y398/402A | CAGAGCAAGATCACGCTGAGACAGACGCCACAACTGGCGGCGAG |
| Y398F | GAGAACAGAGCAAGATCACTTTGAGACAGACTACACAACTGG |
| Y402F | GCAAGATCACTATGAGACAGACTTCACAACTGGCGGCGAGTCCTG |
| Y398/402F | CAGAGCAAGATCACTTTGAGACAGACTTCACAACTGGCGGCGAG |
| Y398D | GAGAACAGAGCAAGATCACGATGAGACAGACTACACAACTGG |
| Y402D | GCAAGATCACTATGAGACAGACGACACAACTGGCGGCGAGTCCTG |
| Y398/402D | CAGAGCAAGATCACGATGAGACAGACGACACAACTGGCGGCGAG |
Mass Spectrometric Analysis
Trypsin Digestion—GST-cOcl-CWT, tyrosine-phosphorylated by c-Src, was suspended in 10% acetonitrile in 10 mm ammonium bicarbonate, pH 8.5, and incubated at 37 °C overnight with TPCK-treated trypsin (enzyme to substrate molar ratio of 1:10). The digest was passed through 0.45-μm filters. Clear supernatant was lyophilized to dryness. Air-dried samples were equilibrated in an aqueous solution containing 0.1% trifluoroacetic acid and desalted by passing through C-18 ZipTip (Millipore, Bedford, MA) using the manufacturer's protocol. Peptides extracted from the ZipTip were subjected to phosphopeptide extraction.
Extraction of Phosphopeptides—The phosphopeptides from trypsin digestion of Tyr-phosphorylated GST-cOcl-C were isolated using a phosphopeptide isolation kit (Pierce). Phosphopeptides were bound to immobilized gallium matrix at acidic pH (<3.5) and eluted in 50 mm ammonium bicarbonate at pH 10. Phosphopeptide extracts were then subjected to MALDI and LC/MS/MS analysis.
MALDI-TOF—Phosphopeptide extracts were dried under vacuum and reconstituted in 2 μl of matrix (α-cyano-4-hydroxycinnamic acid) and spotted for crystallization. Crystals were analyzed for mass by MALDI-TOF using Voyager Biospectrometry work station DE (delayed extraction technology) (Perseptive Biosystems Inc., Framingham, MA) and Data Explorer (Perseptive Biosystems). A Prescriptive Biosystems MALDI time-of-flight instrument incorporating a nitrogen laser (Laser Science, Newton, MA) was used to obtain MALDI mass spectra. Samples solubilized in 85% acetic acid and mixed (1:3 v/v) with α-cyano-4-hydroxycinnamic acid matrix were spotted in 1-μl aliquots and air-dried. Typically, 100–250 laser shots were used to obtain one mass spectrum. Mass scale was calibrated with peptide internal standards.
LC/MS/MS Analysis—Sequence analysis of tryptic peptides was performed by injecting 3 μl of the ZipTip-purified sample onto a capillary C-18 LC column on-line with a Finnigan LCQDECA (Thermoquest, San Jose, CA) ion-trap mass analyzer that is equipped with a nanoelectrospray ionization source. The capillary C-18 column was prepared in-house using New Objective Pico Frit (360-μm outer diameter, 75-μm inner diameter, 15-μm tip, 10.4-cm length) and Magic C18AQ packing material (5-μm beads, 200 A° pores). The peptides were fractionated using 0.1% formic acid in water as solvent A and 90% acetonitrile as solvent B. The acquired spectra were visualized using Qual-browser in the X-Calibur software suite. Raw data thus obtained was analyzed against a protein data base generated from Swissprot using the Sequest software suite (Sequest Technologies Inc., Lisle, IL).
Antibodies—Mouse monoclonal anti-ZO-1, rabbit polyclonal anti-ZO-1, and rabbit polyclonal anti-ZO-3 were purchased from Zymed Laboratories Inc., and AlexaFluor 488-conjugated anti-mouse IgG antibody was from Molecular Probes (Eugene, OR). Anti-phosphotyrosine antibody conjugated with horseradish peroxidase (HRP) was from BD Transduction Laboratories. HRP-conjugated mouse monoclonal anti-GST antibody was from Upstate Biotechnology. Cy3-conjugated anti-rabbit IgG, HRP-conjugated anti-mouse IgG, and HRP-conjugated anti-rabbit IgG antibodies were purchased from Sigma.
Cell Culture and Transfection
Caco-2, Rat-1, and MDCK cells were cultured in Dulbecco's modified Eagle's medium from Invitrogen and supplemented with 10% fetal bovine serum, 1 mm sodium pyruvate, and 2 mm glutamine as per the ATCC guidelines. MDCK cells were seeded on 6-well plates a day before transfection to achieve 50–60% confluency. The cells were transfected using 1 ml of antibiotic-free Dulbecco's modified Eagle's medium containing 10% fetal bovine serum, 1 μg of DNA plasmid (empty vector pEGFP or vector carrying hOclWT or its mutants), 1 μl of Plus reagent, and 3 μl of Lipofectamine-R for each well. After 20 h, the cell monolayers were trypsinized and seeded onto 100-mm plates. The cells were subjected to G418 selection (0.7 mg/ml) for 2 weeks. Resistant cells were sorted to obtain only GFP-expressing cells by fluorescence-activated cell sorter. Cells were maintained in the medium that was supplemented with 0.3 mg/ml G418. Rat-1 cells were transfected using FuGENE reagent as per the manufacturer's protocol, and the GFP-positive cells were sorted by fluorescence-activated cell sorter. Stably transfected cells were selected using G418 as described above.
Immunofluorescence Microscopy
Cell monolayers (12-mm transwells) were washed with phosphate-buffered saline and fixed in acetone-methanol (1:1) at 0 °C for 5 min. Cell monolayers were blocked in 3% nonfat milk in TBST (20 mm Tris, pH 8.0, containing 150 mm NaCl and 0.5% Tween 20) and incubated for 1 h with primary antibodies (rabbit polyclonal anti-ZO-1 and mouse monoclonal anti-GFP) followed by incubation for 1 h with secondary antibodies (Cy3-conjugated anti-rabbit IgG and AlexaFluor 488-conjugated anti-mouse IgG). The fluorescence was visualized using a Zeiss LSM 5 laser scanning confocal microscope, and images from Z-series sections (1 μm) were collected by using Zeiss LSM 5 Pascal Confocal Microscopy Software (Release 3.2). Images were stacked using the software, Image J (NIH), and processed by Adobe Photoshop (Adobe Systems Inc., San Jose, CA).
Occludin Phosphorylation in Vitro
Recombinant GST-cOcl-CWT or GST-hOcl-CWT (5 μg) was incubated with 500 ng of active c-Src in 250 μl of kinase buffer (50 mm Hepes, pH 7.4, 1 mm EDTA, 0.2% β-mercaptoethanol, 3 mm MgCl2) containing 100 μm ATP at 30 °C for 3 h on a shaking incubator. Control reactions were done in the absence of ATP.
GST Pulldown Assay
To determine the interaction of occludin with ZO-1 and ZO-3, GST-hOcl-C GST-cOcl-C (2.5–10 μg) was incubated with Caco-2 whole cell extract made in phosphate-buffered saline containing 0.2% Triton X-100, 1 mm sodium vanadate, and 10 mm sodium fluoride for 16 h at 4 °C on an inverter. GST-occludin-C (GST-conjugated C-terminal tail of occludin) was pulled down with 20 μl of 50% GSH-agarose slurry at 4 °C for 1 h. The amounts of ZO-1 and ZO-3 bound to GSH-agarose were determined by immunoblot analysis. Nonspecific binding was determined by carrying out the binding with GST.
Immunoblot and Densitometric Analysis
Proteins were separated by 7% SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. Membranes were blotted for ZO-1, ZO-3, and p-Tyr by using specific antibodies in combination with HRP-conjugated anti-mouse IgG or HRP-conjugated anti-rabbit IgG antibodies. HRP-conjugated anti-GST antibody was used for immunoblot analysis of GST or GST-occludin. The blot was developed using ECL chemiluminescence method (Amersham Biosciences). Quantitation was performed by densitometric analysis of specific bands on immunoblots by using the software, Image J.
Hydrogen Peroxide Treatment and Paracellular Permeability
MDCK cell monolayers that stably express GFP-hOclWT, GFP-hOclY398A/Y402A, or GFP-hOclY398D/Y402D were exposed to varying concentrations (20–2500 μm) of hydrogen peroxide for 2 h, and paracellular permeability was evaluated by measuring the unidirectional flux of inulin as described before (11).
TJ Assembly by Calcium Switch
MDCK cell monolayers that stably express GFP-hOclWT, GFP-hOclY398A/Y402A, or GFP-hOclY398D/Y402D were incubated overnight with low calcium medium followed by calcium replacement as described before (29). TJ assembly was evaluated by measuring transepithelial electrical resistance, inulin permeability, and confocal microscopy.
Immunoprecipitation
GFP was immunoprecipitated from cells under native or denatured conditions as described before (29). Anti-GFP immunocomplexes at native conditions were immunoblotted for ZO-1, whereas complexes under denatured conditions were immunoblotted for p-Tyr.
Statistics
Comparison between two groups was made by Student's t tests for grouped data. Significance in all tests was set at 95% or greater confidence level.
RESULTS
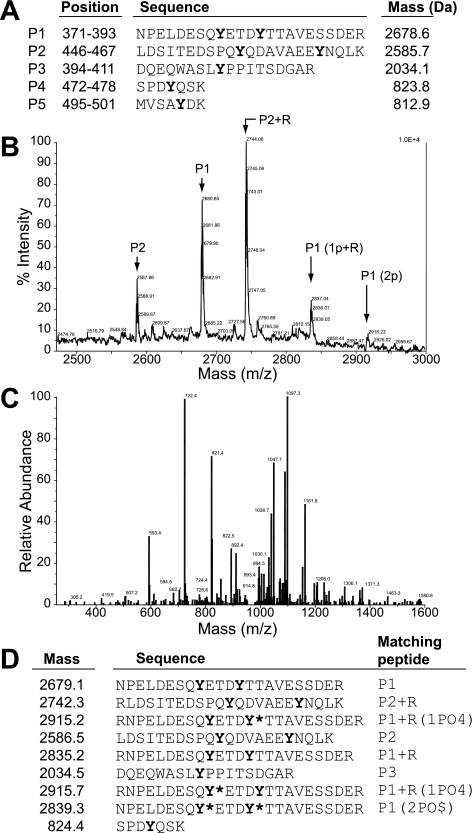
Tyr-379 and Tyr-383 in Chicken Occludin Are the Sites of Phosphorylation by c-Src—A previous study showed that Tyr phosphorylation of occludin C-terminal domain by c-Src resulted in the loss of its interaction with ZO-1 (25). In the present study we identified the phosphorylation sites in occludin C-terminal domain by mass spectrometric analysis. GST-fused C-terminal region (150 amino acids) of chicken occludin (GST-cOcl) was prepared and phosphorylated by incubation with c-Src and ATP. Tyr-phosphorylated GST-cOcl-C was digested with trypsin. Generation of five different tryptic peptides containing Tyr residues was predicted (Fig. 1A); the mass of these peptides was expected to increase by 80 Da with phosphorylation of each Tyr residue. MALDI mass spectrometric analysis of the phosphopeptide extracts from the tryptic digest detected several phosphopeptides with masses slightly deviated from the predicted mass analysis (Fig. 1B). Fig. 1C shows the ion fragmentation spectrum from LC/MS/MS analysis of the phosphopeptide with a mass of 2918 Da. The sequences of peptides as determined by LC/MS/MS analyses of the phosphopeptide extract are summarized in Fig. 1D. Three different Tyr-phosphorylated peptides were identified. All three peptides were identified as the derivatives of tryptic peptide, P1 with single or double phosphorylation of Tyr residues. These results determine that two Tyr residues in occludin C-terminal region corresponding to the sequence of P1 were singly or doubly phosphorylated. These two Tyr residues correspond to Tyr-379 and Tyr-383 in chicken occludin.
FIGURE 1.
Identification of phosphorylation sites in occludin. GST-cOcl-CWT was phosphorylated by incubation with c-Src in the presence of ATP and subjected to digestion by trypsin. A, predicted peptide fragments (P1–P5) that contained one or two Tyr residues generated by trypsin digestion of GST-cOcl-CWT. The position of peptides in the sequence of occludin and their predicted mass are listed. B, phosphopeptides extracted from trypsin digests were subjected to mass analyses by MALDI mass spectrometry. P1(1p+R) represents P1 tryptic peptide with one phospho-Tyr and one additional Arg residue as a result of misdigestion by trypsin, and P1(2p) represents the P1 peptide with two phospho-Tyr residues. C, the ion fragmentation spectrum for P1(2p) as obtained by LC/MS/MS analyses. D, the summary of peptides sequenced by LC/MS/MS analysis. Peptides corresponding to these are listed in panel A, and their phosphorylation states are provided. Asterisks represent the phosphorylated Tyr residues.
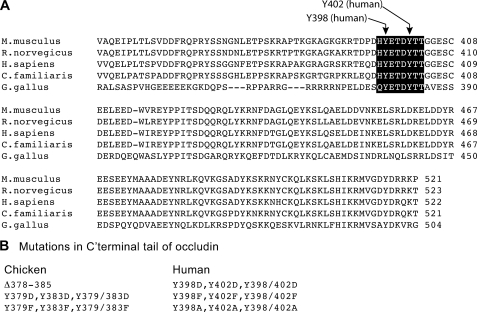
Sequence alignment of occludin from different species (Fig. 2A) demonstrated that Tyr-379 and Tyr-383 are located in a highly conserved sequence of occludin (YETDYTT) and that Tyr-398 and Tyr-402 are the corresponding Tyr residues in human occludin. Therefore, we induced mutations in chicken and human occludin C-terminal region (cOcl-C and hOcl-C). The YETDYTT was deleted or the tyrosine residues in this region were subjected to point mutation in cOcl-C and hOcl-C (Fig. 2B) and inserted into pGEX2T vector to generate GST-fused mutant proteins. Tyr-398 and Tyr-402 in full-length human occludin in pEGFP vector were mutated to phenylalanine or aspartic acid and expressed in Rat-1 or MDCK cells as GFP fusion proteins.
FIGURE 2.
Mutation of occludin. A, the alignment of amino acid sequence of occludin from different mammalian species indicate that the Tyr residues phosphorylated by c-Src correspond to Tyr-379 and Tyr-383 in chicken occludin and Tyr-398 and Tyr-402 in human occludin. B, list of the mutations induced in the C-terminal region of chicken and human occludin.
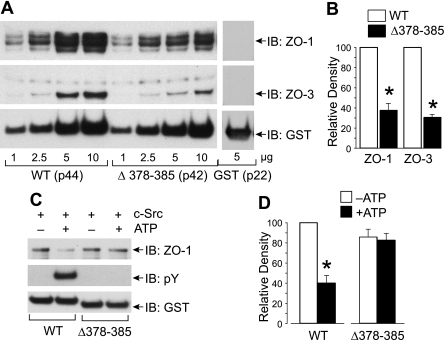
Deletion of YEDYTT in Chicken Occludin C-terminal Region Prevents Phosphorylation and Attenuates Regulation of ZO-1 Binding—The ability of GST-cOcl-C or GST-cOcl-C(Δ378–385) to bind ZO-1 was evaluated by GST pulldown assay. Both GST-cOcl-CandGST-cOcl(Δ378–385)showeddose-dependent binding to ZO-1 and ZO-3 (Fig. 3A). However, ZO-1 binding to GST-cOcl(Δ378–385) was low at higher doses compared with the ZO-1 binding to GST-cOcl-C under similar conditions (Fig. 3B). Incubation with c-Src in the presence of ATP induced Tyr phosphorylation of GST-cOcl-C, whereas no phosphorylation was detected in GST-cOcl(Δ378–385) (Fig. 3C). Incubation of GST-cOcl-C with c-Src in the presence of ATP significantly reduced ZO-1 binding (Fig. 3, C and D), whereas ZO-1 binding was similar for GST-cOcl(Δ378–385) that was incubated with c-Src in the presence or absence of ATP.
FIGURE 3.
Deletion mutation of occludin prevents phosphorylation and attenuates regulation of ZO-1 binding. A, varying amounts of GST-cOcl-CWT or GST-cOcl-C(Δ378–385) was analyzed for ZO-1 binding by GST pulldown assay using Caco-2 cell extract. GST pulldown was immunoblotted (IB) for ZO-1, ZO-3, and GST. Binding to GST (5 μg) was performed as a control. The labels p44, p42, and p22 correspond to the molecular weight of GST-cOcl-CWT, GST-cOcl-C(Δ378–385), and GST, respectively. B, densitometric analysis of ZO-1 and ZO-3 binding to 5 μg of wild type (WT) or mutant (Δ378–385) occludin. Values are the mean ± S.E. (n = 3). Asterisks indicate the values that are significantly (p < 0.05) different from corresponding value for WT group. C, GST-cOcl-C and GST-cOcl-C(Δ378–385), 2.5 μg, were incubated with c-Src in the absence or presence of ATP and analyzed for ZO-1 binding by GST pulldown assay. GST pulldown assays were immunoblotted for ZO-1, p-Tyr, and GST. D, densitometric analysis of ZO-1 bands from three different experiments described in panel B. In each experiment, ZO-1 band density for GST-cOcl-CWT incubated without ATP was designated to 100, and corresponding bands in other groups were normalized as a percent of that number. Values are the mean ± S.E. (n = 3). The asterisk indicates the value that is significantly (p < 0.05) different from corresponding value for -ATP group.
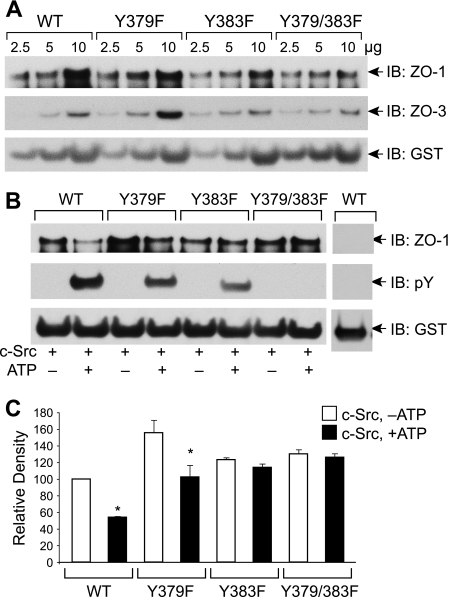
Y379F and Y383F Mutation of Chicken Occludin Attenuates Its Phosphorylation and Regulation of ZO-1 Binding— GST pulldown assay for ZO-1 binding showed that GST-cOcl-CWT, GST-cOcl-CY379F, GST-cOcl-CY383F, and GST-cOcl-CY379F/Y383F bind ZO-1 and ZO-3 in a dose-dependent manner (Fig. 4A). Incubation with c-Src in the presence of ATP showed a partial phosphorylation of single mutants, GST-cOcl-CY379F and GST-cOcl-CY383F, whereas phosphorylation was undetectable in the double mutant, GST-cOcl-CY379F/Y383F (Fig. 4B). ZO-1 binding was not significantly different among unphosphorylated occludin (Fig. 4, B and C), except that ZO-1 binding of GST-cOcl-CY379F was slightly greater than that of GST-cOcl-CWT. Incubation in the presence of ATP and c-Src resulted in a reduced ZO-1 binding by GST-cOcl-CWT and GST-cOcl-CY379F (Fig. 4, B and C). However, incubation with c-Src in the presence of ATP did not alter the ZO-1 binding by GST-cOcl-CY383F and GST-cOcl-CY379F/Y383F (Fig. 4, B and C).
FIGURE 4.
Y379F and Y383F mutations in chicken occludin attenuate c-Src-mediated Tyr phosphorylation and regulation of ZO-1 binding. A, GST-cOcl-CWT, GST-cOcl-CY379F, GST-cOcl-CY383F, and GST-cOcl-CY379/383F were analyzed for ZO-1 binding by GST pulldown assay. GST pulldown assays were immunoblotted (IB) for ZO-1, ZO-3, and GST. B, GST-cOcl-C, GST-cOcl-CY379F, GST-cOcl-CY383F, and GST-cOcl-CY379/383F were incubated with c-Src in the absence or presence of ATP. Five μg of phosphorylated and non-phosphorylated occludins were analyzed for ZO-1 binding by GST pulldown assay. GST pulldown assays were immunoblotted for ZO-1, p-Tyr, and GST. Control binding was performed by using 5 μg of GST. C, densitometric analysis of ZO-1 bands from three different experiments described in panel B. In each experiment, ZO-1 band density for GST-cOcl-CWT incubated without ATP was designated to 100, and corresponding bands in other groups were normalized as a percent of that number. Values are the mean ± S.E. (n = 3). Asterisks indicate the values that are significantly (p < 0.05) different from corresponding value for -ATP group.
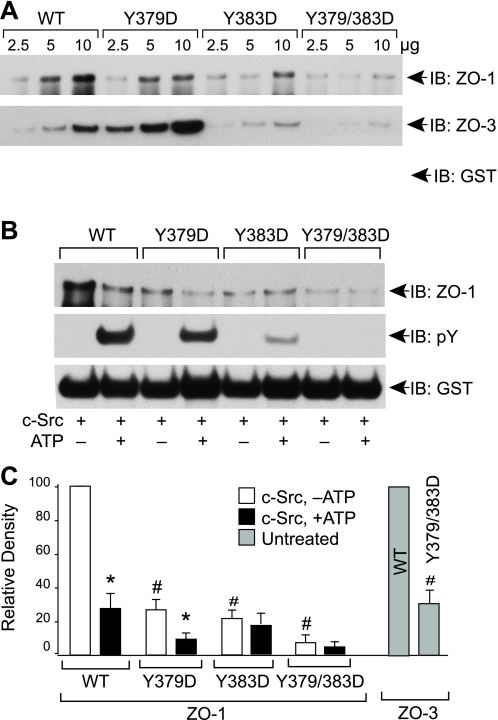
Y379D and Y383D Mutation in Chicken Occludin Attenuates Its Binding to ZO-1—GST pulldown assay showed a dose-dependent binding of GST-cOcl-CWT, GST-cOcl-CY379D, GST-cOcl-CY383D, and GST-cOcl-CY379/383D to ZO-1 and ZO-3 (Fig. 5A). However, the ZO-1 binding to GST-cOcl-CY379D, GST-cOcl-CY383D, and GST-cOcl-CY379/383D was significantly low as compared with - ZO-1 binding to GST-cOcl-CWT (Fig. 5A). ZO-3 binding to GST-cOcl-CY383D and GST-cOcl-CY379/383D were significantly low compared with ZO-3 binding to GST-cOcl-CWT, whereas ZO-3 binding to GST-cOcl-CY379D was significantly greater. c-Src-induced Tyr-phosphorylation of GST-cOcl-CY379D and GST-cOcl-CY383D was low compared with Tyr phosphorylation of GST-cOclWT, and the Tyr-phosphorylation was absent in GST-cOcl-CY379/383D double mutant (Fig. 5B). Incubation with c-Src in the presence of ATP resulted in reduced ZO-1 binding to GST-cOcl-CWT and GST-cOcl-CY379D (Fig. 5, B and C). However, incubation of GST-cOcl-CY383D and GST-cOcl-CY379/383D with c-Src in the presence of ATP did not alter ZO-1 binding (Fig. 5, B and C).
FIGURE 5.
Y379D and Y383D in chicken occludin attenuates its binding to ZO-1. A, GST-cOcl-CWT, GST-cOcl-CY379D, GST-cOcl-CY383D, and GST-cOcl-CY379/383D were analyzed for ZO-1 binding by GST pulldown assay. GST pulldown assays were immunoblotted (IB) for ZO-1 and GST. B, GST-cOcl-C, GST-cOcl-CY379D, GST-cOcl-CY383D, and GST-cOcl-CY379/383D were incubated with c-Src in the absence or presence of ATP. Ten μg each of phosphorylated and non-phosphorylated occludins were analyzed for ZO-1 binding by GST pulldown assay. GST pulldown assays were immunoblotted for ZO-1, p-Tyr, and GST. C, densitometric analysis of ZO-1 bands from three different experiments described as in panel B and selected ZO-3 bands in panel A. In each experiment, ZO-1 (or ZO-3) band density for GST-cOcl-C incubated without ATP was designated to 100, and corresponding bands in other groups were normalized as a percent of that number. Values are the mean ± S.E. (n = 3). Asterisks indicate the values that are significantly (p < 0.05) different from corresponding value for -ATP group, and the symbols # indicate the values that are significantly different (<0.05) from corresponding value for WT group.
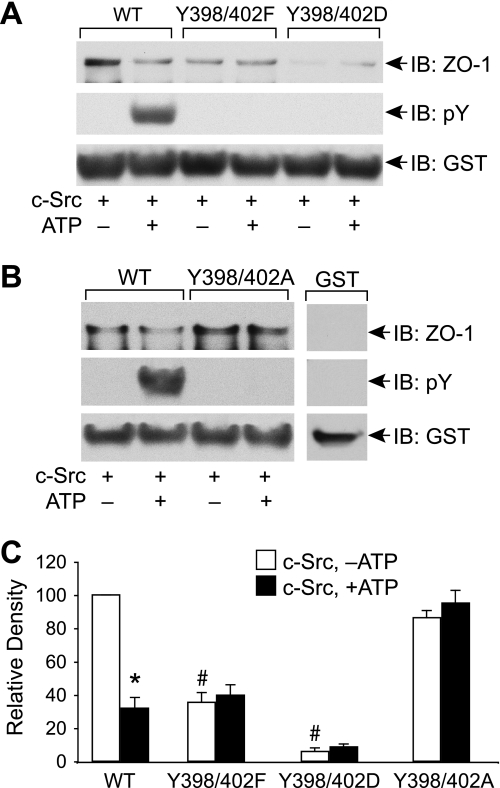
Mutation of Tyr-398 and Tyr-402 in Human Occludin Prevents Phosphorylation and Alters ZO-1 Binding—The sequence analysis indicated that Tyr-398 and Tyr-402 are the residues in human occludin that correspond to Tyr-379 and Tyr-383 in chicken occludin. Therefore, we mutated Tyr-398 and Tyr-402 in hOcl-C. Similar to GST-cOcl-CWT, incubation with c-Src in the presence of ATP induced Tyr phosphorylation of GST-hOcl-CWT, whereas phosphorylation was undetectable in GST-hOcl-CY398F/Y402F and GST-hOcl-CY398D/Y402D mutants (Fig. 6A). GST pulldown assay showed that GST-hOcl-CWT binds to ZO-1, and this binding was reduced by incubation with c-Src in the presence of ATP. GST-hOcl-CY398D/Y402D showed only a trace amount of ZO-1 binding. ZO-1 binding to GST-hOcl-CY398F/Y402F was lower than GST-hOcl-CWT; however, the ZO-1 binding was not further reduced by incubation with c-Src in the presence of ATP (Fig. 6, A and C). Unlike reduced binding to GST-hOcl-CY398F/Y402F, the ZO-1 binding to GST-hOcl-CY398A/Y402A was similar to that of GST-hOcl-CWT (Fig. 6B). Once again, GST-hOcl-CY398A/Y402A showed no Tyr phosphorylation or regulation of ZO-1 binding when incubated with c-Src in the presence of ATP (Fig. 6B). Densitometric analysis (Fig. 6C) confirmed that ZO-1 binding to GST-hOcl-CY398A/Y402A and GST-hOcl-CY398D/Y402D is significantly lower than that of GST-hOcl-CWT, whereas the binding to GST-hOcl-CY398A/Y402A was similar to that of GST-hOcl-CWT. Furthermore, incubation with c-Src in the presence of ATP significantly reduced ZO-1 binding to GST-hOcl-CWT but not to GST-hOcl-CY398F/Y402F, GST-hOcl-CY398A/Y402A, or GST-hOcl-CY398D/Y402D when compared with corresponding ZO-1 binding in the absence of ATP.
FIGURE 6.
Mutation of Tyr-398 and Tyr-402 in human occludin attenuates its phosphorylation and altered regulation of ZO-1 binding. A, GST-hOcl-CWT, GST-hOcl-CY398F/Y402F, and GST-hOcl-CY398D/Y402D were incubated with c-Src in the absence or presence of ATP. Five μg of phosphorylated or non-phosphorylated occludin was analyzed for ZO-1 binding by GST pulldown assay. GST pulldown assays were immunoblotted (IB) for ZO-1, p-Tyr, and GST. B, five μg of phosphorylated or non-phosphorylated GST-hOcl-C and GST-hOcl-CY398A/Y402A were incubated with c-Src in the absence or presence of ATP and analyzed for ZO-1 binding by GST pulldown assay. GST pulldown assays were immunoblotted for ZO-1, p-Tyr, and GST. C, densitometric analysis of ZO-1 bands from three different experiments described in panel B.In each experiment, ZO-1 band density for GST-cOcl-C incubated without ATP was designated to 100, and corresponding bands in other groups were normalized as a percent of that number. Values are the mean ± S.E. (n = 3). The asterisk indicates the value that is significantly (p < 0.05) different from corresponding value for -ATP group. # indicates the values that are significantly (p < 0.05) different from value for non-phosphorylated wild type occludin.
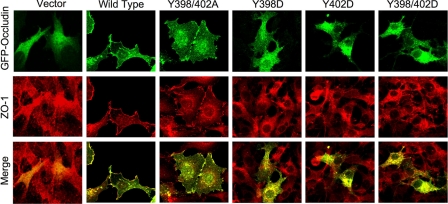
Y398D and Y402D Mutation in Human Occludin Prevents Its Localization at Plasma Membrane and Cell-Cell Contact Sites in Rat-1 Cells—The regulation of ZO-1 binding by Tyr-398 and Tyr-402 raised the question of whether phosphorylation of Tyr-398 and/or Tyr-402 of occludin affects its localization at the TJs. To determine the effect of mutation of Tyr-398 and Tyr-402 on the distribution of occludin at the plasma membrane and the intercellular junctions, we transfected Rat-1 cells (occludin null) with GFP-hOcl, GFP-hOclY398A/Y402A, and GFP-hOclY398D/Y402D and visualized the cells by confocal microscopy. GFP-hOclWT and GFP-hOclY398A/Y402A were localized to both the plasma membrane and the intracellular compartment (Fig. 7). A greater level of occludin was found at the cell-cell contact sites, which was associated with the redistribution of ZO-1 at the cell-cell contact and the plasma membrane. In contrast, GFP-hOclY398D/Y402D was localized exclusively at the intracellular compartment with no trace of distribution at the plasma membrane or cell-cell contact sites (Fig. 7).
FIGURE 7.
Y398D and Y402D mutations in human occludin prevents its localization at the plasma membrane and cell-cell contact sites in Rat-1 cells. GFP-hOclWT, GFP-cOclY398A/Y402A, GFP-cOclY398D, GFP-cOclY402D, GFP-cOclY398D/Y402D, and pEGFP empty vector were expressed in Rat-1 cells. Subcellular localization was examined by immunofluorescence confocal microscopy by double labeling for GFP and ZO-1.
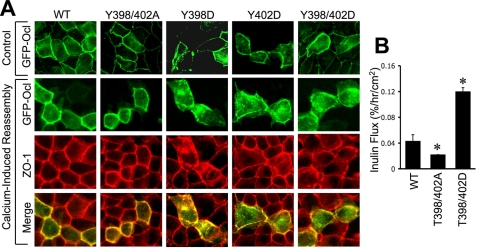
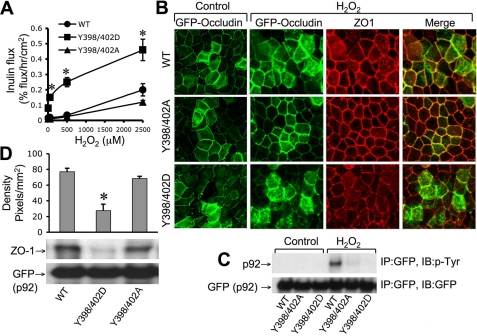
Y398D/Y402D Mutation of Occludin Delays Its Assembly at the TJs and Sensitizes MDCK Cells for TJ Disruption by Hydrogen Peroxide—Unlike Rat-1 cells, in MDCK cell monolayers, GFP-hOclY398D/Y402D appeared at the intercellular junctions. However, during the calcium switch-induced assembly of TJs, GFP-hOclY398D/Y402D localized predominantly at the intracellular compartment, whereas GFP-hOclWT and GFP-hOclY398A/Y402A appeared at the intercellular junctions 1 h after calcium replacement (Fig. 8A). The inulin permeability in cell monolayers that express GFP-hOclY398D/Y402D was significantly greater than those in cell monolayers expressing GFP-hOclWT or GFP-hOclY398A/Y402A (Fig. 8B). As reported before (11), hydrogen peroxide induced a dose-dependent increase in inulin permeability in MDCK cell monolayers that stably express GFP-hOclWT (Fig. 9A). A hydrogen peroxide-induced increase in inulin permeability was significantly lower in cell monolayers that express GFP-hOclY398A/Y402A, whereas it was significantly higher in cells expressing GFP-hOclY398D/Y402D. Incubation of cell monolayers that express GFP-hOclWT, GFP-hOclY398A/Y402A, and GFP-hOclY398D/Y402D with 500 μm hydrogen peroxide for 1 h increased inulin permeability (% flux/h/cm2) from 0.025 ± 0.005 to 0.035 ± 0.01, 0.02 ± 0.01 to 0.025 ± 0.006, and 0.07 ± 0.007 to 0.25 ± 0.03, respectively. These observations were confirmed by analyzing the junctional distribution of GFP and ZO-1 after hydrogen peroxide treatment. Hydrogen peroxide induced a slight redistribution of GFP in cells expressing GFP-hOclWT, whereas the hydrogen peroxide-induced redistribution of GFP from the junctions was much more dramatic in cells that express GFP-hOclY398D/Y402D (Fig. 9B). ZO-1 distribution in hydrogen peroxide-treated cell monolayers paralleled the distribution of GFP.
FIGURE 8.
Y398D and Y402D mutations in human occludin prevents its assembly at the intercellular junctions in MDCK cells. A, 360q-length human occludin, GFP-hOclWT, GFP-hOclY398A/Y402A, GFP-cOclY398D, GFP-cOclY402D, and GFP-hOclY398D/Y402D were expressed in MDCK cells. Subcellular localization was examined 1 h after calcium replacement by immunofluorescence confocal microscopy by double labeling for GFP and ZO-1. B, inulin permeability was measured in cell monolayers expressing GFP-hOclWT, GFP-hOclY398A/Y402A, and GFP-hOclY398D/Y402D. Values are the mean ± S.E. (n = 4). Asterisks indicate the values that are significantly (p < 0.05) different from corresponding value for GFP-hOclWT.
FIGURE 9.
Y398D/Y402D mutation of occludin sensitizes MDCK cell monolayers for hydrogen peroxide-induced disruption of TJs. A and B, MDCK cell monolayers that express GFP-hOclWT, GFP-hOclY398A/Y402A, or GFP-hOclY398D/Y402D were exposed to varying concentrations of hydrogen peroxide. Inulin permeability was measured 1 h after hydrogen peroxide (A), and fixed cell monolayers were double-stained for GFP and ZO-1 by the immunofluorescence method (B). C, MDCK cell monolayers that express GFP-hOclWT, GFP-hOclY398A/Y402A, or GFP-hOclY398D/Y402D were incubated with or without (control) hydrogen peroxide for 1 h. Anti-GFP immunocomplexes prepared under denatured conditions were immunoblotted (IB) for p-Tyr and GFP. The arrow with p92 label corresponds to GFP-occludin. IP, immunoprecipitates. D, anti-GFP immunocomplexes prepared under non-denaturing conditions from MDCK cells expressing wild type or mutant occludins were immunoblotted for ZO-1 and GFP. Density of ZO-1 bands from three different experiments was measured. The arrow with p92 label corresponds to GFP-occludin.
Tyr-398- and Tyr-402-phosphorylated Occludin Results in Loss of Interaction with ZO-1 in MDCK Cell Monolayers—To confirm the H2O2-induced phosphorylation of occludin in the cell, we evaluated H2O2-induced Tyr-phosphorylation of GFP-hOclWT, GFP-hOclY398A/Y402A, and GFP-hOclY398D/Y402D in MDCK cell monolayers. Immunoprecipitation of GFP followed by immunoblot analysis in H2O2-treated cells expressing GFP-hOclWT indicated that occludin was phosphorylated on Tyr residues (Fig. 9C). However, GFP-hOclY398A/Y402A and GFP-hOclY398D/Y402D were not Tyr-phosphorylated in H2O2-treated cells.
Coimmunoprecipitation studies (Fig. 9D) indicated that ZO-1 coimmunoprecipitated with GFP-OclWT and GFP-hOclY398A/Y402A, whereas ZO-1 failed to co-immunoprecipitate with GFP-hOclY398D/Y402D, indicating that phosphorylation of Tyr-398 and Tyr-402 results in loss of interaction with ZO-1 in MDCK cells.
DISCUSSION
A significant body of evidence suggests that Tyr phosphorylation of TJ proteins may play an important role in the regulation of epithelial TJs. Occludin is highly phosphorylated on Ser and Thr residues in the intact epithelium, and Tyr phosphorylation is undetectable (38). Previous studies, however, demonstrated that occludin undergoes Tyr phosphorylation during the disruption of TJs by hydrogen peroxide (11, 39). Furthermore, a recent in vitro study demonstrated that Tyr phosphorylation of the C-terminal region of occludin reduces its ability to interact with ZO-1, a TJ plaque protein (25). In the present study we identified the Tyr phosphorylation sites in the C-terminal region of occludin and demonstrated their role in regulation of ZO-1 binding.
The Tyr phosphorylation sites in the C-terminal region of chicken occludin were determined by mass spectrometric analysis of phospho-occludin. Mass analysis of phosphopeptide extracts from tryptic digests detected the presence of one phosphopeptide corresponding to the predicted peptide fragment of chicken occludin (amino acids 371–393) with phospho-Tyr residues. There are two Tyr residues within this sequence (Tyr-379 and 383), which were found to be phosphorylated as the mass of this peptide was 160 daltons greater than the predicted mass value. Another phosphopeptide detected by MALDI showed a molecular mass of 2915.7, which is 156 daltons greater than the predicted mass of the monophosphate peptide fragment. LC/MS/MS analysis determined that this peptide corresponds to the sequence 370–393 of chicken occludin with an extra Arg (Arg-370) at the N terminus compared with the predicted 371–393 fragment. This is possibly caused by a misdigestion by trypsin due to the presence of sequential Arg residues in this region of the occludin sequence. LC/MS/MS analysis also detected two types of 2915.2-dalton peptides; one in which Tyr-379 was phosphorylated and another in which Tyr-383 was phosphorylated. Therefore, the sequences of all three phosphopeptides identified in the study demonstrate that Tyr-379 and Tyr-383 are the phosphorylation sites in chicken occludin; Tyr-398 and Tyr-402 are the corresponding tyrosines in human occludin. These two tyrosines are located in a highly conserved sequence of occludin HYETDYTT. BLAST analysis of this sequence demonstrated that this is a unique motif that is not present in other proteins, including claudins.
Deletion of HYETDYTT (378–385) from chicken occludin abrogated c-Src-induced Tyr-phosphorylation of the occludin C-terminal region, confirming the mass spectrometric data that Tyr-379 and Tyr-383 are the phosphorylation sites in chicken occludin. Point mutation of Tyr-379 or Tyr-383 to phenylalanine resulted in a partial decrease in c-Src-induced Tyr-phosphorylation. Decrease in Tyr phosphorylation was greater in Y383F mutants compared with that in Y379F mutants, suggesting that Tyr-383 is a preferred phosphorylation site. Double mutation of Tyr-379 and Tyr-383 abolished c-Src-induced Tyr phosphorylation. Similarly, mutation of Tyr-398 and Tyr-402 in the C-terminal region of human occludin also abrogated the c-Src-induced Tyr phosphorylation. Previous studies showed that c-Src plays an important role in hydrogen peroxide-induced disruption of TJs and barrier dysfunction in Caco-2 cell monolayers (11). Expression of inactive c-Src significantly reduced hydrogen peroxide-induced Tyr-phosphorylation of occludin and TJ disruption, suggesting that c-Src-induced Tyr-phosphorylation may be involved in this process. The present study suggests that hydrogen peroxide may induce phosphorylation of Tyr-379 and Tyr-383 in Caco-2 cells. A previous study demonstrated that Tyr phosphorylation of the C-terminal region of occludin results in the loss of its interaction with ZO-1 (25). ZO-1, a major adaptor protein of TJs, interacts with C-terminal region of occludin on one hand and with actin cytoskeleton on the other (12, 14).
The interaction between the C-terminal region of occludin and ZO-1 is crucial for the assembly and the maintenance of occludin at the TJs (14). Truncation of the C-terminal region of occludin resulted in a loss of its interaction with ZO-1 and prevented its assembly into TJs. In the present study we determined the role of phosphorylation of specific Tyr residues in the C-terminal region of occludin in the regulation of its interaction with ZO-1. GST pulldown assays demonstrated that C-terminal regions of both chicken and human occludin bind to ZO-1. Deletion of the sequence HYETDYTT in chicken occludin resulted in a significant reduction in binding to ZO-1 at higher concentrations of occludin; however, at low concentrations the deletion mutant bound to ZO-1 at a similar level to that of the wild type occludin. When wild type occludin C-terminal domain was incubated with c-Src and ATP, there was a significant reduction in ZO-1 binding; however, this was not observed with the deletion mutant, indicating that the phosphorylation of Tyr-379 and Tyr-383 is important in the regulation of interaction between occludin and ZO-1. This was confirmed by point mutations of Tyr-379 and -383. Y379F mutation partially reduced c-Src-induced regulation of ZO-1 binding, whereas Y383F or Y379F/Y383F mutation completely attenuated c-Src-induced regulation of ZO-1 binding. Similarly, Y398F/Y402F mutation in human occludin attenuated c-Src-induced regulation of ZO-1 binding. However, Y398F/Y402F mutation by itself resulted in significant reduction in ZO-1 binding. On the other hand, Y398A/Y402A mutation did not affect ZO-1 binding in the absence or presence of c-Src. Therefore, the results of this study demonstrate that Tyr-398 and Tyr-402 are important in regulation of ZO-1 binding by human occludin.
The crystal structure of the occludin C-terminal region (383–522) has been determined recently (15). This coiled-coil region C-terminal to the Tyr phosphorylation sites does bind to ZO-1 quite well. This indicates that the main function of the phosphorylation is not ZO-1 binding; rather, it plays a role in the regulation of ZO-1 binding. Similar to ZO-1 binding, ZO-3 binding to GST-hOcl-C was also altered by mutation of Tyr-398 and -402. Interestingly, ZO-3 binding to Y398F mutant was greater than its binding to GST-hOcl-CWT. However, at present, the reason for this enhanced binding is not clear and needs further studies.
To determine the effect of Tyr-398 or Tyr-402 phosphorylation on the assembly of occludin into intercellular junctions, we induced point mutations to GFP-tagged full-length human occludin. The GFP-hOclWT and its mutants, GFP-hOclY398/Y402A, GFP-hOclY398/Y402D, and the corresponding single mutants were transfected into Rat-1 fibroblasts or MDCK cells. Confocal immunofluorescence microscopy demonstrated that the GFP-hOclWT and its Y398/Y402A mutant were localized at the plasma membrane of Rat-1 cells, forming intercellular contact sites. Rat-1 cells express high levels of ZO-1, but it is predominantly localized in the intracellular compartment. However, transfection of GFP-hOclWT or GFP-hOclY398/Y402A induced a recruitment of ZO-1 at the plasma membrane and the intercellular contact sites. Y398A/Y402A mutation did not alter the distribution of occludin at the plasma membranes. However, Y398D, Y402D, and Y398/Y402D mutants of occludin failed to localize at the plasma membrane or cell-cell contact sites; rather, they were distributed in the intracellular compartment. This indicates that mimicking the phosphorylation of Tyr-398 and Tyr-402 by mutation to aspartic acid results in the loss of its ability to assemble at the plasma membranes and cell-cell contact sites. This may be due to the loss of its ability to bind to ZO-1.
A significant portion of GFP-occludin in vesicular structure did appear near the plasma membrane, suggesting that the mutation did not result in a defect in its ability to integrate into the plasma membrane; rather, these occludin mutants are internalized into the cells due to the lack of their interaction with ZO-1 and inability to integrate into TJ structure. Both single mutants, Y398D and Y402D, similarly failed to localize at the intercellular junctions, indicating that phosphorylation of either Tyr residues is enough to alter its ability to bind ZO-1 and integrate into TJs. In vitro phosphorylation and ZO-1 binding studies indicated that Tyr-383 is more important than Tyr-379 in chicken occludin. However, this is in contrast to cell data, which shows that both Tyr-398 and Tyr-402 in human occludin are important for its localization at the cell-cell contact sites. This may be explained by the lower preference of c-Src for Tyr-379 compared with Tyr-383. In the cell, Tyr-379 may be phosphorylated by some other tyrosine kinase.
In MDCK cells, wild type and Y398A/Y402A mutant occludin were localized at the intercellular junctions. However, during the early stages of TJ assembly by calcium replacement, we saw a delay in the organization of GFP-hOclY398D/Y402D at the intercellular junctions. One hour after calcium replacement, Y398D/Y402D mutant occludin was distributed predominantly in the intracellular compartment, whereas WT and Y398A/Y402A mutant occludin were organized at the intercellular junctions. This confirmed that phosphorylation of Tyr-398 and Tyr-402 of occludin does prevent its ability to integrate into the TJs. The organization of Y398D/Y402D mutant at the intercellular junctions may be mediated by dimerization of mutant with the endogenous occludin. Expression of Y398D/Y402D mutant of occludin did disrupt the junctional distribution of ZO-1. This dominant negative effect is evident only at higher levels of mutant expression. The expression of mutant in relation to endogenous occludin is difficult to assess. However, ZO-1 redistribution was seen only in cells with higher level of mutant expression. Therefore, it is not clear whether such an effect can be seen at endogenous levels of phospho-occludin.
Furthermore, MDCK cell monolayers that express Y398D/Y402D mutant of occludin were dramatically more sensitive to hydrogen peroxide-induced disruption of barrier function, whereas cell monolayers expressing Y398A/Y402A mutant occludin showed significant resistance to hydrogen peroxide compared with cell monolayers expressing wild type occludin. The present study also shows that hydrogen peroxide failed to induce Tyr phosphorylation in MDCK cell monolayers, demonstrating that Tyr-398 and Tyr-402 are the phosphorylation sites in hydrogen peroxide-treated cells. The loss of co-immunoprecipitation of ZO-1 with GFP-hOclY398D/Y402D in MDCK cells confirms our observation made in in vitro studies that phosphorylation of Tyr-398 and Tyr-402 does prevent its interaction with ZO-1.
As shown in Fig. 9, the GFP-hOclY398D/Y402D eventually tends to organize at the intercellular junctions, although not as discretely as GFP-hOclWT or GFP-hOclY398A/Y402A. This is possibly due to oligomerization of GFP-hOclY398D/Y402D with the endogenous occludin. The inulin flux in hydrogen peroxide-treated cell monolayers that express GFP-hOclY398D/Y402D is significantly higher than that in cells that express GFP-hOclWT or GFP-hOclY398A/Y402A. The mechanism for greater sensitivity to hydrogen peroxide or delayed assembly is not clear at this point. However, we speculate that mixed oligomerization of GFP-OclY398D/Y402D and endogenous occludin facilitates the dissociation of ZO-1 by hydrogen peroxide due to weak interaction between GFP-OclY398D/Y402D with ZO-1; this may work synergistically with the hydrogen peroxide-induced Tyr phosphorylation of endogenous occludin and loss of its interaction with ZO-1. In summary, this study identifies Tyr-398 and Tyr-402 as the phosphorylation sites in human occludin and demonstrates that phosphorylation of these Tyr residues results in the loss of interaction between occludin and ZO-1 and attenuation of its integration into the epithelial TJs.
This work was supported, in whole or in part, by National Institutes of Health Grants R01-DK55532 and R01-AA12307. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: TJ, tight junction; HRP, horseradish peroxidase; ZO-1, ZO-2, and ZO-3, zonula occludens 1, 2, and 3; GST, glutathione S-transferase; p-Tyr, phosphotyrosine; GFP, green fluorescence protein; TPCK, tosylphenylalanyl chloromethyl ketone; MDCK, Madin-Darby canine kidney cells; MALDI-TOF, matrix-assisted laser desorption ionization time-of-flight; WT, wild type; LC/MS/MS, liquid chromatography/tandem mass spectroscopy.
References
- 1.Gonzalez-Mariscal, L., Betanzos, A., Nava, P., and Jaramillo, B. E. (2003) Prog. Biophys. Mol. Biol. 81 1-44 [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez-Mariscal, L., Lechuga, S., and Garay, E. (2007) Prog. Histochem. Cytochem. 42 1-57 [DOI] [PubMed] [Google Scholar]
- 3.Berkes, J., Viswanathan, V. K., Savkovic, S. D., and Hecht, G. (2003) Gut 52 439-451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laukoetter, M. G., Bruewer, M., and Nusrat, A. (2006) Curr. Opin. Gastroenterol. 22 85-89 [DOI] [PubMed] [Google Scholar]
- 5.Coyne, C. B., Vanhook, M. K., Gambling, T. M., Carson, J. L., Boucher, R. C., and Johnson, L. G. (2002) Mol. Biol. Cell 13 3218-3234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balkovetz, D. F. (2006) Am. J. Physiol. 290 F572-F579 [DOI] [PubMed] [Google Scholar]
- 7.Ma, T. Y., Iwamoto, G. K., Hoa, N. T., Akotia, V., Pedram, A., Boivin, M. A., and Said, H. M. (2004) Am. J. Physiol. 286 G367-G376 [DOI] [PubMed] [Google Scholar]
- 8.Stevens, C., Walz, G., Singaram, C., Lipman, M. L., Zanker, B., Muggia, A., Antonioli, D., Peppercorn, M. A., and Strom, T. B. (1992) Dig. Dis. Sci. 37 818-826 [DOI] [PubMed] [Google Scholar]
- 9.Wang, F., Schwarz, B. T., Graham, W. V., Wang, Y., Su, L., Clayburgh, D. R., Abraham, C., and Turner, J. R. (2006) Gastroenterology 131 1153-1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rao, R. K., Baker, R. D., and Baker, S. S. (1999) Biochem. Pharmacol. 57 685-695 [DOI] [PubMed] [Google Scholar]
- 11.Basuroy, S., Sheth, P., Kuppuswamy, D., Balasubramanian, S., Ray, R. M., and Rao, R. K. (2003) J. Biol. Chem. 278 11916-11924 [DOI] [PubMed] [Google Scholar]
- 12.Rao, R. K. (2008) Front. Biosci. 13 7210-7226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneeberger, E. E., and Lynch, R. D. (2004) Am. J. Physiol. 286 C1213-C1228 [DOI] [PubMed] [Google Scholar]
- 14.Furuse, M., Itoh, M., Hirase, T., Nagafuchi, A., Yonemura, S., Tsukita, S., and Tsukita, S. (1994) J. Cell Biol. 127 1617-1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li, Y., Fanning, A. S., Anderson, J. M., and Lavie, A. (2005) J. Mol. Biol. 352 151-164 [DOI] [PubMed] [Google Scholar]
- 16.Itoh, M., Morita, K., and Tsukita, S. (1999) J. Biol. Chem. 274 5981-5986 [DOI] [PubMed] [Google Scholar]
- 17.Wittchen, E. S., Haskins, J., and Stevenson, B. R. (1999) J. Biol. Chem. 274 35179-35185 [DOI] [PubMed] [Google Scholar]
- 18.Haskins, J., Gu, L., Wittchen, E. S., Hibbard, J., and Stevenson, B. R. (1998) J. Cell Biol. 141 199-208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Itoh, M., Furuse, M., Morita, K., Kubota, K., Saitou, M., and Tsukita, S. (1999) J. Cell Biol. 147 1351-1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhong, Y., Saitoh, T., Minase, T., Sawada, N., Enomoto, K., and Mori, M. (1993) J. Cell Biol. 120 477-483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Citi, S., Sabanay, H., Jakes, R., Geiger, B., and Kendrick-Jones, J. (1998) Nature 333 272-275 [DOI] [PubMed] [Google Scholar]
- 22.Keon, B. H., Schafer, S., Kuhn, C., Grund, C., and Franke, W. W. (1996) J. Cell Biol. 134 1003-1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyoshi, J., and Takai, Y. (2005) Adv. Drug Delivery Rev. 57 815-855 [DOI] [PubMed] [Google Scholar]
- 24.Andreeva, A. Y., Krause, E., Muller, E. C., Blasig, I. E., and Utepbergenov, D. I. (2001) J. Biol. Chem. 276 38480-38486 [DOI] [PubMed] [Google Scholar]
- 25.Kale, G., Naren, A. P., Sheth, P., and Rao, R. K. (2003) Biochem. Biophys. Res. Commun. 302 324-329 [DOI] [PubMed] [Google Scholar]
- 26.Nusrat, A., Chen, J. A., Foley, C. S., Liang, T. W., Tom, J., Cromwell, M., Cliff, Q., and Randall, J. M. (2000) J. Biol. Chem. 275 29816-29822 [DOI] [PubMed] [Google Scholar]
- 27.Chen, Y. H., Lu, Q., Goodenough, D. A., and Jeansonne, B. (2002) Mol. Biol. Cell 13 1227-1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basuroy, S., Seth, A., Elias, B., Naren, A. P., and Rao, R. (2006) Biochem. J. 393 69-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seth, A., Sheth, P., Elias, B. C., and Rao, R. K. (2007) J. Biol. Chem. 282 11487-11498 [DOI] [PubMed] [Google Scholar]
- 30.Stuart, R. O., Sun, A., Panichas, M., Hebert, S. C., Brenner, B. M., and Nigam, S. K. (1994) J. Cell. Physiol. 159 423-433 [DOI] [PubMed] [Google Scholar]
- 31.Sheth, P., Basuroy, S., Li, C., Naren, A. P., and Rao, R. K. (2003) J. Biol. Chem. 278 49239-49245 [DOI] [PubMed] [Google Scholar]
- 32.Nusrat, A., Giry, M., Turner, J. R., Colgan, S. P., Parkos, C. A., Carnes, D., Lemichez, E., Boquet, P., and Madara, J. L. (1995) Proc. Natl. Acad. Sci. U. S. A. 92 10629-10633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takaishi, K., Sasaki, T., Hirokazu, K., Nishioka, H., and Takai, Y. (1997) J. Cell Biol. 139 1047-1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saitou, M., Furuse, M., Sasaki, H., Schulzke, J. D., Fromm, M., Takano, H., Noda, T., and Tsukita S. (2000) Mol. Biol. Cell 11 4131-4142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujimoto, K. (1995) J. Cell Sci. 108 3443-3449 [DOI] [PubMed] [Google Scholar]
- 36.Furuse, M., Hirase, T., Itoh, M., Nagafuchi, A., Yonemura, S., Tsukita, Sa., and Tsukita, Sh. (1993) J. Cell Sci. 123 1777-1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ando-Akatsuka, Y., Saitou, M., Hirase, T., Kishi, M., Sakakibara, A., Itoh, M., Yonemura, S., Furuse, M., and Tsukita, S. (1996) J. Cell Biol. 133 43-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakakibara, A., Furuse, M., Saitou, M., Ando-Akatsuka, Y., and Tsukita, S. (1997) J. Cell Biol. 137 1393-1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rao, R. K., Basuroy, S., Rao, V. U., Karnaky, K. J., and Gupta, A. (2002) Biochem. J. 368 471-481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Atkinson, K. J., and Rao, R. K. (2001) Am. J. Physiol. 280 G1280-G1288 [DOI] [PubMed] [Google Scholar]