Abstract
Helicobacter pylori VacA toxin contributes to the pathogenesis and severity of gastric injury. We found that incubation of AZ-521 cells with VacA resulted in phosphorylation of protein kinase B (Akt) and glycogen synthase kinase-3β (GSK3β) through a PI3K-dependent pathway. Following phosphorylation and inhibition of GSK3β,β-catenin was released from a GSK3β/β-catenin complex, with subsequent nuclear translocation. Methyl-β-cyclodextrin (MCD) and phosphatidylinositol-specific phospholipase C (PI-PLC), but not 5-nitro-2-(3-phenylpropylamino)-benzoic acid (NPPB) and bafilomycin A1, inhibited VacA-induced phosphorylation of Akt, indicating that it does not require VacA internalization and is independent of vacuolation. VacA treatment of AZ-521 cells transfected with TOPtkLuciferase reporter plasmid or control FOPtkLucifease reporter plasmid resulted in activation of TOPtkLuciferase, but not FOPtkLucifease. In addition, VacA transactivated the β-catenin-dependent cyclin D1 promoter in a luciferase reporter assay. Infection of AZ-521 cells by a vacA mutant strain of H. pylori failed to induce phosphorylation of Akt and GSK3β, or release of β-catenin from a GSK3β/β-catenin complex. Taken together, these results support the conclusion that VacA activates the PI3K/Akt signaling pathway, resulting in phosphorylation and inhibition of GSK3β, and subsequent translocation ofβ-catenin to the nucleus, consistent with effects of VacA on β-catenin-regulated transcriptional activity. These data introduce the possibility that Wnt-dependent signaling might play a role in the pathogenesis of H. pylori infection, including the development of gastric cancer.
Although persistent infection by Helicobacter pylori is accepted as a major cause of gastroduodenal diseases, the cellular pathways responsible for the different outcomes such as peptic ulcer disease, gastric lymphoma, or gastric adenocarcinoma have not been defined. Variation in manifestations of H. pylori infection in different populations suggest effects of strains differing in virulence, or interactions involving the organism, environmental factor(s), and the host. Many H. pylori strains isolated from patients contain the cagA gene (cytotoxin-associated gene A) as well as produce the vacuolating cytotoxin, VacA. Additional H. pylori products, including urease, OipA, the neutrophil-activating protein NapA, adhesins, heat-shock protein, and lipopolysaccharide, appear to be involved in virulence (1–3).
VacA is a protein toxin with a molecular mass of about 90 kDa, whereas the native toxin is an oligomer of about 1,000 kDa (4). Although a clear functional association between VacA and clinical outcome of any type of gastroduodenal disease has not been found, oral administration of VacA causes gastric mucosal damage in mice (5, 6), suggesting that VacA may contribute to epithelial cell injury or peptic ulceration in H. pylori-infected humans.
VacA has multiple effects on susceptible cells, including vacuolation, mitochondrial damage, and inhibition of T cell proliferation (7). These pleiotropic effects of VacA appear to result from activation of different signal transduction pathways. With regard to apoptosis, we have shown that VacA did not directly induce cytochrome c release from mitochondria. Rather, VacA stimulated Bax activation, which resulted in cytochrome c release and cell death. Although VacA internalization was necessary for vacuolation and Bax activation, VacA-induced Bax activation was independent of vacuole formation, indicating that these activities might be functionally independent (8). In addition, we and others reported that VacA induced alterations in protein phosphorylation patterns, including effects on Erk and p38 mitogen-activated protein kinase (MAPK)2 (9, 10), which did not require toxin internalization (11) and were not necessary for vacuolation and Bax activation. VacA-dependent MAPK activation, in the absence of toxin internalization, led to induction of COX-2, but not of IL-8, by gastric epithelial AZ-521 cells (9, 12) and expression of IL-8 and monocyte chemoattractant protein-1 (MCP-1) by human promonocytic U937 and peripheral blood mononuclear cells (13). Although the pleiotropic effects of VacA are cell specific, these results suggest that VacA selectively activates kinases (e.g. MAPKs), thereby stimulating prostaglandin E2 (PGE2) production, facilitating proliferation of AZ-521 cells or chemokine production by U937 cells, and leading to an inflammatory response in vivo. Sustained VacA exposure, however, also caused mitochondrial damage and apoptotic cell death. Cells appear to respond to VacA in a manner similar to that seen with stressors (e.g. UV irradiation, heat shock, changes in osmolality, oxidative stress, production of inflammatory cytokines) by activating pathways that protect cells from damage. If the stress caused by VacA is excessive, it appears that cells undergo apoptosis.
More recently, it has become evident that glycogen synthase kinase-3 (GSK3) is a crucial, and often central, regulatory component of many cellular pathways, including apoptosis, cell cycle, cell polarity and migration, and gene expression (14). This multitasking by GSK3 is achieved by its phosphorylation of proteins in diverse signal transduction pathways.
Here we report that VacA stimulated protein kinase B (Akt) activity via activation of PI3K, resulting in increased phosphorylation of its substrate, GSK3, with subsequent release of β-catenin from a GSK3β/β-catenin complex and its translocation to the nucleus, leading to activation of the cyclin D1 promoter. Activation of this GSK3β-dependent pathway has been shown to play an important role in promoting pathological changes seen in cancer (15) and appears to be important in the pathogenesis of gastric diseases seen in H. pylori infection.
EXPERIMENTAL PROCEDURES
Purification of VacA—The toxin-producing H. pylori strain ATCC49503 was the source of VacA for purification by our published procedure (10). In brief, after growth of H. pylori in Brucella broth containing 0.1% β-cyclodextrin at 37 °C for 3–4 days with vigorous shaking in a controlled microaerobic atmosphere of 10% O2 and 10% CO2, VacA was precipitated from culture supernatant with 50% saturated ammonium sulfate. Precipitated proteins were dialyzed against Reaction buffer (10 mm KCl, 0.3 mm NaCl, 0.35 mm MgCl2, 0.125 mm EGTA, 1 mm HEPES, pH 7.3) and applied to an anti-VacA-specific IgG antibody column equilibrated with Reaction buffer. After washing the column with Reaction buffer, VacA was eluted with 50 mm glycine-HCl buffer, pH 1.0, which was promptly neutralized with 1 m Tris-HCl, pH 10. After gel filtration on Superose 6HR 10/30 equilibrated with TBS buffer (60 mm Tris-HCl buffer, pH 7.7, containing 0.1 m NaCl), purified VacA was stored at -20 °C. This purified VacA was activated by acidic elution from an anti-VacA-specific IgG antibody column.
Western Blotting—Human gastric adenocarcinoma cell line AZ-521 (Culture Collection of Health Science Research Resources Bank, Japan Health Sciences Foundation), which were grown in EMEM containing 10% FCS under 5% CO2 at 37 °C, were incubated with 120 nm VacA at 37 °C for indicated times. After incubation, cell lysates were subjected to SDS-PAGE in 10% gels, and then transferred to PVDF membranes for Western blotting using anti-phospho-Thr-308 Akt, phospho-Ser-473 Akt, phospho-Ser-9 GSK3β, Akt, or GSK3β antibodies (products of Cell Signaling). To avoid the enzymatic effects of endogenous phosphatases and proteases during cell lysis, 1 mm Na3VO4 and 50 mm NaF were added to block phosphatase activity and 1 mm PMSF and leupeptin (10 μg/ml) were added to inhibit proteases. Density was measured by scanning using Image J software.
Transfection with PI3K p110α Isoform siRNA—AZ-521 cells (1 × 105 cells) were seeded and grown overnight. The cells were transiently transfected with siRNA for PI 3-kinase p110α (200 pmol, the targeting sequence; ggugaaagacgauggacaa, product of B-Bridge International, Inc.) or nonspecific siRNA as a negative control (200 pmol, the sequence; gauaacgaguaaacgggag, product of B-Bridge International, Inc.) by using Lipofectamine RNAiMAX transfection reagent (Invitrogen, Carlsbad, CA) in medium (500 μl) without FCS, according to the manufacturer's directions. Following incubation for 5 h, the medium was replaced and fresh medium containing 10% FCS was added, followed by 24-h incubation. Silencing of the p110α gene was determined by measuring p110α protein expression by Western blot analysis using anti-p110α antibody (Cell Signaling). The transfected cells were incubated with 120 nm VacA at 37 °C for indicated times. After incubation, the cells were lysed. The lysates were subjected to SDS-PAGE in 10% gels, and then transferred to PVDF membranes for Western blot analysis with anti-phospho-Ser-473 Akt, Akt, p110α, or β-actin antibodies.
Effects of Various Inhibitors on VacA Activation of Akt in AZ-521 Cells—AZ-521 cells were treated with MCD (37 °C, 60 min) or LY294002 (37 °C, 30 min) at indicated concentrations. After treatment with inhibitors, the cells were incubated with 120 nm VacA at 37 °C for 30 min and then lysed. Lysate samples were subjected to SDS-PAGE in 10% gels, transferred to PVDF membranes for Western blotting, and probed with anti-phospho-Ser-473 Akt or Akt antibodies.
Immunoprecipitation of GSK3β/β-Catenin Complex Dissociation—AZ-521 cells were incubated with 120 nm VacA or iVacA for 0 to 240 min. After incubation, cells were solubilized with lysis buffer (50 mm Tris-HCl, pH 7.5, containing 150 mm NaCl, 20% glycerol, 1% Triton X-100, 1 μg/ml leupeptin, 1 μg/ml aprotinin, and 1 mm phenylmethylsulfonyl fluoride) and incubated on ice for 30 min. The lysates were analyzed by immunoprecipitation using anti-β-catenin mouse monoclonal antibody (Transduction Laboratories, BD). The precipitates were subjected to SDS-PAGE in 9% gels and transferred to PVDF membranes. Next, GSK3β and β-catenin were quantified by Western blot analysis using anti-GSK3β rabbit monoclonal antibodies (rabbit monoclonal antibody 27C10, product of Cell Signaling), and anti-β-catenin rabbit polyclonal antibody (1:1,000; H-102, product by Santa Cruz Biotechnology), respectively. These experiments were repeated three times.
β-Catenin Translocation from Cytosol to Nucleus—AZ-521 cells were incubated with 120 nm VacA or iVacA for 0, 30, and 60 min. After incubation, the cells were fixed at room temperature for 10 min in PBS containing 2% paraformaldehyde. The fixed cells were treated for 5 min with 0.1% Triton X-100 for membrane permeabilization and blocked with 4% Block Ace (in PBS, Snow Brand Milk Products, Tokyo, Japan) for 30 min. The fixed cells were studied by triple-immunostaining using anti-β-catenin mouse monoclonal antibodies (Transduction Laboratories, BD) and anti-GSK3β rabbit monoclonal antibodies (Cell Signaling) as primary antibodies in TBS containing 1% bovine serum albumin (BSA). After treatment with the respective primary antibodies, cells were incubated with secondary antibodies in TBS containing 1% BSA, utilizing anti-mouse antibodies conjugated with Alexa Fluor 488 or anti-rabbit antibodies conjugated with Alexa Fluor 546, respectively. The samples were analyzed with confocal microscopy using Leica Confocal Scanning System.
Luciferase Reporter Assay—Luciferase activity was measured using the Dual-Luciferase reporter assay (product of Promega), according to the manufacturer's directions. TOPtkLuciferase and FOPtkLuciferase reporter constructs carrying 3 copies of wild-type Tcf/Lef-binding sites and mutated Tcf/Lef-binding sites, respectively, were placed upstream of a luciferase reporter gene (16, 17). In brief, TOPtkLuciferase plasmids (1 μg), FOPtkLuciferase plasmids (1 μg), Cyclin D1Luciferase plasmids (1 μg), or pRL-TK vector plasmids (0.1 μg) was introduced into AZ-521 cells using Transfast transfection reagents (product of Promega) as described previously (18). The cells were incubated with EMEM containing 10% FCS for 12 h. After transfection with plasmid, the medium was replaced with new medium without FCS and then incubated for 24 h. After AZ-521 cells were treated with VacA for the indicated times, cells were washed with 500 μl of PBS and lysed by adding 100 μl of lysis buffer (Promega). After incubation for 15 min at room temperature, the lysate was centrifuged (15,000 × g, 5 min, 4 °C), and the supernatant was harvested and assayed with LAR II solution, which was provided a substrate for Firefly luciferase or with Stop and Glo solution, which was provided a substrate for Renilla luciferase. Luciferase activity was normalized to the pRL-TK luciferase activity.
Infection of AZ-521 Cells with H. pylori—H. pylori standard strain ATCC43504 and its vacA mutant strain were used. VacA mutants were constructed as previously described (19) with the exception that we used a kanamycin resistance gene cassette in this study. Before challenging AZ-521 cells, H. pylori strains were cultured in Brucella broth supplemented with 5% FCS under microaerobic conditions for 12–24 h at 37 °C with vigorous shaking, and then incubated with AZ-521 cells (5 × 105 cells) at a multiplicity of infection (MOI) of 100 for 6 h. After incubation in a 5% CO2 atmosphere for the time indicated in each figure, phosphorylation of Akt and GSK3β in infected cells was determined by Western blotting. Dissociation of GSK3β/β-catenin complex was assessed by quantification of GSK3β and β-catenin by Western blotting after immunoprecipitation of H. pylori-infected cell lysates using anti-β-catenin mouse monoclonal antibody.
Statistical Analysis—To establish the significance of the results, the Student's t test was used for numerical data. Fisher's exact test or χ2 test was used for categorical data as appropriate. A p value less than 0.05 was considered statistically significant.
RESULTS
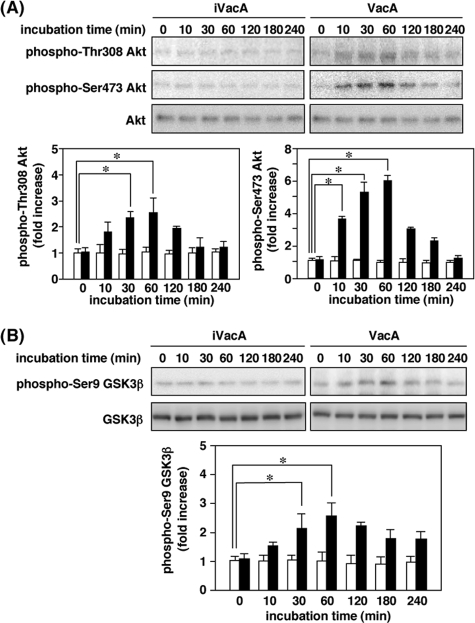
VacA Activation of Akt and Subsequent GSK3 Phosphorylation— The PI3K/Akt signaling pathway, activated in response to insulin and many other growth factors, often is a major regulator of GSK3 through phosphorylation by Akt of an inhibitory serine residue (20). To determine whether VacA induces Akt phosphorylation, AZ-521 cells were incubated with 120 nm VacA. We observed that VacA stimulated phosphorylation of Akt at regulatory residues Thr-308 and Ser-473, consistent with activation of Akt activity (Fig. 1A). Cells incubated with heat-inactivated VacA showed low or undetectable levels of Akt phosphorylation. Phosphorylation of both Thr-308 and Ser-473 was clearly evident within a 60-min incubation with VacA and declined thereafter. Addition of anti-VacA IgG blocked VacA-stimulated Akt phosphorylation (data not shown), suggesting that Akt phosphorylation and subsequent effects were not due to the presence of contaminants in the purified VacA (e.g. endotoxin). In rat fibroblast-like synoviocytes and human skin fibroblasts, Akt has been shown to participate in signaling pathways that lead to translocation of NF-κB to the nucleus and increased NF-κB-dependent transcription (21). In AZ-521 cells, however, we found that VacA did not enhance nuclear translocation of NF-κB (supplemental Fig. S1). In contrast, the increase in phosphorylation of another potential downstream substrate, GSK3β, at Ser-9 paralleled the rise in phosphorylation of Thr-308 and Ser-473 and was maximal at 30–60 min and declined within 120 min (Fig. 1B), suggesting that VacA may cause phosphorylation and inhibition of GSK3β activity by Akt activation.
FIGURE 1.
VacA-induced Akt phosphorylation leading to GSK3β phosphorylation at Ser9. A, AZ-521 cells were incubated with 120 nm VacA or heat-inactivated VacA (iVacA) at 37 °C for the indicated times. After incubation, cell lysates were analyzed by SDS-PAGE (10% gels), followed by Western blot analyses using anti-phospho-Thr-308 Akt, phospho-Ser-473 Akt, and Akt antibodies. Results are representative of three independent experiments. Quantification of phospho-Thr-308 Akt and phospho-Ser-473 Akt obtained with VacA (filled bars) and iVacA (open bars) was performed by densitometry. Data are means ± S.E. of values from triplicate experiments, with an n = 3 per experiment. Statistical significance: *, p < 0.01. B, AZ-521 cells were incubated with 120 nm VacA (filled bars) or iVacA (open bars) at 37 °C for indicated times. Cell lysates were prepared at indicated incubation times and subjected to Western blot analyses using anti-phospho-Ser-9 GSK3β and GSK3β antibodies. Results are representative of three independent experiments. Quantification of phospho-Ser-9 GSK3β obtained with VacA (filled bars) and iVacA (open bars) was determined by densitometry. Data are means ± S.E. of values from triplicate experiments, with an n = 3 per experiment. Statistical significance: *, p < 0.05.
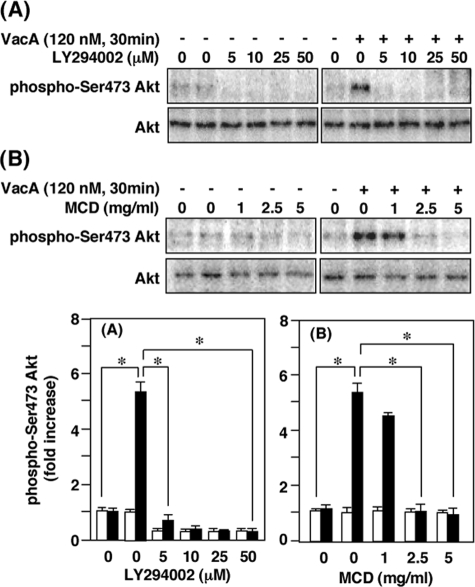
Effects of LY294002 and MCD on VacA Activation of Akt Phosphorylation—In the presence of PI3K inhibitor LY294002, VacA-treated cells and control cells showed equivalent and reduced levels of Akt phosphorylation at Ser-473 (Fig. 2A), demonstrating that blocking PI3K activity pharmacologically resulted in inhibition of both basal phosphorylation of Akt as well as VacA activation of Akt. To define further the mechanism by which PI3K activity regulates Akt phosphorylation, we asked whether the inhibitory effect of LY294002 could be reproduced with an inhibitor of VacA cellular effects. Blocking VacA clustering in lipid rafts with MCD, a cholesterol-depleting reagent which disrupts lipid rafts, inhibited VacA-induced Akt activation in AZ-521 cells (Fig. 2B). Taken together, these data identify lipid rafts and the PI3K/Akt pathway as participating in VacA inhibition of GSK3β in AZ-521 cells.
FIGURE 2.
Effect of various inhibitors on VacA-induced phosphorylation of Akt in AZ-521 cells. AZ-521 cells were pretreated with LY294002 for 30 min (A) or MCD for 60 min (B) at the indicated concentrations prior to incubation with or without 120 nm VacA. After incubation for 30 min, cell lysates were prepared and subjected to Western blot analyses using anti-phospho-Ser-473 Akt and Akt antibodies. Results are representative of three independent experiments. Quantification of phospho-Ser-473 Akt obtained with (filled bars) or without VacA (open bars) was determined by densitometry. Data are means ± S.E. of values from triplicate experiments, with an n = 3 per experiment. Statistical significance: *, p < 0.05.
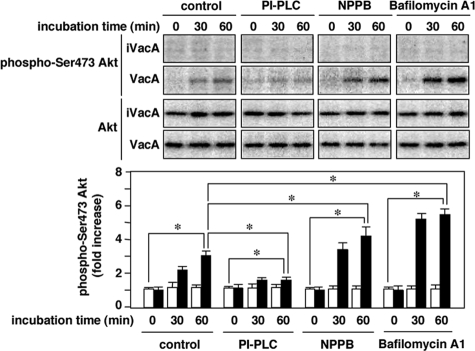
Effects of 5-Nitro-2-(3-phenylpropylamino)-benzoic Acid (NPPB), Bafilomycin A1, and Phosphatidylinositol-specific Phospholipase C (PI-PLC) on VacA Activation of Akt Phosphorylation—In addition to MCD, it is well known that NPPB (22), which disrupts anion channels, or bafilomycin A1 (23), which is a vacuolar-type H+ ATPase inhibitor, inhibited VacA internalization followed by vacuolation. In addition, PI-PLC, which cleaves the glycolipid of GPI-anchored proteins, inhibited VacA translocation to lipid rafts, VacA internalization, and VacA-induced vacuolation (11, 24). Consistent with inhibition of VacA effects by MCD, PI-PLC inhibited VacA-induced Akt phosphorylation, whereas NPPB and bafilomycin A1 had no inhibitory effect (Fig. 3). These results support our hypothesis that Akt phosphorylation induced by VacA requires VacA translocation to lipid rafts, but does not require VacA internalization or vacuolation.
FIGURE 3.
Effect of PI-PLC, NPPB, or Bafilomycin A1 on VacA-induced Akt phosphorylation in AZ-521 cells. AZ-521 cells were pretreated with PI-PLC (1 unit/ml, 60 min), NPPB (50 μm, 30 min), or Bafilomycin A1 (5 nm, 30 min) at the indicated concentrations. After treatment, the cells were incubated with 120 nm VacA or iVacA at 37 °C for 30 and 60 min. Following incubation, the cell lysates were subjected to SDS-PAGE in 10% gels and transferred to PVDF membranes for Western blotting with anti-phospho-Ser-473 Akt and Akt antibodies. Quantification of phospho-Ser-473 Akt obtained with VacA (filled bars) and iVacA (open bars) was determined by densitometry. Data are means ± S.E. of values from triplicate experiments, with an n = 3 per experiment. Statistical significance: *, p < 0.05.
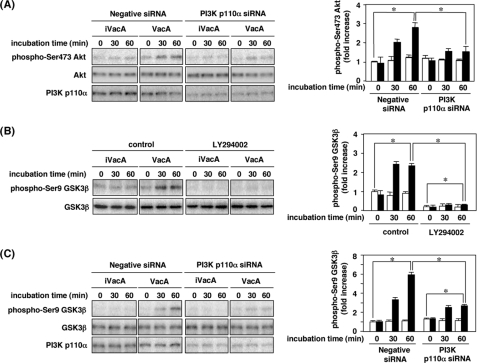
Inhibition of VacA Activation of Akt Phosphorylation and GSK3β Phosphorylation by PI3K p110α Isoform Silencing and PI3K Inhibitor—To address whether PI3K is crucial for VacA stimulation of Akt phosphorylation, AZ-521 cells were transfected with PI3K p110α siRNA. Reduced p110α expression in AZ-521 cells, treated with p110α-siRNA, resulted in suppression of Akt phosphorylation in response to VacA (Fig. 4A). To characterize further the mechanism by which PI3K activity regulates GSK3β phosphorylation, we examined whether the inhibitory effects of LY294002 and PI3K p110α silencing reduced VacA activation of GSK3β phosphorylation. Both LY294002 and PI3K p110α silencing blocked VacA-induced GSK3β phosphorylation in AZ-521 cells, suggesting that PI3K activation is critical for GSK3β phosphorylation (Fig. 4, B and C).
FIGURE 4.
Effect of PI3K p110α siRNA on VacA-induced Akt phosphorylation and effects of LY294002 and silencing of the PI3K gene on VacA-induced phosphorylation at Ser-9 of GSK3β. A, AZ-521 cells were transiently transfected with siRNA for PI3K p110α or negative siRNA as a control as described under “Experimental Procedures.” The transfected cells were incubated with 120 nm VacA at 37 °C for up to 60 min. After incubation, cell lysates were subjected to SDS-PAGE in 10% gels, and then transferred to PVDF membranes for Western blot analysis with the antibodies against phospho-Ser-473 Akt, Akt, and PI3K p110α. Quantification of phospho-Ser-473 Akt obtained with VacA (filled bars) and iVacA (open bars) was determined by densitometry scan analysis. Data are means ± S.E. of values from triplicate experiments, with an n = 3 per experiment. Statistical significance: *, p < 0.05. B, AZ-521 cells were pretreated with 50 μm LY294002 for 30 min before incubation with 120 nm VacA or iVacA at 37 °C. After incubation with VacA or iVacA for indicated times, the cell lysates were applied to SDS-PAGE in 10% gels. The separated proteins were transferred to PVDF membranes, and subjected to Western blot analysis with anti-phospho-Ser-9 GSK3β and GSK3β antibodies. Quantification of phospho-Ser-9 GSK3β was determined by densitometry for VacA (filled bars) and iVacA (open bars). Data are means ± S.E. of values from triplicate experiments, with an n = 3 per experiment. Statistical significance: *, p < 0.05. C, AZ-521 cells were transiently transfected with 200 pmol of siRNA for PI3K p110α or negative siRNA as negative control as described under “Experimental Procedures.” The transfected cells were incubated with 120 nm VacA or iVacA at 37 °C for indicated times. After incubation, the cell lysates were subjected to SDS-PAGE in 10% gels, and then transferred to PVDF membranes for Western blot analysis with anti-phospho-Ser-9 GSK3β, GSK3β, and PI3K p110α antibodies. Quantification of phospho-Ser-9 GSK3β obtained with VacA (filled bars) and iVacA (open bars) was determined by densitometry. Data are means ± S.E. of values from triplicate experiments, with an n = 3 per experiment. Statistical significance: *, p < 0.05.
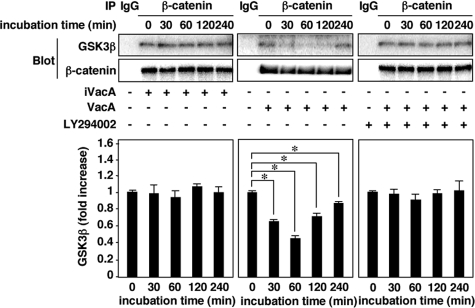
VacA Induces Dissociation of the GSK3β/β-Catenin Complex—GSK3β is one of the key elements of the Wnt pathway, which governs β-catenin homeostasis. Inhibition of GSK3 kinase activity might lead to stabilization of β-catenin and up-regulation of Lef-1/Tcf family of transcription factors, representing a potential mitogenic stimulus (25, 26). Wnt treatment disassembles the Axin/GSK3β complex, thus decreasing GSK3β phosphorylation of both β-catenin and Axin. Whereas unphosphorylated β-catenin is stabilized, unphosphorylated Axin is subject to proteasomal degradation, further reducing Axin/GSK3β complex levels (14, 27, 28). Therefore, we next assessed whether VacA induces dissociation of GSK3β and β-catenin from a GSK3β/β-catenin complex in AZ-521 cells. Western blot presents data compatible with dissociation of GSK3β and β-catenin from a GSK3β/β-catenin complex in AZ-521 cells incubated with VacA (Fig. 5). Blocking VacA-induced dissociation of GSK3β/β-catenin complex with LY294002 suggests the involvement of PI3K/Akt pathway in this process.
FIGURE 5.
GSK3β/β-catenin complexes are dissociated by VacA in AZ-521 cells. AZ-521 cells were incubated with 120 nm VacA or iVacA for 0–240 min in the presence or absence of 50 μm LY294002. After incubation, the cells were solubilized with lysis buffer and incubated on ice for 30 min. The lysates were available for immunoprecipitation using anti-β-catenin mouse monoclonal antibody (Transduction Laboratories, BD). The precipitates were subjected to SDS-PAGE in 8% gels and transferred to PVDF membranes. Then, GSK3β and β-catenin were quantified by Western blot analysis using anti-GSK3β rabbit monoclonal antibody (1:1,000; Cell Signaling) and anti-β-catenin rabbit polyclonal antibody (1:1,000; H-102, Santa Cruz Biotechnology), respectively. Quantification of phospho-Ser-9 GSK3β obtained with VacA (filled bars) and iVacA (open bars) was determined by densitometry. Data are means ± S.E. of values from triplicate experiments, with an n = 3 per experiment. Statistical significance: *, p < 0.01.
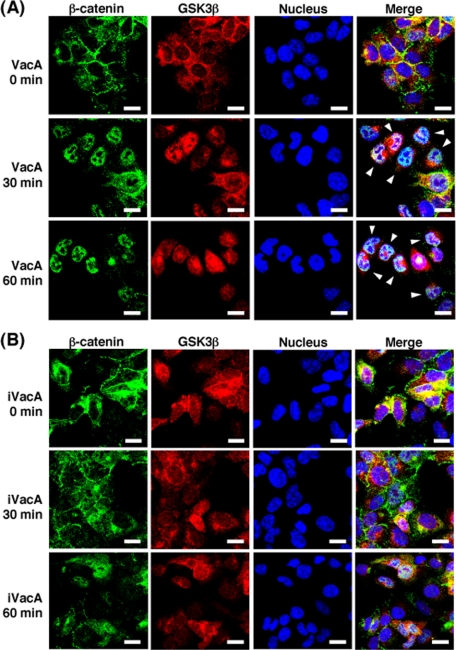
VacA Induced β-Catenin Translocation to Nucleus—VacA induced translocation of β-catenin to the nucleus as visualized by immunostaining with anti-β-catenin antibody (Fig. 6A), whereas heat-inactivated VacA did not cause β-catenin translocation (Fig. 6B).
FIGURE 6.
Confocal microscopy analysis of β-catenin translocation to nucleus from cytosol. AZ-521 cells were incubated with 120 nm VacA (A) or iVacA (B) at 37 °C for 0, 30 and 60 min. After incubation, the cells were fixed using 2% paraformaldehyde at room temperature, treated with 0.1% Triton X-100, and blocked with Block Ace. The fixed cells were stained with DAPI for 5 min, and were subjected to triple immunostaining using anti-β-catenin monoclonal antibodies and anti-GSK3β monoclonal antibodies as primary antibodies. After treatment with the respective primary antibodies, cells were incubated with the secondary anti-mouse antibodies, conjugated with Alexa Fluor 488, and anti-rabbit antibodies conjugated with Alexa Fluor 546, respectively. Data are representative of three experiments. Scale bar, 5 μm.
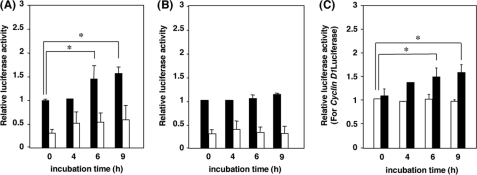
β-Catenin-dependent Gene Transcription—To demonstrate the activation of β-catenin-dependent gene transcription by VacA, we examined the effect of VacA on β-catenin-dependent transcriptional activity of AZ-521 cells transfected with TOPtkLuciferase reporter plasmid or control FOPtkLuciferase reporter plasmid. VacA induced activation of TOPtkLuciferase, but not FOPtkLucifease (Fig. 7, A and B). In addition, VacA also transactivated the β-catenin-dependent cyclin D1 promoter in a luciferase reporter assay, suggesting that VacA stimulates β-catenin signaling, which is one of the major signal transduction pathway involved in development of gastric cancer and in the induction of cyclin D1 expression (Fig. 7C).
FIGURE 7.
Effect of VacA on β-catenin-Tcf-mediated transcription and cyclin D1 promoter activity. AZ-521 cells were transfected with the luciferase reporter plasmid TOPtkLuciferase (filled bars) or FOPtkLuciferase (open bars), and internal control pRL-TK, followed by incubation with 120 nm VacA (A) or iVacA (B) for indicated times. Luciferase activities were measured using the Dual-Luciferase Reporter Assay System. Relative luciferase activities were compared with activities obtained for TOPtkLuciferase at 0 min in the presence of toxin. Data are means ± S.E. of values from triplicate experiments, with an n = 3 per experiment. Statistical significance: *, p < 0.01. C, AZ-521 cells were transfected with a reporter plasmid containing the cyclin D1 promoter-luciferase gene or a control empty vector. After transfection, cells were incubated with VacA (filled bars) or iVacA (open bars). Relative luciferase activities were compared with activities obtained at 0 min in the presence of iVacA. Data are means ± S.E. of values from triplicate experiments, with an n = 3 per experiment. Statistical significance: *, p < 0.01.
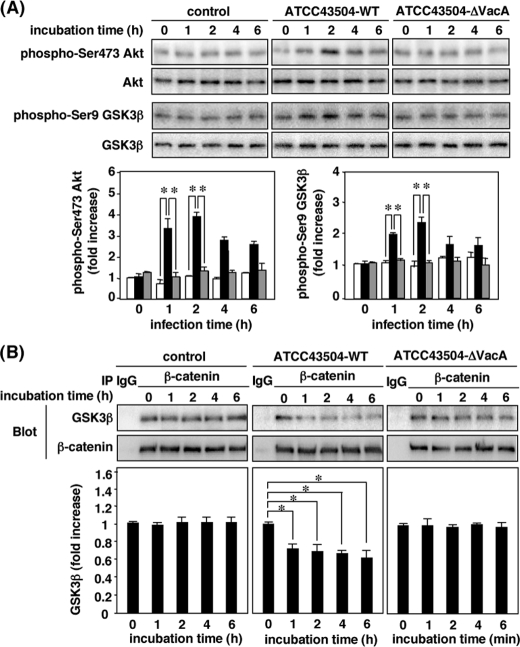
Phosphorylation of Akt and GSK3β in AZ-521 Cells Infected with H. pylori Strain Containing a VacA Gene—To determine whether production of VacA by H. pylori induces phosphorylation of Akt and GSK3β, AZ-521 cells were infected with H. pylori ATCC43504 (WT strain) or its isogenic VacA knock-out mutant strain (ΔVacA strain). After challenging AZ-521 cells with WT strain, greater phosphorylation of Akt at Ser-473 and GSK3β at Ser-9 was observed, with dissociation of β-catenin from the GSK3β/β-catenin complex, whereas a ΔVacA strain did not have similar effects, implying that VacA production by H. pylori is responsible for Akt activation leading to GSK3β phosphorylation (Fig. 8).
FIGURE 8.
Phosphorylation of Akt and GSK3β and dissociation of GSK3β/β-catenin complexes in AZ-521 cells infected with H. pylori, but not an isogenic VacA-knock-out mutant strain. AZ-521 cells were infected for 6 h with H. pylori ATCC43504 (WT strain) or its isogenic VacA-knock-out mutant strain (ΔVacA strain). Cells incubated without infection (uninfected cells) were used as a negative control. A, after incubation, phosphorylation of Akt and GSK3β in cells was determined by Western blot analysis with anti-phospho-Ser-473 Akt, Akt phospho-Ser-9 GSK3β, and GSK3β antibodies. Quantification of phospho-Ser-473 Akt and phospho-Ser-9 GSK3β obtained with control (open bars), WT strain (black bars), and ΔVacA strain (gray bars) was determined by densitometry. Data are means ± S.E. of values from triplicate experiments, with an n = 3 per experiment. Statistical significance: *, p < 0.05. B, after incubation, proteins from cell lysates were immunoprecipitated using anti-β-catenin antibody, followed by SDS-PAGE in 8% gels and Western blotting with anti-β-catenin and GSK3β antibodies. Relative densities of GSK3β, as determined by densitometry, were compared with densities obtained at 0 min. Data are means ± S.E. of values from triplicate experiments, with an n = 3 per experiment. Statistical significance: *, p < 0.05.
DISCUSSION
VacA enhances PGE2 production by AZ-521 cells through induction of COX-2 expression via the p38 MAP kinase/ATF-2 cascade without any effect on the COX-2 gene promoter at its NF-κB site (12). Consistent with previous findings, in AZ-521 cells, VacA did not affect intracellular Ca2+-release and NF-κB translocation to the nucleus (supplemental Fig. S1) despite the fact that in U937 cells, VacA increased IL-8 production by activation of the p38 MAP kinase via intracellular Ca2+-release, leading to activation of the transcription factors, ATF-2, CREB, as well as NF-κB (13). In addition, VacA initiated an intracellular signaling pathway, leading to the inactivation of NF-AT in Jurkat T cells by blocking increases in intracellular Ca2+. Thus, it appears that effects of VacA on these transcription factors can be cell type-specific and regulated through different mechanisms.
Because it is well known that PI3K and Akt participate in signaling pathways induced by endotoxin that lead to NF-κB activation (29), we examined whether, in AZ-521 cells, VacA affects Akt phosphorylation and found that VacA stimulated phosphorylation of Akt as well as its downstream substrate GSK3β (Fig. 1), but did not stimulate NF-κB activation. In agreement, pharmacologic studies using LY294002 and PI3K p110α silencing blocked VacA-induced GSK3β phosphorylation in AZ-521 cells, suggesting that VacA stimulated phosphorylation of Akt and GSK3β through PI3K-dependent pathways (Fig. 4). VacA induced changes in protein phosphorylation, including Erk and p38 MAPKs (9, 10), which did not require toxin internalization (11). VacA-induced phosphorylation of Akt and GSK3β through PI3K-dependent pathways may not be due to Erk and p38 MAPKs because it was not blocked by a specific inhibitor of p38 MAP kinase activity, SB203580, and with a MEK inhibitor, PD98059, which is known to block the Erk1/2 signal cascade (supplemental Fig. S2). Although MCD and PI-PLC were inhibitory, NPPB and bafilomycin A1 had no effect on VacA-induced phosphorylation of Akt (Fig. 3), indicating that VacA-induced signaling pathways leading to Akt phosphorylation do not require VacA internalization and are independent of vacuolation caused by VacA (8), as is VacA-induced p38 MAP kinase activation (11). It has been reported that ammonium chloride enhances several cellular effects of VacA, with the most notable being the potentiation of cellular vacuolation because it might be possible to detect subtle effects of VacA on endocytic processes that are obscured by the extensive changes in cellular architecture associated with VacA-induced vacuolation (30). Although ammonium chloride (5 mm) stimulated VacA-induced vacuolation in AZ-521 cells about 2-fold at 60 min of incubation under our experimental condition, it did not affect phosphorylation of Akt and GSK3β (supplemental Fig. S3).
Infection of AZ-521 cells with a vacA mutant strain of H. pylori failed to induce Akt phosphorylation (Fig. 8), suggesting that VacA is responsible for activation of the PI3K/Akt pathway. Thus, the finding that VacA-induced activation of PI3K/Akt pathway negatively regulates GSK3β function is also reasonable because it has been reported that GSK3β is necessary for the full transcriptional activity of NF-κB (31), although the inhibition of GSK3β may not interfere with all actions of NF-κB. In agreement, in AZ-521 cells treated with VacA, nuclear translocation of NF-κB did not differ from non-treated control cells (Fig. S1).
GSK3β does not have specific subcellular localization motifs, but various extracellular signals can impact the interaction of GSK3 with its target substrates. Canonical Wnt signaling functions primarily by regulating GSK3β to promote the rapid, cytosolic accumulation of the multifunctional protein β-catenin. In quiescent cells, β-catenin is principally found in association with cytoskeletal components (e.g. E-cadherin), where it functions to orchestrate actin dynamics and regulate cell adhesion, cell-cell interactions, and intercellular communication. In addition to its role as a cytoskeletal component, β-catenin can also function as a transcriptional co-factor; complexes of β-catenin and Lef/Tcf transcription factors serve as nuclear activators (32) of gene sets that promote specific developmental pathways or tumorigenesis (28). Confocal microscope visualization with anti-β-catenin antibody revealed that VacA induced translocation of β-catenin to the nucleus (Fig. 6), suggesting that PI3K/Akt-dependent pathways induced by VacA stimulate GSK3β phosphorylation and thus promote the accumulation of an uncomplexed, cytosolic population of β-catenin (Fig. 5) that may be available for interaction with and activation of Lef/Tcf family members after its nuclear translocation (Fig. 9).
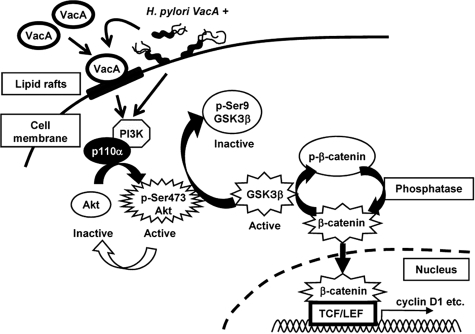
FIGURE 9.
VacA or an H. pylori vacA-positive strain induced activation of PI3K p110α/Akt pathway leading to inactivation of GSK3β and enhanced transcriptional activity of β-catenin. VacA or an H. pylori vacA-positive strain activated the PI3K/Akt signaling pathway, resulting in phosphorylation and inhibition of GSK3β, and subsequent translocation of β-catenin to the nucleus, consistent with effects of VacA on β-catenin-regulated transcriptional activity.
Indomethacin, which is an inhibitor of COX, reducing its production of prostanoids, significantly delayed epithelial regeneration in vivo (33). In contrast to the action of indomethacin, VacA induced COX-2 expression, resulting in enhancement of PGE2 production in AZ-521 cells during early VacA action (12), even though VacA eventually is responsible for gastric injury through induction of cell detachment from basal membrane and apoptosis after longer exposure to toxin. Although the involvement of the COX-2/PGE2 pathway in the regulation of cell survival and death by VacA remains controversial, here, we found that the increasing incubation times with VacA dramatically increased both the phosphorylation of Akt and GSK3β, suggesting that the PI3K/Akt pathway was necessary for inhibition of GSK3β function during early VacA action. Indeed, its inhibition was sufficient to dissociate a GSK3β/β-catenin complex during 2 h incubation with VacA. However, longer exposure (over 4 h) with VacA resulted in increased GSK3β/β-catenin complex levels compared with a short exposure for 2 h (Fig. 5). At that time, Akt was no longer active, which resulted in a decrease in the inhibition of phosphorylation of GSK3β on Ser-9 and hence, in a higher GSK3β activity. GSK3 inhibition reverted the prolonged activation of transcriptional function of β-catenin; after prolonged exposure to VacA, more β-catenin was observed in the nucleus compared with a short exposure, consistent with increased β-catenin transcriptional activity, which may contribute expression of a growth-promoting protein such as cyclin D1 (Fig. 7) (34). Interestingly, it has been reported that the activation of the Wnt/β-catenin signaling is found in about 30% of gastric cancer (35), suggesting that Wnt activation may be involved in the pathogenesis of gastric cancer. Consistent with this finding, Oshima et al. (36) recently showed that cooperation of Wnt signaling and PGE2-dependent pathways results in gastric cancer in a transgenic mouse model.
In conclusion, VacA induced two effects on β-catenin, with activation and nuclear accumulation following a short incubation, which depends on an active PI3K/Akt pathway and an inactive GSK3β, whereas prolonged incubation with VacA results in inactivation of Akt and activation of GSK3β, which then down-regulates β-catenin activity.
Supplementary Material
Acknowledgments
We thank K. Maeda, K. Tamura, and K. Shiraishi for skillful assistance, and I. Kato (Chiba University School of Medicine) for helpful discussions. We thank M. Vaughan of the Translational Medicine Branch, NHLBI, National Institutes of Health (Bethesda, MD) for helpful discussions and critical review of the manuscript.
This work was supported, in whole or in part, by the Intramural Research Program of the NHLBI, National Institutes of Health (to J. M.). This work was also supported by Grants-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement”in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
Footnotes
The abbreviations used are: MAPK, mitogen-activated protein kinase; EMEM, Eagle's minimal essential medium; FCS, fetal calf serum; Lef, leukocyte enhancer factor; NaF, sodium fluoride; PBS, phosphate-buffered saline; PVDF, polyvinylidine difluoride; Tcf, T-cell factor; PI3K, phosphatidylinositol 3-kinase; MCD, methyl-β-cyclodextrin; PGE, prostaglandin E; GSK, glycogen synthase kinase; PLC, phospholipase C; NPPB, 5-nitro-2-(3-phenylpropylamino)-benzoic acid.
References
- 1.Atherton, J. C. (2006) Annu. Rev. Pathol. 1 63-96 [DOI] [PubMed] [Google Scholar]
- 2.Kusters, J. G., van Vliet, A. H., and Kuipers, E. J. (2006) Clin. Microbiol. Rev. 19 449-490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D'Elios, M. M., Montecucco, C., and de Bernard, M. (2007) Clin. Chim. Acta 381 32-38 [DOI] [PubMed] [Google Scholar]
- 4.Manetti, R., Massari, P., Burroni, D., de Bernard, M., Marchini, A., Olivieri, R., Papini, E., Montecucco, C., Rappuoli, R., and Telford, J. L. (1995) Infect. Immun. 63 4476-4480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Telford, J. L., Ghiara, P., Dell'Orco, M., Comanducci, M., Burroni, D., Bugnoli, M., Tecce, M. F., Censini, S., Covacci, A., Xiang, Z. Y., Papini, E., Montecucco, C., Parente, L., and Rappuoli, R. (1994) J. Exp. Med. 179 1653-1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujikawa, A., Shirasaka, D., Yamamoto, S., Ota, H., Yahiro, K., Fukuda, M., Shintani, T., Wada, A., Aoyama, N., Hirayama, T., Fukamachi, H., and Noda, M. (2003) Nat. Genet. 33 375-381 [DOI] [PubMed] [Google Scholar]
- 7.Cover, T. L., and Blanke, S. R. (2005) Nat. Rev. Microbiol. 3 320-332 [DOI] [PubMed] [Google Scholar]
- 8.Yamasaki, E., Wada, A., Kumatori, A., Nakagawa, I., Funao, J., Nakayama, M., Hisatsune, J., Kimura, M., Moss, J., and Hirayama, T. (2006) J. Biol. Chem. 281 11250-11259 [DOI] [PubMed] [Google Scholar]
- 9.Boncristiano, M., Paccani, S. R., Barone, S., Ulivieri, C., Patrussi, L., Ilver, D., Amedei, A., D'Elios, M. M., Telford, J. L., and Baldari, C. T. (2003) J. Exp. Med. 198 1887-1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakayama, M., Kimura, M., Wada, A., Yahiro, K., Ogushi, K., Niidome, T., Fujikawa, A., Shirasaka, D., Aoyama, N., Kurazono, H., Noda, M., Moss, J., and Hirayama, T. (2004) J. Biol. Chem. 279 7024-7028 [DOI] [PubMed] [Google Scholar]
- 11.Nakayama, M., Hisatsune, J., Yamasaki, E., Nishi, Y., Wada, A., Kurazono, H., Sap, J., Yahiro, K., Moss, J., and Hirayama, T. (2006) Infect. Immun. 74 6571-6580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hisatsune, J., Yamasaki, E., Nakayama, M., Shirasaka, D., Kurazono, H., Katagata, Y., Inoue, H., Han, J., Sap, J., Yahiro, K., Moss, J., and Hirayama, T. (2007) Infect. Immun. 75 4472-4481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hisatsune, J., Nakayama, M., Isomoto, H., Kurazono, H., Mukaida, N., Mukhopadhyay, A. K., Azuma, T., Yamaoka, Y., Sap, J., Yamasaki, E., Yahiro, K., Moss, J., and Hirayama, T. (2008) J. Immunol. 180 5017-5027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim, L., and Kimmel, A. R. (2006) Curr. Drug Targets. 7 1411-1419 [DOI] [PubMed] [Google Scholar]
- 15.Giles, R. H., van Es, J. H., and Clevers, H. (2003) Biochim. Biophys. Acta 1653 1-2 [DOI] [PubMed] [Google Scholar]
- 16.Tago, K., Nakamura, T., Nishita, M., Hyodo, J., Nagai, S., Murata, Y., Adachi, S., Ohwada, S., Morishita, Y., Shibuya, H., and Akiyama, T. (2000) Genes Dev. 14 1741-1749 [PMC free article] [PubMed] [Google Scholar]
- 17.Murata-Kamiya, N., Kurashima, Y., Teishikata, Y., Yamahashi, Y., Saito, Y., Higashi, H., Aburatani, H., Akiyama, T., Peek, R. M., Jr., Azuma, T., and Hatakeyama, M. (2007) Oncogene 26 4617-4626 [DOI] [PubMed] [Google Scholar]
- 18.Kurashima, Y., Murata-Kamiya, N., Kikuchi, K., Higashi, H., Azuma, T., Kondo, S., and Hatakeyama, M. (2008) Int. J. Cancer. 122 823-831 [DOI] [PubMed] [Google Scholar]
- 19.Lu, H., Wu, J. Y., Kudo, T., Ohno, T., Graham, D. Y., and Yamaoka, Y. (2005) Mol. Biol. Cell 16 4954-4966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grimes, C. A., and Jope, R. S. (2001) Prog. Neurobiol. 65 391-426 [DOI] [PubMed] [Google Scholar]
- 21.Romashkova, J. A., and Makarov, S. S. (1999) Nature 401 86-90 [DOI] [PubMed] [Google Scholar]
- 22.Szabò, I., Brutsche, S., Tombola, F., Moschioni, M., Satin, B., Telford, J. L., Rappuoli, R., Montecucco, C., Papini, E., and Zoratti, M. (1999) EMBO J. 18 5517-5527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papini, E., Bugnoli, M., De Bernard, M., Figura, N., Rappuoli, R., and Montecucco, C. (1993) Mol. Microbiol. 7 323-327 [DOI] [PubMed] [Google Scholar]
- 24.Ricci, V., Galmiche, A., Doye, A., Necchi, V., Solcia, E., and Boquet, P. (2000) Mol. Biol. Cell 11 3897-3909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galceran, J., Hsu, S. C., and Grosschedl, R. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 8668-8673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hovanes, K., Li, T. W., Munguia, J. E., Truong, T., Milovanovic, T., Lawrence Marsh, J., Holcombe, R. F., and Waterman, M. L. (2001) Nat. Genet. 28 53-57 [DOI] [PubMed] [Google Scholar]
- 27.Salic, A., Lee, E., Mayer, L., and Kirschner, M. W. (2000) Mol. Cell. 5 523-532 [DOI] [PubMed] [Google Scholar]
- 28.Moon, R. T., Bowerman, B., Boutros, M., and Perrimon, N. (2002) Science 296 1644-1646 [DOI] [PubMed] [Google Scholar]
- 29.Yum, H. K., Arcaroli, J., Kupfner, J., Shenkar, R., Penninger, J. M., Sasaki, T., Yang, K. Y., Park, J. S., and Abraham, E. (2001) J. Immunol. 167 6601-6608 [DOI] [PubMed] [Google Scholar]
- 30.Li, Y., Wandinger-Ness, A., Goldenring, J. R., and Cover, T. L. (2004) Mol. Biol. Cell 15 1946-1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoeflich, K. P., Luo, J., Rubie, E. A., Tsao, M. S., Jin, O., and Woodgett, J. R. (2000) Nature 406 86-90 [DOI] [PubMed] [Google Scholar]
- 32.Takemaru, K., Yamaguchi, S., Lee, Y. S., Zhang, Y., Carthew, R. W., and Moon, R. T. (2003) Nature 422 905-909 [DOI] [PubMed] [Google Scholar]
- 33.Szabó, I. L., Pai, R., Jones, M. K., Ehring, G. R., Kawanaka, H., and Tarnawski, A. S. (2002) Exp. Biol. Med. 227 412-424 [DOI] [PubMed] [Google Scholar]
- 34.Doucas, H., Garcea, G., Neal, C. P., Manson, M. M., and Berry, D. P. (2005) Eur. J. Cancer. 41 365-379 [DOI] [PubMed] [Google Scholar]
- 35.Clements, W. M., Wang, J., Sarnaik, A., Kim, O. J., MacDonald, J., Fenoglio-Preiser, C., Groden, J., and Lowy, A. M. (2002) Cancer Res. 62 3503-3506 [PubMed] [Google Scholar]
- 36.Oshima, H., Matsunaga, A., Fujimura, T., Tsukamoto, T., Taketo, M. M., and Oshima, M. (2006) Gastroenterology 131 1086-1095 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.