Abstract
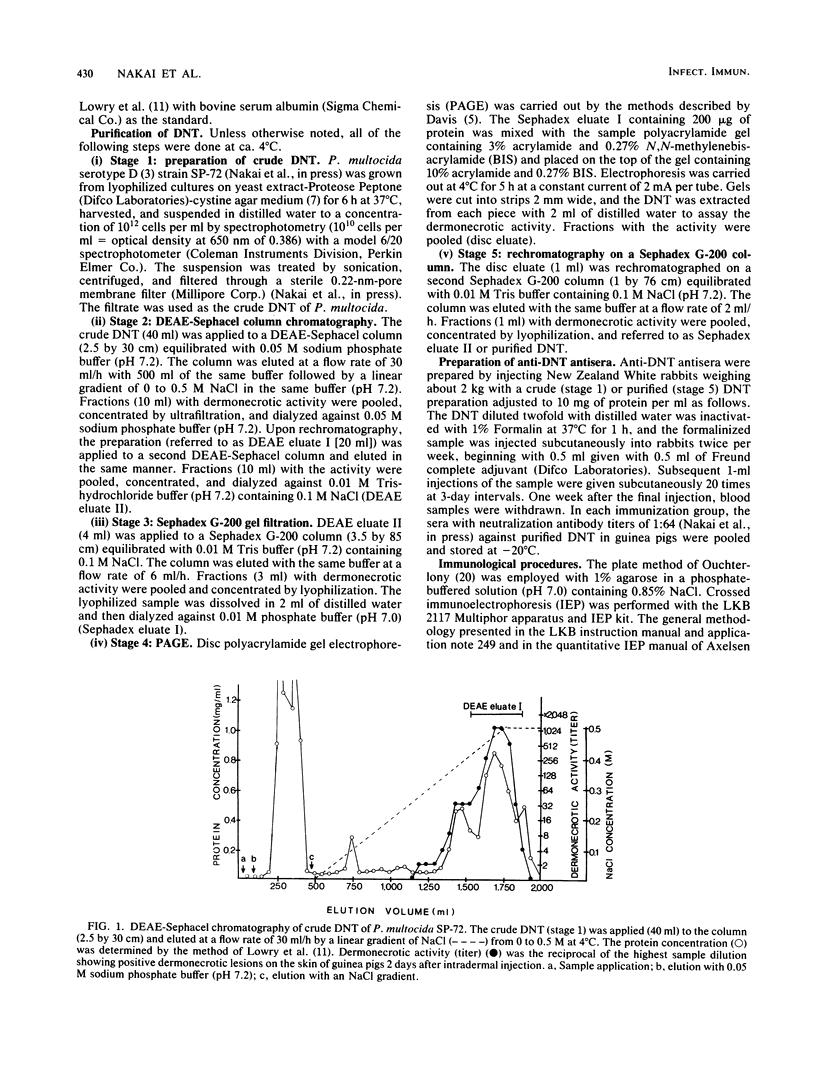
A procedure was developed to purify dermonecrotic toxin (DNT) from a sonic extract of a serotype D strain of Pasteurella multocida. Sonic extract containing DNT was applied to a DEAE-Sephacel column and eluted by a linear gradient of NaCl. Upon rechromatographing, fractions with dermonecrotic activity for guinea pigs were applied on a second Sephacel column, and a pooled fraction with the toxic activity was filtered through a Sephadex G-200 column. Pooled fractions with the toxic activity were subjected to polyacrylamide disc gel electrophoresis (PAGE), and the toxic substance was eluted from each sliced gel. Eluted fractions with the toxic activity were rechromatographed on a second Sephadex G-200 column, and a pooled fraction with high dermonecrotic activity was referred to as a purified DNT. The activity of purified DNT was increased by 1,000 times, and the average yield was about 1.8%. The purified DNT was homogeneous as determined by Ouchterlony double immunodiffusion, crossed immunoelectrophoresis, and thin-layer isoelectric focusing in polyacrylamide gels and gave a single band on PAGE and sodium dodecyl sulfate-PAGE. The molecular weight of the toxin was ca. 160,000 as determined by sodium dodecyl sulfate-PAGE. The isoelectric point of the toxin was ca. 4.7 to 4.8. Amino acid analysis of the purified DNT revealed that the toxin was composed of characteristically high proportions of glutamic acid, aspartic acid, glycine, proline, alanine, and leucine. The minimal necrotizing dose of the toxin was about 1 ng of protein, and the 50% lethal dose per mouse was 0.2 micrograms. The purified DNT was heat labile and sensitive to inactivation by trypsin, Formalin, and glutaraldehyde.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANERJEA A., MUNOZ J. Antigens of Bordetella pertussis. II. Purification of heat-labile toxin. J Bacteriol. 1962 Aug;84:269–274. doi: 10.1128/jb.84.2.269-274.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARTER G. R. Studies on Pasteurella multocida. I. A hemagglutination test for the identification of serological types. Am J Vet Res. 1955 Jul;16(60):481–484. [PubMed] [Google Scholar]
- Cowell J. L., Hewlett E. L., Manclark C. R. Intracellular localization of the dermonecrotic toxin of Bordetella pertussis. Infect Immun. 1979 Sep;25(3):896–901. doi: 10.1128/iai.25.3.896-901.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Eliás B., Krüger M. Epizootologische Untersuchungen der Rhinitis atrophicans des Schweines. III. Untersuchungen zu den Eigenschaften des Bordetella bronchiseptica-Exotoxins. Zentralbl Veterinarmed B. 1983 Jun;30(5):333–340. [PubMed] [Google Scholar]
- Eliás B., Krüger M., Rátz F. Epizootiologische Untersuchungen der Rhinitis atrophicans des Schweines. II. Biologische Eigenschaften der von Schweinen isolierten Bordetella bronchiseptica-Stämme. Zentralbl Veterinarmed B. 1982 Sep;29(8):619–635. [PubMed] [Google Scholar]
- Hanada M., Shimoda K., Tomita S., Nakase Y., Nishiyama Y. Production of lesions similar to naturally occurring swine atrophic rhinitis by cell-free sonicated extract of Bordetella bronchiseptica. Nihon Juigaku Zasshi. 1979 Feb;41(1):1–8. doi: 10.1292/jvms1939.41.1. [DOI] [PubMed] [Google Scholar]
- Iida T., Okonogi T. Lienotoxicity of Bordetella pertussis in mice. J Med Microbiol. 1971 Feb;4(1):51–61. doi: 10.1099/00222615-4-1-51. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maeda M., Shimizu T. Nasal infection of Alcaligenes bronchisepticus (Bordetella bronchiseptica) and lesions in newborn rabbits. Natl Inst Anim Health Q (Tokyo) 1975 Spring;15(1):29–37. [PubMed] [Google Scholar]
- Miniats O. P., Johnson J. A. Experimental atrophic rhinitis in gnotobiotic pigs. Can J Comp Med. 1980 Oct;44(4):358–365. [PMC free article] [PubMed] [Google Scholar]
- NAMIOKA S., MURATA M. Serological studies on Pasteurella multocida. I. A simplified method for capsule typing of the organism. Cornell Vet. 1961 Oct;51:498–521. [PubMed] [Google Scholar]
- NAMIOKA S., MURATA M. Serological studies on Pasteurella multocida. III. O antigenic analysis of cultures isolated from various animals. Cornell Vet. 1961 Oct;51:522–528. [PubMed] [Google Scholar]
- Nakagawa M., Shimizu T., Motoi Y. Pathology of experimental atrophic rhinitis in swine infected with Alcaligenes bronchisepticus or Pasteurella multocida. Natl Inst Anim Health Q (Tokyo) 1974 Summer;14(2):61–71. [PubMed] [Google Scholar]
- Nakase Y., Takatsu K., Tateishi M., Sekiya K., Kasuga T. Heat-labile toxin of Bordetella pertussis purified by preparative acrylamide gel electrophoresis. Jpn J Microbiol. 1969 Dec;13(4):359–366. doi: 10.1111/j.1348-0421.1969.tb00479.x. [DOI] [PubMed] [Google Scholar]
- ONOUE K., KITAGAWA M., YAMAMURA Y. CHEMICAL STUDIES ON CELLULAR COMPONENTS OF BORDETELLA PERTUSSIS. III. ISOLATION OF HIGHLY POTENT TOXIN FROM BORDETELLA PERTUSSIS. J Bacteriol. 1963 Oct;86:648–655. doi: 10.1128/jb.86.4.648-655.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen K. B., Barfod K. The aetiological significance of Bordetella bronchiseptica and Pasteurella multocida in atrophic rhinitis of swine. Nord Vet Med. 1981 Dec;33(12):513–522. [PubMed] [Google Scholar]
- Rutter J. M. Virulence of Pasteurella multocida in atrophic rhinitis of gnotobiotic pigs infected with Bordetella bronchiseptica. Res Vet Sci. 1983 May;34(3):287–295. [PubMed] [Google Scholar]
- Sawata A., Kume K. Nasal turbinate atrophy in young mice inoculated with Bordetella bronchiseptica of pig origin. Am J Vet Res. 1982 Oct;43(10):1845–1847. [PubMed] [Google Scholar]
- Sawata A., Nakai T., Tuji M., Kume K. Dermonecrotic activity of Pasteurella multocida strains isolated from pigs in Japanese field. Nihon Juigaku Zasshi. 1984 Apr;46(2):141–148. doi: 10.1292/jvms1939.46.141. [DOI] [PubMed] [Google Scholar]
- Sekiya K., Munoz J. J., Nakase Y. Effect of dermonecrotic toxin of Bordetella pertussis on the spleen of CFW and C57BL/10ScN mice. Microbiol Immunol. 1982;26(10):971–977. doi: 10.1111/j.1348-0421.1982.tb00244.x. [DOI] [PubMed] [Google Scholar]
- Shimizu T., Nakagawa M., Shibata S., Suzuki K. Atrophic rhinitis produced by intranasal inoculation of Bordetella bronchiseptica in hysterectomy produced colostrum-deprived pigs. Cornell Vet. 1971 Oct;61(4):696–705. [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Yokomizo Y., Shimizu T. Adherence of Bordetella bronchiseptica to swine nasal epithelial cells and its possible role in virulence. Res Vet Sci. 1979 Jul;27(1):15–21. [PubMed] [Google Scholar]






