Abstract
Nucleotide incorporation and extension opposite N2-ethyl-Gua by DNA polymerase ι was measured and structures of the DNA polymerase ι-N2-ethyl-Gua complex with incoming nucleotides were solved. Efficiency and fidelity of DNA polymerase ι opposite N2-ethyl-Gua was determined by steady state kinetic analysis with Mg2+ or Mn2+ as the activating metal. DNA polymerase ι incorporates dCMP opposite N2-ethyl-Gua and unadducted Gua with similar efficiencies in the presence of Mg2+ and with greater efficiencies in the presence of Mn2+. However, the fidelity of nucleotide incorporation by DNA polymerase ι opposite N2-ethyl-Gua and Gua using Mn2+ is lower relative to that using Mg2+ indicating a metal-dependent effect. DNA polymerase ι extends from the N2-ethyl-Gua:Cyt 3′ terminus more efficiently than from the Gua:Cyt base pair. Together these kinetic data indicate that the DNA polymerase ι catalyzed reaction is well suited for N2-ethyl-Gua bypass. The structure of DNA polymerase ι with N2-ethyl-Gua at the active site reveals the adducted base in the syn configuration when the correct incoming nucleotide is present. Positioning of the ethyl adduct into the major groove removes potential steric overlap between the adducted template base and the incoming dCTP. Comparing structures of DNA polymerase ι complexed with N2-ethyl-Gua and Gua at the active site suggests movements in the DNA polymerase ι polymerase-associated domain to accommodate the adduct providing direct evidence that DNA polymerase ι efficiently replicates past a minor groove DNA adduct by positioning the adducted base in the syn configuration.
N2-Ethylguanine (N2-ethyl-Gua)2 is an acetaldehyde-derived DNA adduct generated from the reduction of acetaldehyde with 2′-deoxyguanosine-3′-monophosphate (1). Humans are exposed to acetaldehyde from the environment and through the formation of acetaldehyde by the oxidation of ethanol (2). N2-Ethyl-Gua has been detected in the DNA of both alcoholic and nonalcohol drinkers (2, 3). Ethanol is classified as a human carcinogen, and acetaldehyde is known to contribute to the formation of malignant tumors (4). The formation of N2-ethyl-Gua during the reduction of acetaldehyde could cause ethanol-related cancers (5).
The ethyl moiety of N2-ethyl-Gua is predicted to project into the minor groove of duplex DNA. The N2-ethyl-Gua adduct is a strong block to DNA replication by replicative DNA polymerases in vitro and in cells (6, 7). Structures of bacteriophage DNA polymerase (pol) RB69, a homolog of human DNA pol α, indicate a possible mechanism of N2-ethyl-Gua blocked DNA replication. The structures reveal a DNA-binding motif that contacts the DNA minor groove and functions as an important safeguard to replication fidelity (8). The blocking of replicative DNA pols by N2-ethyl-Gua could arise when the ethyl group, protruding into the minor groove, disrupts protein:DNA contacts involved in the proposed “checking mechanism” (8). N2-Ethyl-Gua also has a high mis-coding potential during DNA replication with the Klenow fragment of Escherichia coli DNA pol I (9). Mutations caused by N2-ethyl-Gua range from single base deletions to transversions (10).
The Y family DNA polymerases η, ι, and κ replicate through adducted DNA templates (6, 11–13) and an open, more rigid active site contributes to lesion bypass (14). The multitude of Y family DNA pols suggests that a variety of mechanisms might be utilized by these polymerases during lesion bypass dependent upon the nature of the specific DNA adducts. Structural data indicate that DNA pol ι rotates unadducted template purines from the anti to syn conformation in ternary complexes and forms hydrogen bonds between the Hoogsteen edge of the template base and the Watson-Crick edge of the incoming nucleotide (15–17). Kinetic studies show that DNA pol ι has increased efficiency and fidelity during nucleotide insertion opposite template purines (11, 18–20). Similarly, rotation of the template base to the syn conformation is observed in the structure of DNA pol ι complexed with the 1,N6-ethenodeoxyadenosine lesion, allowing correct nucleotide insertion but not subsequent extension opposite the adduct (21). Rotation of the purine base at the active site of DNA pol ι would allow for efficient bypass of DNA adducts at the N2 of Gua by repositioning the adduct into the major groove and removing potential steric overlap between the lesion and incoming nucleotide. Thus, DNA pol ι could be involved in the bypass of the minor groove DNA adduct N2-ethyl-Gua.
The DNA polymerases utilize two divalent metal ions for activation of catalysis (22–24). The metals play a role in binding and positioning of the incoming nucleotide and in determining fidelity during catalysis (24, 25). The Mg2+ ion is often used as the activating metal for DNA polymerization studies in vitro (24). The Mn2+ ion also binds to and activates DNA polymerases but frequently results in decreased fidelity of the replicative DNA polymerases (26, 27). Recently, the Mn2+ ion has been shown to increase the efficiency and fidelity of nucleotide incorporation by DNA pol ι opposite a template Thy nucleotide (28).
The strong blocking effect of N2-ethyl-Gua to the replicative DNA polymerases and its possible role in alcohol-related cancers have prompted our studies on bypass of N2-ethyl-Gua by the Y family DNA pol ι. These data provide new insights into replication bypass of the ethanol-derived N2-ethyl-Gua adduct with potential carcinogenic consequences. The structures of DNA pol ι complexed with N2-ethyl-Gua containing DNA provide direct evidence for the initial anti position of the N2-ethyl-Gua that is subsequently rotated into the syn position upon binding the correct Cyt nucleotide but not upon binding the incorrect Thy nucleotide. The N2-ethyl moiety is easily accommodated in the major groove binding pocket of DNA pol ι by the specific repositioning of Lys309 located in a loop of the PAD domain. The Lys309 hydrogen bonding to the 5′ phosphate of the N2-ethyl-Gua template base in the anti orientation repositions to accommodate the ethyl side chain. This repositioning of Lys309 defines the available space for accommodation of relatively small adducts such as the alkyl lesions at the N2 position of Gua for efficient replication past these lesions by DNA pol ι. Furthermore, we show that when Mn2+ is the activating divalent metal, DNA pol ι bypass of N2-ethyl-Gua occurs with increased efficiency but reduced fidelity compared with Mg2+, demonstrating that Mn2+ could play an important role in modulating efficiency and fidelity of lesion bypass of minor groove purine adducts like N2-ethyl-Gua by the Y family DNA polymerases. Consequences of Mn2+ as the activating metal and flexibility of the DNA pol ι PAD domain for efficient bypass of N2-ethyl-Gua are discussed.
EXPERIMENTAL PROCEDURES
Oligonucleotides—N2-Ethyl-Gua phosphoramidites and template oligonucleotides were prepared as described previously (6). Three DNA primer oligonucleotides, 5′-(6-FAM)-GCTCCGGAACCC-3′, 5′-(6-FAM)-GCTCCGGAACCCTT-3′, and 5′-(6-FAM)-GCTCCGGAACCCTTC-3′, were purchased from Operon Biotechnologies, Inc. (Huntsville, AL). For crystallization experiments, a self-annealing DNA oligonucleotide containing the N2-ethyl-Gua adduct and a dideoxy CMP at the 3′ end, 5′-TCTXGGGTCCTAGGACCddC-3′ (where X = N2-ethyl-Gua), was synthesized by Midland Certified Reagents (Midland, TX) using the supplied N2-ethyl-Gua phosphoramidites. Synthesis was carried out using cyanoethyl phosphoramidite chemistry, and protecting groups were removed by hydrolysis with concentrated ammonium hydroxide (20). The oligo was purified by reverse phase high performance liquid chromatography (mass calculated = 5503.7, mass observed = 5504.9).
Expression and Purification of Human DNA Polymerase ι— The recombinant catalytic fragment of human DNA pol ι (amino acids 1–420) was made as an maltose-binding protein-DNA pol ι fusion protein with a PreScission Protease cleavage site seven residues from the DNA pol ι N-terminal methionine. The PreScission Protease recognition sequence and DNA pol ι coding sequence were verified by DNA sequencing. The plasmid constructs were transformed into E. coli BL21(DE3) Rosetta 2 cells (Novagen) for overexpression. Cells were grown to an A600 = 0.5 at 37 °C and quickly cooled on ice to 17 °C. After induction with 1 mm isopropyl β-d-thiogalactopyranoside, the cells were allowed to grow for 15 h at 17 °C. Cell extracts were prepared and the maltose-binding protein-DNA pol ι fusion protein was bound to an amylose resin in buffer containing 20 mm Tris-HCl (pH 7.5), 1 mm EDTA, and 200 mm NaCl. The fusion protein was cleaved overnight by on-column incubation with PreScission Protease at 4 °C. The recovered DNA pol ι was purified to homogeneity using phosphocellulose chromatography.
Assays—For primer extension assays the DNA primer (12-mer) was hybridized to the 32-mer DNA template and added to reactions containing 20 mm Tris-HCl (pH 7.5), 2 mm dithiothreitol, 100 μm dNTP, 10 nm DNA pol ι, and the amount of MgCl2 or MnCl2 indicated in the figure legends. Incubations were for 15 min at 37 °C and reactions were quenched with EtOH. Samples were dried and resuspended in 5 μl of a 95% formamide/dye solution. Extension products were separated on 23% urea-polyacrylamide gels, and imaged with a PhosphorImager (Molecular Dynamics) and quantified using ImageQuant software.
For the kinetic assays, the site-specific insertion procedure of Boosalis et al. (29) was used. DNA primers (14-mer for insertion and 15-mer for extension) were hybridized to the 32-mer DNA templates and added to reactions containing 20 mm Tris-HCl (pH 7.5), 2 mm dithiothreitol, 2 mm MgCl2 or 0.075 mm MnCl2, 50 nm primer-template, and 0.625 nm DNA pol ι (Mg2+-activated reactions), or 0.2 nm DNA pol ι (Mn2+-activated reactions). The amounts of DNA pol ι in reactions yielded ∼20% extended product maximally. Incubations were for 10 min at 37 °C and reactions were processed as described above. All extended product bands were used to determine kinetic parameters (Km and kcat values) by non-linear regression using SigmaPlot 8.02 software (SPSS Science, Inc.). Relative insertion frequencies were calculated as 1/[(kcat/KM,correct)/(kcat/KM,incorrect)].
Crystallization of Human DNA Pol ι—The purified catalytic fragment of DNA pol ι was dialyzed into 20 mm NaPO4 monobasic, 1 mm EDTA, 1 mm dithiothreitol, and 150 mm NaCl and concentrated to ∼11 mg/ml. The DNA pol ι was mixed at a 1:1.2 molar ratio with the N2-ethyl-Gua containing DNA oligonucleotide. To study ternary complexes, MgCl2 and dCTP (or dTTP) were added to final concentrations of 10 and 20 mm, respectively. Crystals grew from solutions described by Nair et al. (16) containing 0.2–0.4 m (NH4)2SO4, 12.5–15% PEG 5000 monomethyl ether, and 0.1 m MES (pH 6.5). Crystal trays were kept at 4 °C and diffraction quality crystals appeared in 1–3 days. The crystals belonged to space group P6522 and had cell dimensions of a = b = 98.53 Å, c = 202.35 Å for dCTP containing crystals, and a = b = 98.64 Å, c = 202.23 Å for dTTP containing crystals, and α = β = 90°, γ = 120°. For data collection crystals were step soaked for 5 min in mother liquor solutions containing 0–25% glycerol and flash frozen in liquid nitrogen.
Structure Determination and Refinement—X-ray diffraction data were collected using CuKα radiation from an in-house MicroMax 007 generator on a Saturn 92 CCD detector (Rigaku). The data were indexed, integrated, and scaled using d*TREX (30), and phases were calculated using molecular replacement. Molecular replacement with Phaser (31) generated a unique solution using DNA pol ι (Protein Data Bank code 2ALZ) minus DNA as a search model. Electron density maps calculated to 2.5 Å (dCTP) and 2.9 Å (dTTP) showed clear density around the N2-ethyl-Gua lesion. The dCTP ternary structure showed good electron density for the incoming nucleotide. Electron density for incoming dTTP could not be seen except for the γ phosphate, which was included in the model. Models were built in COOT (32) and refined in REFMAC5 using translesion systhesis refinement (33, 34). The refined model converged to an Rcryst = 23.2% and Rfree = 28.6% for the dCTP-containing complex and Rcryst = 23.6% and Rfree = 28.2% for the dTTP-containing complex. Ramachandran plots for the refined models show good stereochemistry, with 87.4 (dCTP-containing) and 88.6% (dTTP-containing) of residues in the favored regions and 0.0 (dCTP-containing) and 0.0% (dTTP-containing) in the disallowed regions. Figures were prepared using PyMol (35).
RESULTS
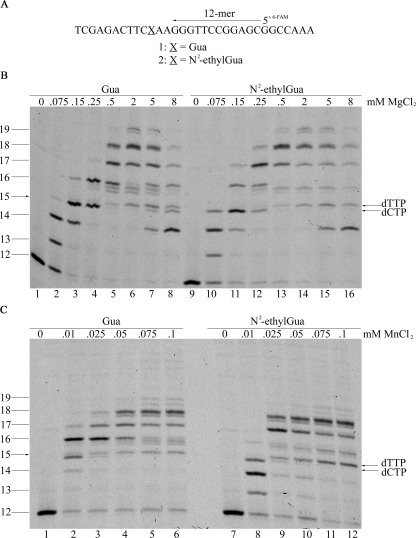
Primer Extension Reactions—DNA polymerase ι catalyzes bypass of the N2-ethyl-Gua adduct using Mg2+ or Mn2+ as the activating divalent metal ion. DNA polymerases can utilize various activating divalent metals (22–25, 28) and recent evidence indicates that both Mg2+ and Mn2+ are potent activators of DNA pol ι with perhaps Mn2+ being the preferred metal ion for activation (28). The DNA pol ι catalyzed bypass of N2-ethyl-Gua was tested using Mg2+ or Mn2+ as the activating metal (Fig. 1). A 12-mer DNA primer was annealed to 32-mer DNA templates with the primer 3′ terminus positioned three nucleotides from the target N2-ethyl-Gua or Gua (Fig. 1A). Upon incubation of the primer-template with DNA pol ι the 12-mer primer is extended to generate products 13 to 19 nucleotides in length and no full-length 25-nucleotide products are observed (Fig. 1, B and C). These data are consistent with the previously described poor primer extension properties of DNA pol ι, which exhibits especially low efficiency when copying template pyrimidines like those positioned 5′ to the target site in this template design (18, 36, 37). Primer extension reactions performed in the presence of increased concentrations of MgCl2 (Fig. 1B) or MnCl2 (Fig. 1C) show that DNA pol ι exhibits considerable sensitivity to the divalent ion concentration as indicated by the observed products. The maximum level of primer extension was detected using either DNA template at 2 mm MgCl2 (Fig. 1B) and 0.075 mm MnCl2 (Fig. 1C). Higher concentrations of MgCl2 and MnCl2 reduce DNA pol ι-catalyzed extension (Fig. 1, B and C, see also Ref. 28). These data indicate that DNA pol ι-catalyzed primer extension using the N2-ethyl-Gua and Gua DNA templates is similar and that the maximum extension is achieved at a ∼26-fold lower concentration of Mn2+ ion compared with Mg2+.
FIGURE 1.
Extension opposite N2-ethyl-Gua or Gua by DNA Pol ι in the presence of MgCl2 or MnCl2. Primer extension assays were carried out as described under “Experimental Procedures.” The annealed primer (12-mer) and templates containing either N2-ethyl-Gua or Gua (A) were incubated with 10 nm DNA pol ι and increasing concentrations of MgCl2 (B) or MnCl2 (C). Maximal primer extension was observed at 2 mm MgCl2 and 0.075 mm MnCl2. In single nucleotide extension reactions with only dTTP or dCTP using the 14-mer primer, the 15-mer product of dTMP insertion migrates to the position of the upper band and the 15-mer product of dCMP insertion migrates to the position of the lower band (data not shown).
Insertion of the correct dCMP and incorrect dTMP opposite Gua and N2-ethyl-Gua by DNA pol ι is detected using either Mg2+ or Mn2+ as the activating metal. The 15-nucleotide products are detected as a doublet band corresponding to the correct incorporation of two dTMP nucleotides opposite the template adenines to generate the 14-nucleotide products and subsequent incorporation of dCMP or dTMP opposite the target Gua and N2-ethyl-Gua by DNA pol ι (Fig. 1, B and C). The product band corresponding to dCMP insertion opposite template Gua, but not template N2-ethyl-Gua, was detected at the higher MgCl2 concentrations tested suggesting that DNA pol ι extends the N2-ethyl-Gua:Cyt base pair more efficiently than the normal Gua:Cyt base pair when Mg2+ is the activating metal ion (Fig. 1B, compare lanes 7 and 8 to 15 and 16). The presence of the 15-nucleotide product band corresponding to insertion of dTMP opposite the target Gua and N2-ethyl-Gua in the most active primer extension reactions indicates that DNA pol ι extends more efficiently from the correctly base paired Gua:Cyt and N2-ethyl-Gua:Cyt 3′ termini relative to extension from the mispaired Gua:Thy and N2-ethyl-Gua:Thy termini (Fig. 1, B and C). The triplet band corresponding to the 16-nucleotide position indicates additional heterogeneity in the oligonucleotide product 3′ terminal sequence likely resulting from the low level of nucleotide discrimination by DNA pol ι during nucleotide incorporation opposite template cytosines (37).
Efficiency and Fidelity of N2-Ethyl-Gua Bypass by DNA Pol ι—A steady state kinetic assay was used to more precisely quantify the efficiency and fidelity of DNA pol ι bypass of N2-ethyl-Gua compared with Gua in the presence of MgCl2 or MnCl2. Nucleotide insertion reactions were performed in the presence of increased concentrations of dCTP or dTTP using primed templates with the 3′ terminus positioned one nucleotide before the N2-ethyl-Gua or Gua. Extension reactions were performed in the presence of increased concentrations of the next correct nucleotide dGTP using primed templates with the 3′ Cyt or Thy positioned opposite the N2-ethyl-Gua or Gua. These data were quantified and the summary presented in Tables 1 and 2.
TABLE 1.
Nucleotide insertion opposite Gua and N2-ethyl-Gua by DNA pol ι Km and kcat values were determined by quantifying gel band intensities using ImageQuant, and non-linear regression analysis of product versus [dNTP] curves, using SigmaPlot 8.0.2.
| Metal ion | dNTP | Km | kcat | kcat/Km | Relative insertion frequencya |
|---|---|---|---|---|---|
| μm | min–1 | min–1 μm–1 | |||
| At template N2-ethyl-Gua | |||||
| 0.075 mm Mn2+ | |||||
| Cyt | 0.10 ± 0.012 | 425 ± 15 | 4.3 × 103 | 1 | |
| Thy | 0.030 ± 0.014 | 225 ± 25 | 7.5 × 103 | 1/0.6 | |
| 2 mm Mg2+ | |||||
| Cyt | 36 ± 3 | 74 ± 12 | 2.1 × 100 | 1 | |
| Thy | 650 ± 180 | 115 ± 18 | 1.8 × 10–1 | 1/12 | |
| At template Gua | |||||
| 0.075 mm Mn2+ | |||||
| Cyt | 0.15 ± 0.020 | 700 ± 40 | 4.7 × 103 | 1 | |
| Thy | 0.085 ± 0.020 | 200 ± 15 | 2.4 × 103 | 1/2 | |
| 2 mm Mg2+ | |||||
| Cyt | 49 ± 4 | 112 ± 18 | 2.3 × 100 | 1 | |
| Thy | 220 ± 60 | 50 ± 6 | 2.3 × 10–1 | 1/10 |
Relative insertion frequency is calculated as 1/([kcat/Km, correct]/[kcat/Km, incorrect])
TABLE 2.
Extension from Gua:Cyt, Gua:Thy, and N2-ethyl-Gua:Cyt, N2-ethyl-Gua:Thy base pairs by DNA pol ι Km and kcat values were determined by quantifying gel band intensities using ImageQuant, and non-linear regression analysis of product versus [dNTP] curves, using SigmaPlot 8.0.2.
| Metal ion | Base pair | Km | kcat | kcat/Km | Relative extension frequencya |
|---|---|---|---|---|---|
| μm | min–1 | min–1 μm–1 | |||
| At template N2-ethyl-Gua | |||||
| 0.075 mm Mn2+ | |||||
| N2-Et-Gua:Cytb | 0.10 ± 0.012 | 210 ± 7 | 2.1 × 103 | 1 | |
| N2-Et-Gua:Thy | 0.30 ± 0.027 | 1.0 ± 0.02 | 3.3 × 100 | 1/640 | |
| 2 mm Mg2+ | |||||
| N2-Et-Gua:Cyt | 40 ± 1 | 90 ± 4 | 2.3 × 100 | 1 | |
| N2-Et-Gua:Thy | 160 ± 40 | 0.31 ± 0.03 | 1.9 × 10–3 | 1/1180 | |
| At template Gua | |||||
| 0.075 mm Mn2+ | |||||
| Gua:Cyt | 0.25 ± 0.045 | 220 ± 10 | 8.8 × 102 | 1 | |
| Gua:Thy | 1.1 ± 0.09 | 1.3 ± 0.04 | 1.2 × 100 | 1/730 | |
| 2 mm Mg2+ | |||||
| Gua:Cyt | 200 ± 30 | 110 ± 8 | 5.5 × 10–1 | 1 | |
| Gua:Thy | 170 ± 30 | 0.18 ± 0.013 | 1.1 × 10–3 | 1/500 |
Relative extension frequency is calculated as 1/([kcat/Km, correct]/[kcat/Km, incorrect])
Et, ethyl
The DNA pol ι inserts the correct dCMP or incorrect dTMP nucleotide at high efficiency in the presence of Mn2+. The efficiency (kcat/Km) of dCMP insertion by DNA pol ι opposite N2-ethyl-Gua in the presence of Mn2+ is ∼2,000-fold higher than that measured in the presence of Mg2+, and there is a similar high efficiency for dCMP insertion opposite Gua in the presence of Mn2+ compared with Mg2+. The dramatically higher efficiency measured in the presence of Mn2+ can be attributed to a ∼340-fold lower Km value and a ∼6-fold higher kcat value during correct nucleotide incorporation for both DNA templates (Table 1). The efficiency of incorrect dTMP insertion by DNA pol ι opposite N2-ethyl-Gua in the presence of Mn2+ is ∼42,000-fold higher than that in the presence of Mg2+ and ∼10,000-fold higher opposite Gua using Mn2+ compared with Mg2+. Similar to that observed during correct nucleotide insertion, the higher efficiency for incorrect nucleotide insertion measured in the presence of Mn2+ is mostly attributable to a much lower Km value for the nucleotide using both DNA templates. These data suggest that DNA pol ι binds correct and incorrect incoming nucleotides with greater affinity and catalyzes nucleotide addition more rapidly in the Mn2+-activated reaction compared with the Mg2+-activated reaction.
The DNA pol ι exhibits higher fidelity of nucleotide insertion opposite N2-ethyl-Gua and Gua when activated with Mg2+ compared with Mn2+. Relative insertion frequencies calculated for incorrect dTMP opposite N2-ethyl-Gua and Gua in the presence of Mg2+ are lower compared with those measured using Mn2+ indicating an increased level of fidelity in the presence of Mg2+. Of particular note is the 20-fold higher level of nucleotide discrimination observed opposite N2-ethyl-Gua in the presence of Mg2+ compared with Mn2+ indicating a metal-dependent increase in the level of nucleotide discrimination opposite the adducted N2-ethyl-Gua by DNA pol ι. These data are similar to previous observations that Mn2+ causes a decreased fidelity in the replicative DNA polymerases (26, 27, 38, 39).
The DNA pol ι extends 3′ termini positioned opposite the N2-ethyl-Gua with higher efficiency in the presence of Mn2+ compared with Mg2+. The kcat/Km values for extension from the N2-ethyl-Gua:Cyt and Gua:Cyt base pairs indicate a ∼1300-fold higher extension efficiency in the presence of Mn2+ compared with Mg2+ (Table 2). The increased efficiency of extension using Mn2+ with either the adducted or unadducted DNA template is attributable to a ∼600-fold lower Km value for dGMP incorporation and a ∼2.2-fold higher kcat value (Table 2). Extension from the mispaired N2-ethyl-Gua:Thy base pair and from the Gua:Thy base pair is ∼1400-fold more efficient in the presence of Mn2+ compared with Mg2+ (Table 2). Interestingly, the efficiency of extension from a 3′ terminus positioned opposite the N2-ethyl-Gua is ∼3-fold higher than extension from a 3′ terminus opposite Gua using either Mg2+ or Mn2+ (Table 2).
The relative extension frequencies indicate that DNA pol ι distinguishes the correctly paired N2-ethyl-Gua:Cyt from the incorrectly paired N2-ethyl-Gua:Thy better in the presence of Mg2+ compared with Mn2+. The calculated relative extension frequency from 3′ termini opposite N2-ethyl-Gua in the presence of Mg2+ is ∼2-fold lower than that determined in the presence of Mn2+ (Table 2). The relative extension frequencies from 3′ termini positioned opposite the unadducted Gua using Mg2+ is similar to that using Mn2+ indicating that this apparent metal-dependent effect is not detected during extension from 3′ termini positioned opposite Gua.
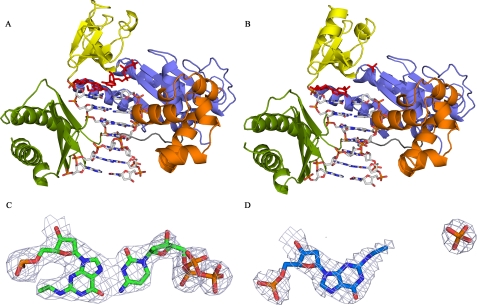
The Structure of DNA Pol ι with N2-Ethyl-Gua in the Active Site— The DNA pol ι bypasses N2-ethyl-Gua by rotating the adducted Gua base into the syn conformation. The crystal structure of N2-ethyl-Gua positioned in the active site of DNA pol ι was solved revealing the N2-ethyl-Gua in the syn conformation when the correct incoming dCTP nucleotide was present and in the anti configuration in the presence of the incorrect dTTP nucleotide (Fig. 2, Table 3). These data provide direct structural evidence that DNA pol ι bypasses the N2-ethyl-Gua adduct by rotating the adducted Gua to position the N2-ethyl adduct into the major groove. The crystal structures of DNA pol ι·N2-ethyl-Gua ternary complexes in the presence of dCTP (Fig. 2A) or dTTP (Fig. 2B) were solved to 2.5- and 2.9-Å resolution, respectively. The data show clear electron density for the ethyl adduct in both complexes (Fig. 2, C and D) as well as clear electron density for incoming dCTP (Fig. 2C). Data collected from crystals grown in the presence of dTTP lack electron density for the incoming nucleotide except around the γ phosphate (Fig. 2D). Electron density around the N2-ethyl-Gua adduct in the presence of incorrect dTTP shows that the template adducted base is in the anti configuration. Thus, our data show that N2-ethyl-Gua is in the syn configuration when paired with the correct dCTP and in the anti configuration when no paired nucleotide is detected. These data parallel the results of Nair et al. (17) who describe the incoming nucleotide as imposing a syn conformation on unadducted templates and extends the relevance of their observation to the N2-ethyl-Gua adducted DNA template.
FIGURE 2.
The structures of DNA pol ι·N2-ethyl-Gua Complexes with incoming dCTP or dTTP. A, the structure of DNA pol ι containing N2-ethyl-Gua and incoming dCTP shows the N2-ethyl-Gua base rotated into the syn configuration. B, the structure of DNA pol ι containing N2-ethyl-Gua and incoming dTTP shows the N2-ethyl-Gua base in the anti configuration and the N2-adduct protruding into the minor groove. C, electron density around the N2-ethyl adduct and incoming dCTP. D, electron density around the N2-ethyl adduct and the γ phosphate of the incoming dTTP.
TABLE 3.
Collection and refinement statistics
| Data collection | DNA pol ι:N2-ethyl-Gua with dCTP | DNA pol ι:N2-ethyl-Gua with dTTP |
|---|---|---|
| Resolution (Å)a | 2.2 (2.28-2.2) | 2.9 (3.0-2.9) |
| No. of measured reflections | 529,938 | 368,190 |
| No. of unique reflections | 19,625 | 13,417 |
| Completeness (%) | 98.9 (90.6) | 100 (100) |
| Redundancy | 17.7 (11.7) | 27.4 (26.3) |
| Rmerge (%)b | 12.2 (40) | 14.4 (32.3) |
| Mean I/σ | 13 (5.1) | 11.8 (4.0) |
| Refinement | ||
| Resolution range (Å) | 15–2.5 | 15–2.9 |
| Reflections | 19,625 | 12,753 |
| Rcryst (%)c | 23.2 | 23.8 |
| Rfree (%)d | 28.6 | 28.2 |
| Root mean square deviation bond lengths (Å) | 0.019 | 0.010 |
| Root mean square deviation bond angles (°) | 1.8 | 1.5 |
| Mean B-factor (Å2) | ||
| Protein | 34.5 | 14.9 |
| DNA | 35.8 | 15.0 |
| H2O | 32.9 | 13.9 |
Values for outermost shells are given in parentheses
Rmerge = Σ|I – 〈I〉|/ΣI, where I is the integrated intensity of a given reflection
Rcryst = Σ||Fobserved| – |Fcalculated||/Σ|Fobserved|
Rfree was calculated using 5% random data omitted from the refinement
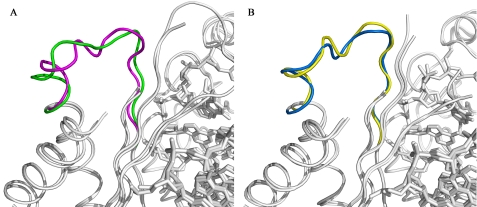
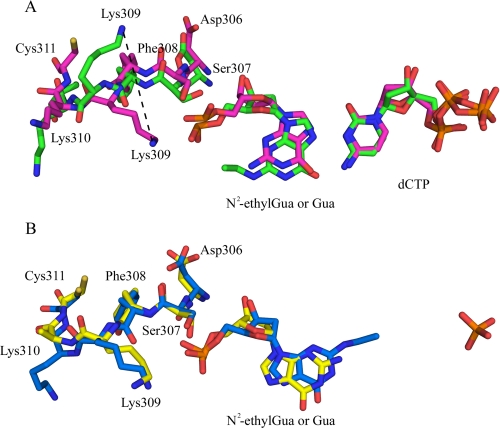
The rotation of N2-ethyl-Gua into the syn conformation and consequent positioning of the N2-ethyl moiety into the major groove disrupts contact between a DNA pol ι residue in the PAD domain and the DNA backbone. The two DNA pol ι·N2-ethyl-Gua complexes generated here were superimposed with the two DNA pol ι·Gua complexes determined by Nair et al. (PDB accession codes 2ALZ and 2FLP (16, 17)) to compare the structures (Fig. 3). As expected, the overall structures are very similar and align with root mean square deviation values of 0.499 Å (structures of DNA pol ι with N2-ethyl-Gua or Gua (2ALZ) in the syn configuration) and 0.466 Å (structures of N2-ethyl-Gua and Gua (2FLP) in the anti configuration). These analyses indicate that residues 306–311, which constitute a loop in the PAD domain, move to accommodate the ethyl adduct when the N2-ethyl-Gua is in the syn conformation in the active site. Detailed views of the DNA pol ι active sites in complex with N2-ethyl-Gua or Gua are shown in Fig. 4. The presence of the ethyl group at the N2 position of the N2-ethyl-Gua, coupled with rotation to the syn conformation, results in a ∼9Å shift in the position of the Lys309 side chain as indicated by the different positions of Lys309 in the presence of syn N2-ethyl-Gua compared with syn Gua (Fig. 4A). This repositioning of Lys309 is not observed when the N2-ethyl-Gua or Gua base is in the anti conformation (Fig. 4B). Thus, the Lys309 side chain hydrogen bonds with the 5′ phosphate of the unadducted Gua in the anti or syn configuration and with the N2-ethyl-Gua in the anti but not the syn configuration.
FIGURE 3.
Superposition of DNA pol ι complexes with anti and syn configurations of N2-ethyl-Gua and Gua. A, comparisons of the PAD domain in DNA pol ι with N2-ethyl-Gua (green) or Gua (PDB code 2ALZ, magenta) rotated into the syn configuration demonstrate the change in position of the backbone atoms of the PAD loop. B, the repositioning of the loop is not observed when comparing structures of DNA pol ι with N2-ethyl-Gua (blue) or Gua (PDB code 2FLP, yellow) rotated into the anti configuration.
FIGURE 4.
Repositioning of Lys309 in the structure of DNA pol ι with N2-ethyl-Gua in the syn configuration. A, the Lys309 side chain in DNA pol ι in complex with N2-ethyl-Gua (green) in the syn configuration shifts ∼9Å relative to the position of Lys309 in DNA pol ι complexed with syn Gua (PDB code 2ALZ, magenta). B, with N2-ethyl-Gua in the anti conformation (blue) Lys309 remains in a similar position relative to the Lys309 in the DNA pol ι·Gua complex (PDB code 2FLP, yellow).
DISCUSSION
The Y family DNA polymerases bypass adducted DNA that would otherwise impede the completion of genomic replication by the replicative DNA polymerases. The Y family DNA polymerase responsible for translesion synthesis is reflected in the structural and catalytic properties of the DNA polymerase and the specific DNA lesion (14). Human DNA pol ι is unique among the DNA polymerases in synthesizing DNA using Hoogsteen base pairing rather than conventional Watson-Crick base pairing (15–17, 19, 21, 40). This DNA polymerase catalytic feature is particularly relevant to the replication of DNA adducts positioned at the exocyclic N2 of Gua (6, 11, 20, 41–43). Our studies show that DNA pol ι inserts the correct dCTP opposite the N2-ethyl-Gua adduct with the same efficiency as opposite Gua. These findings are consistent with previous results demonstrating DNA pol ι catalyzed incorporation opposite N2-isopropyl-Gua and the N2-adducts γ-HOPdGua and reduced γ-HOPdGua (20, 41, 42). Furthermore, our data indicate that DNA pol ι extends from the N2-ethyl-Gua:Cyt 3′ terminus with higher efficiency compared with the Gua:Cyt 3′ terminus supporting previous findings from Choi and Guengerich (11). Together these data indicate that DNA pol ι bypasses N2-ethyl-Gua by incorporation of the correct dCTP followed by increased extension efficiency from the N2-ethyl-Gua:Cyt 3′ terminus. Thus, there is accumulating evidence that DNA pol ι exhibits increased extension efficiencies from adducted 3′ termini as has also been shown from N2-methyl- and N2-isobutyl-Gua adducts and from the reduced γ-HOPdGua (13, 42).
Our structural data provide evidence that DNA pol ι utilizes a Hoogsteen base pairing mechanism for efficient nucleotide incorporation during bypass of N2-ethyl-Gua. The structural analysis shows that N2-ethyl-Gua adopts a syn configuration in the active site of DNA pol ι in the presence of incoming dCTP. Rotation of template purines to the syn configuration has been shown in structures of DNA pol ι complexed with template Gua or Ade, as well as 1,N6-etheno-Ade (15–17, 21). These structural data support Hoogsteen base pairing as the mechanism utilized by DNA pol ι when incorporating nucleotides opposite adducted or unadducted purines. The ability of DNA pol ι to rotate Gua into the syn configuration allows bypass of the minor groove adduct N2-ethyl-Gua. Rotation of the template base repositions the N2-ethyl-Gua adduct into the major groove where there is less steric overlap between the lesion and incoming nucleotide and the residues at the active site. This unique ability of DNA pol ι to utilize Hoogsteen base pairing for efficient nucleotide incorporation opposite adducted template purines makes it a likely candidate DNA polymerase for bypass of minor groove adducts at the N2 of Gua or adducts that disrupt normal Watson-Crick base pairing during DNA replication.
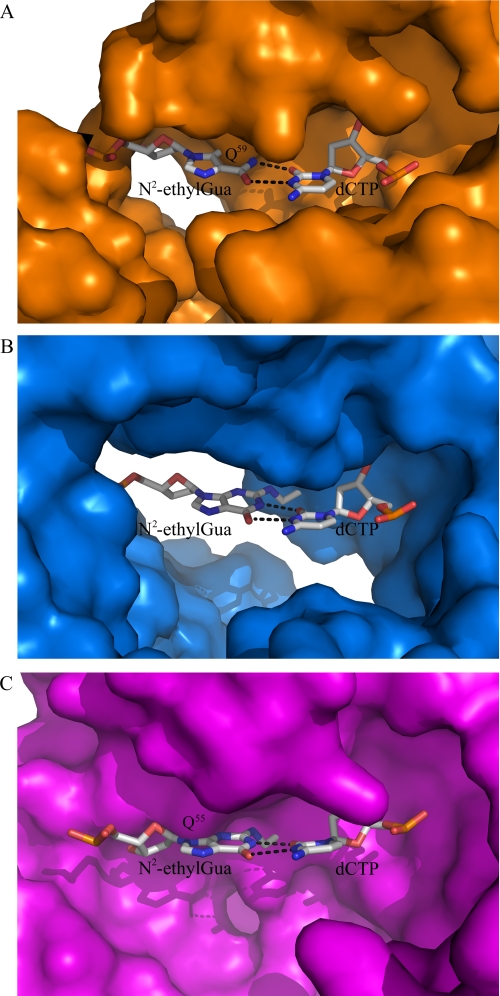
The DNA pol ι is not the only Y family DNA polymerase, however, able to efficiently bypass N2-ethyl-Gua. Kinetic analyses using human DNA pols η and κ have shown that these DNA polymerases will efficiently bypass N2-ethyl-Gua (6, 11). Structures of yeast DNA pol η and human DNA pol κ in complex with DNA show that the template base is in the anti configuration during nucleotide incorporation (44, 45). These kinetic and structural data suggest that DNA pols η and κ utilize a Watson-Crick base pairing mechanism during bypass of N2-ethyl-Gua. A normal Watson-Crick base pair, however, cannot form between N2-ethyl-Gua and incoming dCTP because of steric clash with the ethyl moiety and the O2 atom of Cyt. It is possible that a wobble base pair with hydrogen bonds between the N1 and O6 of N2-ethyl-Gua and the O2 and N3 of the incoming dCTP could form that would avoid steric overlap with the N2-ethyl adduct during dCTP incorporation. A comparison of the Y family DNA polymerase structures in complex with DNA shows that yeast DNA pol η (PDB 2R8J) and human DNA pol κ (PDB 2OH2) are able to accommodate wobble base pair geometry in their active sites, but human DNA pol ι is not (Fig. 5). The DNA pol ι active site more tightly coordinates the template base compared with DNA pols η and κ. Nair et al. (16, 17) have pointed out that this tight coordination, which shortens the C1′-C1′ distance between the sugars of the template nucleotide and incoming dNTP, forces the rotation of the template base into the syn conformation. In the active site of DNA pol ι, Gln59 lies adjacent to the N3 of N2-ethyl-Gua stabilizing the alignment of the template base and incoming dCTP. Modeling an N2-ethyl-Gua:Cyt wobble base pair into the active site of DNA pol ι reveals a ∼20° rotation of the ethyl adduct toward Gln59 causing steric overlap between the lesion and Gln59 (Fig. 5A). The active site of DNA pol κ lacks a corresponding residue to Gln59 leaving the DNA minor groove free of protein contacts and able to accommodate the N2-ethyl-Gua:Cyt wobble base pair (Fig. 5B). In yeast DNA pol η, Gln55 structurally aligns with the Gln59 residue of DNA pol ι but does not coordinate the template base as tightly. The DNA pol η active site is able to accommodate the N2-ethyl-Gua:Cyt wobble base pair (Fig. 5C). These observations demonstrate how different Y family DNA polymerases could facilitate bypass of the N2-ethyl-Gua adduct with or without rotation of the adducted base into the syn configuration.
FIGURE 5.
A N2-ethyl-Gua:Cyt wobble base in the active site of Y family DNA polymerases ι, κ, and η. A, Gln59 in DNA pol ι tightly coordinates the template base and sterically clashes with N2-ethyl-Gua when forming a wobble base pair with the incoming dCTP. B, DNA pol κ lacks a corresponding residue to Gln59 and accommodates the wobble base pair geometry. C, yeast DNA pol η has a Gln55 that corresponds to Gln59 in DNA pol ι but the more open active site accommodates the N2-ethyl-Gua:Cyt wobble base pair.
The rotation of adducted purines from the anti to syn configuration is necessary but not always sufficient for bypass of minor groove adducts by DNA pol ι. The structural data show that DNA pol ι bypasses N2-ethyl-Gua by a rotation of the adducted base and a repositioning of the lesion into the major groove. The ethyl moiety is accommodated in the major groove by changes in the positions of residues in a loop of the DNA pol ι PAD domain. Specifically, the potential for Lys309 to hydrogen bond with the DNA backbone is disrupted. The flexibility of the PAD domain allows accommodation of the ethyl moiety of N2-ethyl-Gua, but may not accommodate adducts as large or larger than N2-methyl(2-naphthyl)-Gua. Steady state kinetic data indicate that the efficiency of DNA pol ι during bypass of N2-alkyl-Gua adducts is decreased at both the incorporation and extension steps when adduct size is increased (11). Assuming the adducted base is rotated into the syn configuration it is possible that the mobility of the PAD domain would limit the ability of DNA pol ι to accommodate larger adducts. Large DNA adducts could disrupt additional contacts between DNA pol ι and the major groove. These observations may explain the lower efficiency of DNA pol ι incorporation and extension measured during bypass of HNE-dGua or N2-Naph-Gua, N2-Anth-Gua, and N2-BP-Gua adducts (11, 43).
Steady state kinetic experiments comparing the efficiency of DNA pol ι in the presence of Mg2+ or Mn2+ reveal that nucleotide insertion and extension catalyzed by DNA pol ι is dramatically activated at low Mn2+ concentrations. Our data show that maximal activation of DNA pol ι opposite Gua or N2-ethyl-Gua is achieved at concentrations of 0.075 mm Mn2+ and higher, similar to what is observed when DNA pol ι copies unadducted DNA templates (28). Furthermore, in the simultaneous presence of Mg2+ and Mn2+ the activity of DNA pol ι during correct nucleotide insertion is intermediate of that observed in the presence of each metal individually and it has been suggested that DNA pol ι might preferentially use Mn2+ even in the presence of excess Mg2+ (28). The effects of Mn2+ on the fidelity of DNA pol ι vary between increased fidelity opposite template Thy to decreased fidelity opposite other unadducted and adducted DNA templates. Our data demonstrate a metal dependent increase in nucleotide discrimination opposite N2-ethyl-Gua in the presence of Mg2+. Thus, these observations suggest that the preferential use of Mn2+ as an activating metal might reduce the fidelity of lesion bypass by DNA pol ι in exchange for increased catalytic efficiency.
The concentration range required for maximum activity of DNA pol ι using Mn2+ is broader than that relative to Mg2+ where maximal activity is observed over a more narrow range of metal ion concentrations. DNA pol ι reaches a maximum activity at 2 mm Mg2+, which decreases at higher Mg2+ ion concentrations. Current estimates indicate that total Mg2+ concentration in the cell ranges between 14 and 20 mm and free Mg2+ in the cytosol between 0.5 and 0.7 mm (46). The nucleus, mitochondrion, and endoplasmic reticulum are important cellular compartments for Mg2+ accumulation and Mg2+ levels are maintained within these cellular compartments by a sensitive regulatory system (46). It is possible that the level of Mg2+ maintained in the nucleus is at an optimal range for the highest activity of DNA pol ι, however, the narrow range of Mg2+ concentrations that result in maximal DNA pol ι activation would limit the efficiency of DNA pol ι catalyzed polymerization. The concentration of Mn2+ in the cell is much lower than Mg2+ and is thought to be around 10 μm (47). Interestingly, there have been several recently identified Mn2+ transporters from both eukaryotes and prokaryotes that are capable of transporting Mn2+ into the cell to bring the concentration to ≥300 μm. This influx of Mn2+ is in response to increased levels of reactive oxygen species (48). Thus, the broad range of Mn2+ concentrations that allow maximal DNA pol ι activity are present in the cell under damaging conditions where the higher efficiency of DNA pol ι opposite DNA lesions would be beneficial. DNA pol ι utilizes low levels of Mn2+ to achieve increased activity during bypass of other DNA adducts besides N2-ethyl-Gua, including a thymine-thymine dimer, an abasic site, a (6-4)TT photoproduct, and a benzo[a]pyrene diol epoxide-deoxy adenine lesion (28). Importantly, we have shown that other Y family DNA polymerases, including DNA pol κ, also have increased activation in the presence of low concentrations of Mn2+.3 Thus, Mn2+ activation of Y family DNA polymerases may be relevant during translesion synthesis where changes in Mg2+ and Mn2+ concentrations under different cellular conditions could contribute to the regulation of Y family DNA polymerase activity during DNA replication.
The atomic coordinates and structure factors (codes 3EPG and 3EPI) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
This work was supported, in whole or in part, by National Institutes of Health Grants GM069962 (to F. W. P.) and CA52881 (to J. C. F.). This work was also supported by American Cancer Society Grant RSG-04-187-01-GMC (to T. H.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: N2-ethyl-Gua, N2-ethylguanine; DNA pol ι, DNA polymerase ι; PAD, polymerase-associated domain; pol, polymerase; MES, 4-morpholineethanesulfonic acid.
M. Pence and F. W. Perrino, unpublished data.
References
- 1.Fang, J. L., and Vaca, C. E. (1995) Carcinogenesis 16 2177-2185 [DOI] [PubMed] [Google Scholar]
- 2.Fang, J. L., and Vaca, C. E. (1997) Carcinogenesis 18 627-632 [DOI] [PubMed] [Google Scholar]
- 3.Matsuda, T., Terashima, I., Matsumoto, Y., Yabushita, H., Matsui, S., and Shibutani, S. (1999) Biochemistry 38 929-935 [DOI] [PubMed] [Google Scholar]
- 4.Baan, R., Straif, K., Grosse, Y., Secretan, B., El Ghissassi, F., Bavard, V., Altieri, A., and Cogliano, V. (2007) Lancet Oncol. 8 292-293 [DOI] [PubMed] [Google Scholar]
- 5.Seitz, H. K., and Becker, P. (2007) Alcohol Res. Health 30 38-47 [PMC free article] [PubMed] [Google Scholar]
- 6.Perrino, F. W., Blans, P., Harvey, S., Gelhaus, S. L., McGrath, C., Akman, S. A., Jenkins, G. S., Lacourse, W. R., and Fishbein, J. C. (2003) Chem. Res. Toxicol. 16 1616-1623 [DOI] [PubMed] [Google Scholar]
- 7.Upton, D. C., Wang, X., Blans, P., Perrino, F. W., Fishbein, J. C., and Akman, S. A. (2006) Chem. Res. Toxicol. 19 960-967 [DOI] [PubMed] [Google Scholar]
- 8.Franklin, M. C., Wang, J., and Steitz, T. A. (2001) Cell 105 657-667 [DOI] [PubMed] [Google Scholar]
- 9.Terashima, I., Matsuda, T., Fang, T. W., Suzuki, N., Kobayashi, J., Kohda, K., and Shibutani, S. (2001) Biochemistry 40 4106-4114 [DOI] [PubMed] [Google Scholar]
- 10.Upton, D. C., Wang, X., Blans, P., Perrino, F. W., Fishbein, J. C., and Akman, S. A. (2006) Mutat. Res. 599 1-10 [DOI] [PubMed] [Google Scholar]
- 11.Choi, J. Y., and Guengerich, F. P. (2006) J. Biol. Chem. 281 12315-12324 [DOI] [PubMed] [Google Scholar]
- 12.Choi, J. Y., and Guengerich, F. P. (2005) J. Mol. Biol. 352 72-90 [DOI] [PubMed] [Google Scholar]
- 13.Choi, J. Y., Angel, K. C., and Guengerich, F. P. (2006) J. Biol. Chem. 281 21062-21072 [DOI] [PubMed] [Google Scholar]
- 14.Prakash, S., Johnson, R. E., and Prakash, L. (2005) Annu. Rev. Biochem. 74 317-353 [DOI] [PubMed] [Google Scholar]
- 15.Nair, D. T., Johnson, R. E., Prakash, S., Prakash, L., and Aggarwal, A. K. (2004) Nature 430 377-380 [DOI] [PubMed] [Google Scholar]
- 16.Nair, D. T., Johnson, R. E., Prakash, L., Prakash, S., and Aggarwal, A. K. (2005) Structure 13 1569-1577 [DOI] [PubMed] [Google Scholar]
- 17.Nair, D. T., Johnson, R. E., Prakash, L., Prakash, S., and Aggarwal, A. K. (2006) Structure 14 749-755 [DOI] [PubMed] [Google Scholar]
- 18.Johnson, R. E., Washington, M. T., Haracska, L., Prakash, S., and Prakash, L. (2000) Nature 406 1015-1019 [DOI] [PubMed] [Google Scholar]
- 19.Johnson, R. E., Haracska, L., Prakash, L., and Prakash, S. (2006) Mol. Cell. Biol. 26 6435-6441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perrino, F. W., Harvey, S., Blans, P., Gelhaus, S., Lacourse, W. R., and Fishbein, J. C. (2005) Chem. Res. Toxicol. 18 1451-1461 [DOI] [PubMed] [Google Scholar]
- 21.Nair, D. T., Johnson, R. E., Prakash, L., Prakash, S., and Aggarwal, A. K. (2006) Nat. Struct. Mol. Biol. 13 619-625 [DOI] [PubMed] [Google Scholar]
- 22.Beese, L. S., Friedman, J. M., and Steitz, T. A. (1993) Biochemistry 32 14095-14101 [DOI] [PubMed] [Google Scholar]
- 23.Beese, L. S., and Steitz, T. A. (1991) EMBO J. 10 25-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang, W., Lee, J. Y., and Nowotny, M. (2006) Mol. Cell 22 5-13 [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Diaz, M., Bebenek, K., Krahn, J. M., Pedersen, L. C., and Kunkel, T. A. (2007) DNA Repair (Amst.) 6 1333-1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sirover, M. A., and Loeb, L. A. (1976) Science 194 1434-1436 [DOI] [PubMed] [Google Scholar]
- 27.Sirover, M. A., and Loeb, L. A. (1976) Proc. Natl. Acad. Sci. U. S. A. 73 2331-2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frank, E. G., and Woodgate, R. (2007) J. Biol. Chem. 282 24689-24696 [DOI] [PubMed] [Google Scholar]
- 29.Boosalis, M. S., Petruska, J., and Goodman, M. F. (1987) J. Biol. Chem. 262 14689-14696 [PubMed] [Google Scholar]
- 30.Pflugrath, J. W. (1999) Acta Crystallogr. D Biol. Crystallogr. 55 1718-1725 [DOI] [PubMed] [Google Scholar]
- 31.McCoy, A. J., Grosse-Kunstleve, R. W., Storoni, L. C., and Read, R. J. (2005) Acta Crystallogr. D Biol. Crystallogr. 61 458-464 [DOI] [PubMed] [Google Scholar]
- 32.Emsley, P., and Cowtan, K. (2004) Acta Crystallogr. D Biol. Crystallogr. 60 2126-2132 [DOI] [PubMed] [Google Scholar]
- 33.Winn, M. D., Murshudov, G. N., and Papiz, M. Z. (2003) Methods Enzymol. 374 300-321 [DOI] [PubMed] [Google Scholar]
- 34.Murshudov, G. N., Vagin, A. A., and Dodson, E. J. (1997) Acta Crystallogr. D Biol. Crystallogr. 53 240-255 [DOI] [PubMed] [Google Scholar]
- 35.Delano, W. L. (2002) PyMol, Delano Scientific, Palo Alto, CA
- 36.Tissier, A., McDonald, J. P., Frank, E. G., and Woodgate, R. (2000) Genes Dev. 14 1642-1650 [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang, Y., Yuan, F., Wu, X., and Wang, Z. (2000) Mol. Cell. Biol. 20 7099-7108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kunkel, T. A., and Loeb, L. A. (1979) J. Biol. Chem. 254 5718-5725 [PubMed] [Google Scholar]
- 39.Rabkin, S. D., Moore, P. D., and Strauss, B. S. (1983) Proc. Natl. Acad. Sci. U. S. A. 80 1541-1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson, R. E., Prakash, L., and Prakash, S. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 10466-10471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Washington, M. T., Minko, I. G., Johnson, R. E., Wolfle, W. T., Harris, T. M., Lloyd, R. S., Prakash, S., and Prakash, L. (2004) Mol. Cell. Biol. 24 5687-5693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolfle, W. T., Johnson, R. E., Minko, I. G., Lloyd, R. S., Prakash, S., and Prakash, L. (2005) Mol. Cell. Biol. 25 8748-8754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolfle, W. T., Johnson, R. E., Minko, I. G., Lloyd, R. S., Prakash, S., and Prakash, L. (2006) Mol. Cell. Biol. 26 381-386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alt, A., Lammens, K., Chiocchini, C., Lammens, A., Pieck, J. C., Kuch, D., Hopfner, K. P., and Carell, T. (2007) Science 318 967-970 [DOI] [PubMed] [Google Scholar]
- 45.Lone, S., Townson, S. A., Uljon, S. N., Johnson, R. E., Brahma, A., Nair, D. T., Prakash, S., Prakash, L., and Aggarwal, A. K. (2007) Mol. Cell 25 601-614 [DOI] [PubMed] [Google Scholar]
- 46.Romani, A. (2007) Arch. Biochem. Biophys. 458 90-102 [DOI] [PubMed] [Google Scholar]
- 47.Kehres, D. G., and Maguire, M. E. (2003) FEMS Microbiol. Rev. 27 263-290 [DOI] [PubMed] [Google Scholar]
- 48.Kehres, D. G., Zaharik, M. L., Finlay, B. B., and Maguire, M. E. (2000) Mol. Microbiol. 36 1085-1100 [DOI] [PubMed] [Google Scholar]