Abstract
The Bacillus subtilis pyr operon is regulated by exogenous pyrimidines by a transcriptional attenuation mechanism. Transcription in vitro from pyr DNA templates specifying attenuation regions yielded terminated and read-through transcripts of the expected lengths. Addition of the PyrR regulatory protein plus UMP led to greatly increased termination. Synthetic antisense deoxyoligonucleotides were used to probe possible secondary structures in the pyr mRNA that were proposed to play roles in controlling attenuation. Oligonucleotides predicted to disrupt terminator structures suppressed termination, whereas oligonucleotides predicted to disrupt the stem of antiterminator stem-loops strongly promoted termination at the usual termination site. Oligonucleotides that disrupt a previously unrecognized stem-loop structure, called the anti-antiterminator, the formation of which interferes with formation of the downstream antiterminator, suppressed termination. We propose that transcriptional attenuation of the pyr operon is governed by switching between alternative antiterminator versus anti-antiterminator plus terminator structures, and that PyrR acts by UMP-dependent binding to and stabilization of the anti-antiterminator.
Keywords: in vitro transcription, pyrimidine biosynthesis, transcriptional regulation
Transcription of the Bacillus subtilis pyrimidine biosynthetic (pyr) operon is regulated by an attenuation mechanism that involves a regulatory protein, PyrR, which is encoded by the first gene of the operon (1, 2). PyrR is thought to act by modulating the function of three attenuators in the operon. These attenuators are located in the pyr 5′ leader, between the first and second genes, and between the second and third genes of the operon. Each attenuation region is predicted to specify mRNA that is capable of folding into a factor-independent transcription terminator (T) stem-loop, the formation of which can be prevented by the formation of a competing and more stable upstream stem-loop, called the antiterminator (AT). The balance between read-through, with consequent expression of downstream genes, versus termination and reduced expression of pyr genes has been proposed to be determined by the interconversion between AT and T structures in the mRNA, mediated in a UMP-dependent manner by the binding of PyrR to a conserved sequence in the 5′ strand of each AT stem. PyrR binding would destabilize the AT secondary structure and favor the formation of the alternative T secondary structure. This model was supported by the analysis of expression of pyr–lacZ fusions in which portions of the 5′ leader specifying the proposed AT and T structures were deleted (1).
Further examination of the conserved putative PyrR binding sequences in the three B. subtilis pyr attenuation regions, together with corresponding sequences from the B. caldolyticus pyr operon (3), revealed that each sequence is capable of folding into a stem-loop in which the most highly conserved region (UCCAGAGAGG in B. subtilis) is located at the top of the structure. The formation of such a stem-loop would in each case disrupt the base pairing between the 5′ and the 3′ strands of the AT stem. Even in the absence of PyrR and UMP, such a structure would tend to favor the downstream T structure and, hence, transcriptional termination. Thus, we have called this putative secondary structure the anti-antiterminator (AAT).
In the present work a simple in vitro transcription system and chemically synthesized antisense deoxyoligonucleotides were used to study the roles of the three putative T, AT, and AAT RNA secondary structures in transcriptional regulation of pyr gene expression in B. subtilis. This approach has been validated by the previous studies of Winkler et al. (4) and Fisher and Yanofsky (5), who used such a system to demonstrate the functional significance of RNA secondary structures in the attenuation control of the Escherichia coli trp operon, for which strong, independent evidence of the existence of the secondary structures is available (6). B. subtilis RNA polymerase was shown to synthesize read-through and terminated transcripts of the expected lengths from pyr DNA templates. Deoxyoligonucleotides chosen to disrupt the various proposed secondary structures in the newly synthesized transcripts by base pairing to them were shown to exert dramatic effects on the ratio of terminated to read-through transcripts that were fully consistent with the proposed roles of these stem-loops in transcriptional regulation. The results lead us to predict that the PyrR–UMP complex exerts its action by binding to and stabilizing the AAT structure of each pyr attenuation region, which in turn stimulates transcriptional termination indirectly by destabilizing a downstream AT structure.
MATERIALS AND METHODS
Plasmids and Templates.
Plasmids pUC19/290 (1), and pLS622 (2) have been described. The plasmids were transformed into E. coli DH5α (GIBCO/BRL) following a published procedure (7), and plasmid DNA was isolated using Qiagen (Chatsworth, CA) columns. PvuII digestion of pUC19/290 was used to generate template 1, which includes the pyr promoter and the untranslated 5′ leader region (attenuation region 1). Template 1 was 591 bp in length and is predicted to produce a 124-nt terminated transcript and a 450-nt read-through transcript. Template 2 was generated by digestion of pLS622 with PvuII. This template consisted of the pyr promoter fused to the 5′ end of the entire pyrR–pyrP intercistronic region (attenuation region 2). Template 2 was 560 bp in length and is predicted to produce a 154-nt terminated transcript and a 324-nt read-through transcript. The two DNA templates were isolated from the corresponding plasmids by agarose gel electrophoresis and electroelution (8).
In Vitro Transcription.
The in vitro transcription assay was a modification of published procedures (8, 9). Transcription reaction mixtures (10 μl) contained 10 nM DNA template, 150 nM B. subtilis sigma A subunit-containing RNA polymerase (kindly provided by John Helmann, Cornell University), 200 μM each ATP, GTP, and CTP, 10 μM UTP, and 5 μCi (1 Ci = 37 GBq) of [α-32P]ATP. Where indicated, 450 nM purified PyrR protein (10) and 25 μM UMP were also added to the reaction mixture. The in vitro transcription reaction buffer contained 40 mM Tris-acetate (pH 7.9), 10 mM Mg2+-acetate, 100 mM K+-acetate, 10 mM 2-mercaptoethanol, 4 mM spermidine, and 2.5% glycerol. Reaction mixtures without RNA polymerase were incubated at 37°C for 5 min, and reactions were initiated by addition of RNA polymerase. After 30 min of incubation at 37°C, 10 μl of stop solution (10 mM EDTA containing 0.1% xylene cyanole and 0.1% bromophenol blue dissolved in 98% formamide) was added to the reaction mixtures. After heating for 5 min at 85°C, the samples were loaded on an 8% polyacrylamide sequencing gel and subjected to electrophoresis at 60 W for 3 h to separate the read-through transcripts from the terminated transcripts. A PhosphorImager (Molecular Dynamics) was used to quantitate the radioactivity in the read-through and terminated transcripts from each reaction.
Deoxyoligonucleotide Synthesis.
Deoxyoligonucleotides used in this study were synthesized from the Triple Helix Synthesizer in the Genetic Engineering Facility at the University of Illinois. The deoxyoligonucleotides that were complementary to the AT structure in the 5′ leader region (attenuation region 1) are denoted as AT11 (+86 to +72), AT12 (+65 to +51), AT13 (+49 to +25), AT14 (+34 to +21), and AT15 (+23 to +1) (Fig. 1). Numbers refer to the pyr DNA sequence to which the deoxyoligonucleotides were complementary, numbering from the start of transcription as +1 (1, 11). AT16 was complementary to part of the pyrB gene (nucleotides +2821 to +2809) and was used as a control. The deoxyoligonucleotides that were complementary to the terminator of attenuation region 1 are denoted as T11 (+119 to +106) and T12 (+101 to +87) (Fig. 1). The deoxyoligonucleotides that were complementary to the AT structure of the pyrR–pyrP intercistronic region (attenuation region 2) are denoted as AT21 (+754 to +731) and AT22 (+740 to +727) (Fig. 2). The deoxyoligonucleotides that were complementary to the terminator of attenuation region 2 are denoted as T23 (+808 to +792) and T24 (+798 to +782) (Fig. 2). The deoxyoligonucleotides that were complementary to the proposed AAT structure in attenuation region 2 are denoted as BL22 (+718 to +685), BL23 (+718 to 702), BL24 (+710 to 685), BL25 (+701 to +685), BL27 (+718 to +699), and BL28 (+718 to +693) (Fig. 3).
Figure 1.
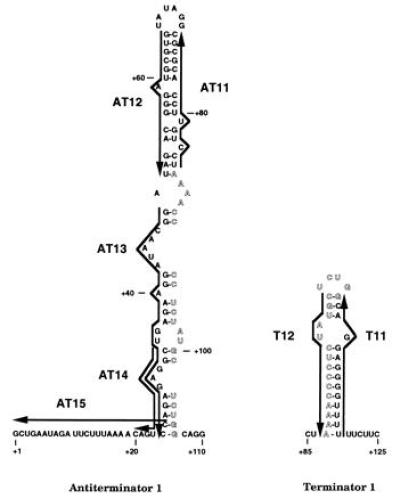
Predicted base pairing by antisense deoxyoligonucleotides used to probe secondary structures of pyr attenuation region 1. The 5′ leader pyr mRNA is shown folded in the putative AT structure (Left) and the alternative T structure (Right). Outlined letters indicate the overlapping portion of the RNA sequence involved in stem-loop formation in both structures. The sequences of the complementary deoxyoligonucleotides (AT11, AT12, AT13, AT14, AT15, T11, and T12) used in this study are indicated by heavy arrows, which denote their 5′ to 3′ direction, next to their complementary sequences in the mRNA.
Figure 2.
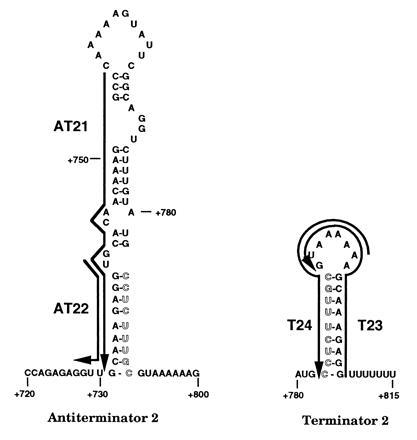
Predicted base pairing by antisense deoxyoligonucleotides used to probe secondary structures of pyr attenuation region 2. Symbols are defined as in Fig. 1.
Figure 3.
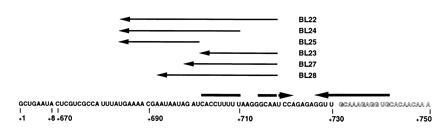
Antisense deoxyoligonucleotides used to probe the putative AAT secondary structure in pyr attenuation region 2. The sequence of the 5′ end of the transcript is shown and indicates the fusion of the first eight nucleotides transcribed from the pyr promoter to the sequence specified by attenuation region 2. Outlined letters indicate portions of the RNA involved in forming the 5′ stem of the downstream AT stem-loop. Thick arrows denote base-paired regions that form the proposed AAT stem-loop. The sequences of the complementary deoxynucleotides (BL22, BL23, BL24, BL25, BL27, and BL28) used in this study are indicated with thin arrows, which denote their 5′ to 3′ direction, above their complementary sequences in the mRNA.
Computer Analysis.
RNA secondary structure predictions were made with the pcfold program (version 3.0). This program was written by Michael Zuker (Washington University, St. Louis) and adapted for the IBM PC by Daniel Brunelle (National Research Council, Ottawa); free energies compiled by Salser (12) were used. The free energies of hybridization of deoxyoligonucleotides to complementary RNA sequences were estimated by calculation of their free energies of hybridization to complementary DNA sequences using the oligo primer-analysis software (version 4.06) from National Biosciences (Plymouth, MN).
RESULTS
AT Structures.
In vitro transcription from a template specifying pyr attenuation region 1 (the 5′ leader region) yielded primarily two transcripts of approximately 450 nt and 120 nt, as predicted for read-through transcripts and transcripts terminating within the uridine tract following the T structure of attenuation region 1, respectively (Fig. 4). PyrR plus 25 μM UMP caused a large increase in transcriptional termination, as expected. Both PyrR and UMP are required for this effect; further description of the effects of PyrR, pyrimidine nucleotides, and other metabolites on pyr transcription in vitro will be published elsewhere. Deoxyoligonucleotides AT11 through AT15, which are complementary to different portions of the attenuator 1 AT structure (Fig. 1), were tested for their effects on the products of transcription, along with deoxyoligonucleotide AT16, which is complementary to part of the pyrB gene and was used as a control. As shown in Fig. 4, those deoxyoligonucleotides that pair to various portions of the 5′ strand of the AT stem (AT12, AT13, and AT14) caused a dramatic increase in the proportion of terminated transcripts, as expected if disruption of the AT structure prevents it from interfering with formation of the downstream T structure. AT14, but not the other deoxyoligonucleotides, also promoted formation of an additional terminated transcript, which was estimated to be about 10 nt longer than the usual terminated transcript (Fig. 4). The reason for the formation of this longer transcript is not known. The control deoxyoligonucleotide AT16 did not have significant effects on transcriptional termination.
Figure 4.
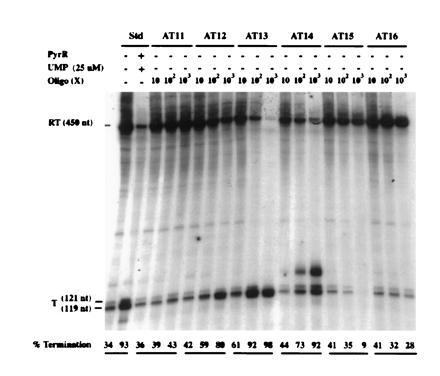
Effects of antisense deoxyoligonucleotides that disrupt pyr AT1 on termination of transcription in vitro. Template 1 was used for transcription (see Materials and Methods), and the radioactive transcripts were analyzed by electrophoresis. Components added to each reaction are shown (Top). Numbers for Oligo indicate the molar excess (fold) of deoxyoligonucleotide over template DNA. Numbers at the bottom of each lane indicate the mol % of terminated transcripts. RT, read-through transcripts; T, terminated transcripts.
Deoxyoligonucleotide AT11, which base pairs to the 3′ strand of the AT stem, caused little or no increase in termination, even though the calculated free energy of its binding to a complementary single-stranded sequence of DNA is essentially the same as that for AT12. A similar difference in the effects of deoxyoligonucleotides that bound to the 3′ versus the 5′ strands of stem-loop structures in the E. coli trp attenuator was observed by Fisher and Yanofsky (5). The asymmetry in effectiveness of AT11 versus AT12, which base pairs to the corresponding portion of the 5′ strand, is predicted if formation of the AT stem-loop is rapid, because it indicates that intramolecular base pairing of the 3′ strand with the adjacent, already synthesized 5′ strand competes more effectively than intermolecular base pairing with AT11. Thus, AT11 is expected to have much less effect than those deoxyoligonucleotides that can base pair with the 5′ strand before the trailing 3′ strand is synthesized.
An analogous set of deoxyoligonucleotides (Fig. 2) was examined for their effects on transcriptional termination from a pyr DNA template specifying attenuation region 2, which is located in the pyrR–pyrP intercistronic region. Transcripts from this template were again of the expected lengths, but a larger fraction of total transcripts in the absence of deoxyoligonucleotides, about 65%, was terminated than was observed with the template for attenuation region 1 (about 30%). Again, addition of PyrR plus UMP promoted termination at the expense of read-through transcription to about 95% of the total (Table 1). Deoxyoligonucleotide AT21 (Fig. 2), which base pairs to the entire 5′ strand of the AT of attenuation region 2, strongly promoted termination. This indicates a similar function for the AT structures in the two attenuation regions. Deoxyoligonucleotide AT22, which base pairs to a shorter segment of the 5′ strand, had no significant effect on termination (Table 1). We suggest that AT22, which is analogous to AT14 in its length and position with respect to the AT structure, may not be able to affect attenuation because of the differences in the stability of the AAT structures between these two regions (see below).
Table 1.
Effects of antisense deoxyoligonucleotides on termination of transcription from B. subtilis pyr templates in vitro
| Oligo added | % termination
|
||||
|---|---|---|---|---|---|
| −PyrR, −UMP | +PyrR, +UMP | −PyrR, −UMP
|
|||
| [Oligo]/[Template]
| |||||
| 10 | 100 | 1000 | |||
| Template 1 | |||||
| None | 32 ± 12 | 91 ± 5 | – | – | – |
| T11 | 35 | 37 | 46 | ||
| T12 | 35 | 32 | 13 | ||
| Template 2 | |||||
| None | 67 ± 4 | 94 ± 3 | – | – | – |
| AT21 | 71 | 90 | 96 | ||
| AT22 | 62 | 62 | 67 | ||
| T23 | 63 | 63 | 67 | ||
| T24 | 60 | 44 | 7 | ||
| BL22 | 61 | 23 | 4 | ||
| BL23 | 66 | 67 | 64 | ||
| BL24 | 59 | 30 | 7 | ||
| BL25 | 67 | 68 | 71 | ||
| BL27 | 67 | 66 | 45 | ||
| BL28 | 67 | 61 | 15 | ||
| BL23 + BL25 | ND | ND | 41 | ||
Oligo, oligonucleotide; ND, not determined. The numbers 10, 100, and 1000 give the molar ratio of deoxyoligonucleotide to DNA template in the experiment.
T Structures.
Deoxyoligonucleotides T12 and T24, which were designed to base pair to the 5′ strand of the putative T structures of attenuation regions 1 and 2, respectively (Figs. 1 and 2), shifted transcription in vitro from the corresponding pyr templates to favor read-through at the expense of termination (Table 1). These deoxyoligonucleotides were equally effective at suppressing termination even when PyrR and UMP were included in the transcription reaction (data not shown). This is the expected result if base pairing disrupts the T stem-loop and interferes with termination. On the other hand, deoxyoligonucleotides T11 and T23, which base pair with the complementary segments of the 3′ strand of the T stems of these attenuators (Figs. 1 and 2), were not able to alter the ratio of terminated to read-through transcripts significantly (Table 1). As with the deoxyoligonucleotides that base pair with the 5′ versus the 3′ strands of the AT stems, highly effective intramolecular base pairing of the upstream 5′ strand of the T stem with the 3′ strand can prevent base pairing with added deoxyoligonucleotides. The results provide evidence for the existence of a T secondary structure in the attenuators and for its role in termination of transcription.
AAT Structures.
As pointed out in the Introduction, each of the mRNAs specified by the three pyr attenuators is capable of forming a third stem-loop, the AAT, the formation of which competes with the AT stem-loop. The possibility that such a structure exists was first detected experimentally using deoxyoligonucleotide AT15 with attenuation region 1 as the template (Figs. 1 and 4). AT15, which is complementary to a putatively single-stranded region 5′ to the AT stem of attenuation region 1, promoted transcriptional read-through instead of termination. We initially expected this deoxyoligonucleotide to behave like the control AT16, but then recognized its ability to disrupt the proposed AAT structure of attenuation region 1. We chose to examine the effects of deoxyoligonucleotides on transcription in vitro from a template specifying attenuation region 2 to test further for the existence of such an AAT structure, because transcription from this attenuation region favors termination. Because the predicted effect of deoxyoligonucleotides that disrupt the AAT structure would be to increase the relative stability of the AT stem-loop and thus favor read-through, attenuation region 2 should be more sensitive to such effects than attenuation region 1, which already favors read-though in the absence of deoxyoligonucleotides. Furthermore, the relative positions of the predicted AAT and AT stem-loops in attenuation region 2 are sufficiently well separated that a number of deoxyoligonucleotides could be designed that would base pair with the stem of the AAT structure but would not be predicted to base pair with any part of the stem of the downstream AT structure (Fig. 3).
The first set of deoxyoligonucleotides examined, called BL22 through BL25, demonstrated that base pairing with both the 5′ strand of the stem of the putative AAT stem-loop and a portion of the single-stranded RNA that precedes it is necessary to suppress termination (Table 1). The longest deoxyoligonucleotide, BL22, which can base pair with the 5′ strand and the 5′ single-stranded sequence, strongly suppressed termination. BL24, which can only base pair with the first half of the 5′ strand of the AAT stem and with the same 5′ single-stranded sequence as BL22, was nearly as effective at suppressing termination as BL22. However, BL23, which can base pair with the same segment of the 5′ stem as BL22 but not to the 5′ single-stranded segment, had no effect on termination. Clearly, base pairing with the 5′ single-stranded segment is needed, but deoxyoligonucleotide BL25, which can base pair to this segment only, also had no effect on termination (Table 1).
The requirement for a deoxyoligonucleotide to base pair with both the 5′ strand of the AAT and a segment of the single-stranded RNA that precedes it if disruption of termination is to take place was probed further with a set of deoxyoligonucleotides that all base paired to the same segment of the 5′ strand of the AAT stem, but base paired to progressively shorter segments of the 5′ single-stranded region (Fig. 3 and Table 1). As described above, BL22, in which 17 bp can form with the 5′ single strand, suppressed termination very effectively, but reducing the number of potential base pairs with this region to 9 (BL28) markedly reduced the effectiveness of the deoxyoligonucleotide, and reduction to 3 bp (BL27) reduced the effect to a barely detectable level. It is essential that the portions of the deoxyoligonucleotide that base pair with the 5′ single strand and the 5′ strand of the stem be joined in a single molecule, as they are in BL22, because neither BL23 nor BL25 (Fig. 3) were effective, and an equimolar mixture of BL23 plus BL25 caused, at best, only a small decrease in termination at the highest concentrations tested (Table 1). We do not believe that the ineffectiveness of BL23, BL25, or BL27 is simply the result of decreased binding affinity of shorter probes, because probes with similar calculated free energies of hybridization (AT12, AT14, T12, and T24) were very effective at altering termination. In Discussion we suggest a kinetic explanation for the requirement for base pairing to a 5′ single-stranded region for effective disruption of the AAT structure.
DISCUSSION
Our original model (1) for the mechanism of transcriptional attenuation of the B. subtilis pyr operon proposed that switching between AT and T secondary structures in three untranslated regions of pyr mRNA determines the balance between read-through versus termination, respectively. The regulatory protein PyrR was proposed to act by binding to the 5′ strand of the AT stem-loop, destabilizing it and favoring formation of the T stem-loop. The results of the present study provide experimental support for the existence of both T and AT stem-loop structures, at least in attenuation regions 1 and 2, and for their postulated roles in regulating the termination of transcription. However, examination of the most highly conserved sequences in the three B. subtilis attenuation regions, as well as in the three corresponding sequences from B. caldolyticus (3), led us to predict that an additional stem-loop structure, the AAT stem-loop, would be formed in the region that also includes the 5′ strand of the AT stem-loop (Fig. 5). This observation led us to propose the following refined model for the role of RNA secondary structures in regulation of attenuation of the pyr operon (Fig. 5). In this model, the balance between transcriptional read-through and termination is determined by switching between the AT stem-loop and the combination of AAT plus T stem-loops, respectively. Furthermore, we propose that PyrR binds specifically to the AAT stem-loop, stabilizing it and hence the AAT-plus-T configuration of the pyr mRNA with consequent termination of transcription. The experiments presented here provide strong evidence for the existence of the AAT structure in attenuation region 2 (as well as an indication of its existence in attenuation region 1, namely the effects of AT15) and indicate that disruption of this structure by deoxyoligonucleotides that base pair to portions of its 5′ stem strongly suppresses termination at the downstream terminator.
Figure 5.
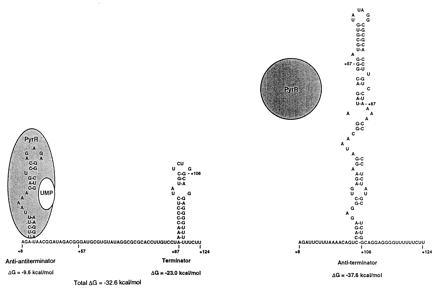
A refined model for transcriptional attenuation of the B. subtilis pyr operon. The RNA sequences shown are from the first pyr attenuation (5′ leader) region. The RNA structure at the right is proposed to be the secondary structure allowing read-through and is the more stable structure when PyrR is not bound. The RNA structure at the left is proposed to favor transcription termination. The free energies of formation of the secondary structures shown do not assume any contribution from PyrR binding. The structure at the left is proposed to be stabilized by binding of the PyrR–UMP complex to the AAT stem-loop, which changes the free energy of formation to a value more negative than −32.6 kcal/mol (1 cal = 4.184 J). PyrR binds to pyr mRNA only when it is complexed with UMP.
The results of in vitro transcription using template 1 and template 2 differed significantly in the frequency of termination in the absence of deoxyoligonucleotides, which was appreciably higher with template 2 (about 65%) than with template 1 (about 30%). It is unlikely that this difference is accounted for by the relative stabilities of the AT versus T structures formed by the two attenuation region RNAs, because their calculated free energies of formation (1) actually predict somewhat greater stability of the AT, relative to the T structure, in attenuation region 2. Furthermore, the fact that deoxyoligonucleotides that base pair with regions upstream of the predicted 5′ stem of both AT structures (e.g. AT15, BL22, BL24) caused decreased termination is inconsistent with the idea that termination is determined only by a balance between AT and T structures. Even if the 5′ stems of the AT stem-loops are longer than predicted by computer analysis, the effects of deoxyoligonucleotides AT15, BL22, and BL24 cannot be explained by assuming that they act to disrupt the AT stem-loop, because that would lead to increased termination, which is the opposite of the observed effect. However, the effects of these deoxyoligonucleotides are readily rationalized by proposing that they disrupt the AAT structure as shown in Fig. 3. If one accepts the existence of AAT structures in the RNA from attenuation regions 1 and 2, the differences in the frequency of termination between the two templates can be readily explained by differences in the predicted free energies of formation of their AAT stem-loops: the AAT of attenuation region 2 is predicted to have a free energy of formation nearly twice as great as the AAT from attenuation region 1 (−18.8 versus −9.6 kcal/mol). A more stable AAT structure should interfere more with AT formation and permit greater termination.
It seems very likely that the frequency of termination will be determined as much by the relative rates of formation and melting of the RNA secondary structures described here as by their free energies of formation. There is much evidence that this is true for other attenuation systems (6). Several experimental observations presented here indicate that kinetic aspects of RNA folding during transcription are important in pyr attenuation as well. We observed that the effects of deoxyoligonucleotides that base pair to the 5′ strands of the AAT, AT, or T stems were always much more effective than those that base pair with the 3′ strands of the same stems. The latter class of deoxyoligonucleotides generally had little effect on transcription termination at the same concentrations that were effective with the former class. We interpreted this result to indicate that intramolecular base pairing within the newly synthesized hairpin structures was able to compete more effectively than intermolecular base pairing by the deoxyoligonucleotides. The deoxyoligonucleotides that were able to base pair to the 5′ strand of the stems were apparently able to base pair very rapidly with these newly synthesized strands before the downstream 3′ strands were completed by RNA polymerase. A second indication of the importance of the kinetics of RNA folding was seen when comparing the effects of deoxyoligonucleotides BL22, BL28, and BL27 in disruption of the AAT structure of attenuation region 2 (Fig. 3 and Table 1). Those deoxyoligonucleotides that were capable of base pairing to a region of the RNA that is predicted to be single stranded and precedes the 5′ strand of the AAT stem-loop were much more effective at disrupting the AAT structure, as judged by their effects on termination of transcription. The longer the sequence that can base pair to this 5′ single-stranded region, the more effective the deoxyoligonucleotide was, even though the region that could base pair to the 5′ strand of the AAT stem itself was the same. These observations lead us to conclude that base pairing and intramolecular secondary structure formation occurs very rapidly in the newly synthesized pyr mRNA and that it occurs while sequences not far downstream are still being synthesized. This suggests that the AAT stem-loop forms very rapidly during transcription. If the PyrR–UMP complex does bind to the AAT structure, part of the effectiveness of the regulatory protein may reside in its ability to kinetically trap this structure before the downstream AT structure is fully formed.
The demonstration of the existence of AAT structures the formation of which promotes transcriptional termination at a downstream terminator leads naturally to the proposal that the PyrR–UMP complex acts to regulate termination by binding to and stabilizing the AAT structures in the pyr attenuation region mRNA. It must be noted that we have not yet demonstrated such binding by direct experimental means. However, there are good reasons to focus on the segment of RNA that forms the AAT structure as a likely PyrR binding site. This sequence is the most highly conserved of all the sequences of the six pyr attenuation regions from B. subtilis and B. caldolyticus (1, 3), and in all cases is predicted to fold into a very similar stem-loop. RNA binding proteins are known in numerous cases to bind to stem-loop structures (13). Evidence for the importance of the highly conserved sequences at the center of the AAT structure in the regulation of pyr expression in B. subtilis was provided by the characterization of a collection of cis-acting regulatory mutations in pyr–lacZ fusion-integrant strains by Ghim and Switzer (14). Mutations selected for defects in the repression of lacZ expression by exogenous pyrimidines in a fusion in which the pyr promoter and 5′ leader attenuation region were linked to lacZ were largely concentrated in the stem and loop of the proposed AAT structure. UMP-dependent binding of PyrR to specific RNA segments from pyr attenuation regions has been recently demonstrated by electrophoretic gel mobility-shift analysis (R.J.T., E. R. Bonner, and R.L.S., unpublished data), and the detailed requirements of RNA sequence and structure required for PyrR binding are being mapped.
At present the best characterized protein factor-dependent transcriptional attenuation system is that for regulation of the trp operon by the trp RNA-binding attenuation protein (TRAP) from B. subtilis (15–19). The overall attenuation mechanism governing the B. subtilis trp and pyr operons is quite similar. In each case binding of the regulatory protein, which is dependent on the presence of the end product metabolite (tryptophan or UMP), disrupts formation of an AT stem-loop and permits formation of a downstream terminator. However, the nature of the RNA binding sites are very different. The trp operon does not appear to contain an AAT secondary structure. TRAP binds only to single-stranded RNA and recognizes a series of up to 11 regularly spaced GAG or UAG triplets (15–18), whereas PyrR appears to require a stem-loop structure with a highly conserved single-stranded loop sequence, although the detailed elements of nucleotide sequence and secondary structure required for PyrR binding are not yet fully defined. Thus, we propose that PyrR stabilizes an RNA secondary structure, whereas TRAP disrupts one. TRAP and PyrR have very different amino acid sequences and quaternary structures as well. TRAP is composed of a remarkable 11-member ring of identical subunits, each of which probably binds to a single G/UAG triplet (15). PyrR is a hexameric protein in which the subunits are arranged with 3-fold and 2-fold symmetry axes (D. R. Tomchick, J. L. Smith, R.J.T., and R.L.S., unpublished data). Finally, PyrR, unlike TRAP, is an enzyme that catalyzes the uracil phosphoribosyltransferase reaction (1). In this case two of the substrates of this reaction, UMP and phosphoribosylpyrophosphate, are also metabolites that regulate transcriptional termination (Y.L. and R.L.S., unpublished data). Thus, very different strategies for protein–RNA recognition appear to have been adopted by mechanistically similar attenuation mechanisms from the same bacterial species.
Acknowledgments
We gratefully acknowledge Dr. John Helmann (Cornell University, Ithaca, NY), whose generous gift of B. subtilis RNA polymerase made this work possible, Dr. Charles L. Turnbough, Jr. (University of Alabama at Birmingham), for assistance in setting up in vitro transcription studies, and both Dr. Helmann and Dr. Turnbough for critical reading of the manuscript before publication. This research was supported by National Institutes of Health Grant GM47112. R.J.T. was supported by National Institutes of Health Cellular and Molecular Biology Training Grant GMO 7283.
Footnotes
Abbreviations: T, terminator; AT, antiterminator; AAT, anti-antiterminator.
References
- 1.Turner R J, Lu Y, Switzer R L. J Bacteriol. 1994;176:3708–3722. doi: 10.1128/jb.176.12.3708-3722.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu Y, Turner R J, Switzer R L. J Bacteriol. 1995;177:1315–1325. doi: 10.1128/jb.177.5.1315-1325.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghim S-Y, Neuhard J. J Bacteriol. 1994;176:3698–3707. doi: 10.1128/jb.176.12.3698-3707.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winkler M E, Mullis K, Barnett J, Stroynowski I, Yanofsky C. Proc Natl Acad Sci USA. 1982;79:2181–2185. doi: 10.1073/pnas.79.7.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher R, Yanofsky C. Nucleic Acids Res. 1984;12:3295–3302. doi: 10.1093/nar/12.7.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landick R, Yanofsky C. In: Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology. Neidhardt F C, editor. Vol. 2. Washington, DC: Am. Soc. Microbiol.; 1987. pp. 1276–1301. [Google Scholar]
- 7.Seidman C E, Struhl K, Sheen J. In: Current Protocols in Molecular Biology. Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. New York: Greene & Wiley; 1994. pp. 1.8.1–1.8.8. [Google Scholar]
- 8.Wilson H R, Archer C D, Liu J, Turnbough C L., Jr J Bacteriol. 1992;174:514–524. doi: 10.1128/jb.174.2.514-524.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimotsu H, Kuroda M I, Yanofsky C, Henner D J. J Bacteriol. 1986;166:461–471. doi: 10.1128/jb.166.2.461-471.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turner R J. Ph.D. thesis. Urbana-Champaign: Univ. of Illinois; 1996. [Google Scholar]
- 11.Quinn C L, Stephenson B T, Switzer R L. J Biol Chem. 1991;266:9113–9127. [PubMed] [Google Scholar]
- 12.Salser W. Cold Spring Harbor Symp Quant Biol. 1977;42:985–1002. doi: 10.1101/sqb.1978.042.01.099. [DOI] [PubMed] [Google Scholar]
- 13.Draper D E. Annu Rev Biochem. 1995;64:593–620. doi: 10.1146/annurev.bi.64.070195.003113. [DOI] [PubMed] [Google Scholar]
- 14.Ghim S-Y, Switzer R L. J Bacteriol. 1996;178:2351–2355. doi: 10.1128/jb.178.8.2351-2355.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antson A A, Otridge J, Brzozowski A M, Dodson E J, Dodson G G, Wilson K S, Smith T M, Yang M, Kurecki T, Gollnick P. Nature (London) 1995;374:693–700. doi: 10.1038/374693a0. [DOI] [PubMed] [Google Scholar]
- 16.Babitzke P, Yanofsky C. Proc Natl Acad Sci USA. 1993;90:133–137. doi: 10.1073/pnas.90.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Babitzke P, Stults J T, Shire S J, Yanofsky C. J Biol Chem. 1994;269:16597–16604. [PubMed] [Google Scholar]
- 18.Babitzke P, Bear D G, Yanofsky C. Proc Natl Acad Sci USA. 1995;92:7916–7920. doi: 10.1073/pnas.92.17.7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gollnick P. Mol Microbiol. 1994;11:991–997. doi: 10.1111/j.1365-2958.1994.tb00377.x. [DOI] [PubMed] [Google Scholar]


