Abstract
The accumulation of unfolded proteins in the endoplasmic reticulum (ER) is caused by many disease-relevant conditions, inducing conserved signaling events collectively known as the unfolded protein response. When ER stress is excessive or prolonged, cell death (usually occurring by apoptosis) is triggered. We undertook a chemical biology approach for investigating mechanisms of ER stress-induced cell death. Using a cell-based high throughput screening assay to identify compounds that rescued a neuronal cell line from thapsigargin-induced cell death, we identified benzodiazepinones that selectively inhibit cell death caused by inducers of ER stress (thapsigargin and tunicamycin) but not by inducers of extrinsic (tumor necrosis factor) or intrinsic (mitochondrial) cell death pathways. The compounds displayed activity in several cell lines and primary cultured neurons. Mechanism of action studies revealed that these compounds inhibit ER stress-induced activation of p38 MAPK and kinases responsible for c-Jun phosphorylation. Active benzodiazepinones suppressed cell death at the level of apoptotic signal kinase-1 (ASK1) within the IRE1 pathway but without directly inhibiting the kinase activity of ASK1 or >400 other kinases tested. Rather, active compounds enhanced phosphorylation of serine 967 of ASK1, promoting ASK1 binding to 14-3-3, an event associated with suppression of ASK1 function. Reducing ASK1 protein expression using small interfering RNA also protected cells from ER stress-induced apoptosis, confirming the importance of this protein kinase. Taken together, these findings demonstrate an essential role for ASK1 in cell death induced by ER stress. The compounds identified may prove useful for revealing endogenous mechanisms that regulate inhibitory phosphorylation of ASK1.
The endoplasmic reticulum (ER)2 plays essential roles in multiple cellular processes required for cell survival and normal cellular function. Multiple disturbances can cause pathological accumulation of unfolded proteins in the ER, triggering an evolutionarily conserved response termed the “unfolded protein response” (UPR) (reviewed in Refs. 1, 2). ER stresses that induce the UPR include the following: (a) disturbances in cellular redox regulation caused by hypoxia, oxidants, or reducing agents that interfere with disulfide bonding of proteins in the lumen of the ER; (b) glucose deprivation, probably by interfering with N-linked protein glycosylation in the ER; (c) aberrations of Ca2+ regulation that impair the functions of Ca2+-dependent ER chaperones such as Grp78, Grp94, and calreticulin; (d) viral infections, which overload the ER with virus-encoded proteins; (e) high fat diet; and (f) inclusion body diseases typical of most chronic neurodegenerative diseases as well as disorders such as inclusion body myositis, which indirectly cause accumulation of unfolded proteins in the ER perhaps by exhausting proteasome capacity (3–5). UPR-associated signaling events are initially intended to reestablish homeostasis and normal ER function, predominantly by activating transcriptional programs that induce expression of genes capable of enhancing the protein folding capacity of the ER and genes for ER-assisted degradation to help clear the ER of unfolded proteins and export them to the cytosol for degradation. However, when the adaptive mechanisms triggered by the UPR fail to compensate, cell death is often induced, typically by apoptosis (reviewed in Refs. 2, 5). Indeed, ER stress-induced cell death has been implicated in a wide variety of diseases, including ischemic injury (stroke and myocardial infarction), heart failure, several neurodegenerative diseases, diabetes, and bipolar disorder, among others (reviewed in Refs. 4, 5). Thus, the identification of small molecule compounds that inhibit cell death induced by ER stress may provide new therapeutic opportunities for a variety of diseases.
The predominant signaling pathways associated with the UPR are initiated by the ER membrane-associated proteins, Ire1α, PERK, and ATF6 (reviewed in Ref. 1). Among these three major UPR signal transduction pathways, the Ire1α pathway has been most clearly connected to cell death, because it leads to activation of stress kinases, including JNK, which is known to phosphorylate and thereby modulate the activities of several Bcl-2 family proteins (6–9). Additionally, downstream elements of all three of these UPR pathways converge on the promoter of the CHOP gene, a bZIP family transcription factor that reportedly induces expression of the pro-apoptotic protein Bim and suppresses expression of anti-apoptotic protein Bcl-2 (8, 10), events that have been causally linked to ER stress-induced apoptosis.
In this study, we describe the identification by high throughput screening (HTS) of benzodiazepinone derivatives that selectively suppress cell death induced by ER stress, without impacting other cell death pathways. These benzodiazepinone compounds inhibit biochemical events associated with the Ire1α pathway, including modulating phosphorylation of apoptotic signaling kinase-1 (ASK1) and inhibiting downstream activation of JNK and p38 MAPK.
EXPERIMENTAL PROCEDURES
Additional experimental procedures are provided as supplemental material.
Reagents—The 50,000-compound Chembridge DIVERset chemical library, salubrinal (ID-5147990, 3-phenyl-N-(2,2,2-trichloro-1-{[(8-quinolinylamino)carbonothioyl] amino}ethyl) acrylamide), and follow up compounds were obtained from Chembridge (San Diego). Thapsigargin (TG) was purchased from Axxora (San Diego). Cycloheximide, VP16, staurosporine, and tunicamycin were from Sigma. TNF-α was from R & D Systems (Minneapolis, MN). ATPlite assay kit was from PerkinElmer Life Sciences. Phosphate-buffered saline (PBS: 3.2 mm Na2HPO4, 0.5 mm KH2PO4, 1.3 mm KCl, 135 mm NaCl, pH 7.4) was from Invitrogen.
Cell Culture—CSM14.1, SNB19, 293T, HeLa, and PPC-1 cells were maintained in DMEM supplemented with 10% FBS, 100 μg/ml streptomycin, 100 IU penicillin, and 1% l-glutamine. OVCAR5, Jurkat, and SW1 cells were maintained in RPMI 1640 medium with 10% FBS, 100 μg/ml streptomycin, 100 IU penicillin, and 1% l-glutamine. PC12 cells were maintained in F-12/DMEM (50:50) with 10% fetal calf serum, 5% FBS, and antibiotics. C17.2 cells were maintained with same media for PC12 cells, but with 10% FBS and 5% fetal calf serum. For routine culture, CSM14.1 cells were incubated at 32 °C, whereas others were at 37 °C.
High Throughput Compound Library Screening—CSM14.1 cells were recovered from cultures by trypsinization and plated in 96-well plates (Greiner catalog number 655074) at 3 × 103 cells per well in 40 μl of phenol red-free DMEM containing 2% FBS, and the plates were incubated overnight at 32 °C in a humidified atmosphere in 95% air, 5% CO2. Using an automated liquid handler (BIOMEK™ FX (Beckman Coulter)), 5 μl of test compounds in 10% DMSO were added to wells to achieve an approximate final concentration of 15 μg/ml. Aliquots of 5 μl of salubrinal (100 μm) in DMSO were delivered to column 1 (wells A–D), whereas 5 μl of 10% DMSO was delivered to column 1 (wells E–H) and column 12 (wells A–H) in every plate. After 2 h of preincubation, all wells except for those in column 12 were treated with 5 μl of TG-containing DMEM to give a final concentration of 15 μm. The wells in column 12 were treated with 5 μl of medium. The plates were returned to the incubator for an additional 20 h. Cell viability was assessed by a cellular ATP content assay (ATPlite, PerkinElmer Life Sciences). The plates were read on an Analyst™ HT (Molecular Device Corp.) multimode plate reader in luminescence mode. Raw values from plates were analyzed in EXCEL software to evaluate survival rates, and imported into CBIS (Chem-Innovation Software, Inc.) for cheminformatics analysis. Wells that received DMSO + TG (column 1, wells F–H) were used as negative controls, whereas wells that received DMSO but no TG (column 12, wells A–H) were used as positive controls for the plate. The % efficacy of rescue for compounds was calculated as follows: % efficacy = ((well value - average of negative control)/(average of positive control - average of negative control))·100 (pubchem.ncbi.nlm.nih.gov). The 384-well assay protocol is available at PubChem (AID = 1222).
Cell Viability Assays—Cell viability was evaluated by cellular ATP levels using a bioluminescence plate reader assay (above) or by annexin V-FITC staining using flow cytometry. For ATP assays, cells at a 105/ml were seeded at 40 μl per well in 96-well polystyrene, flat-bottom, Lumitrac microtiter plates (catalog number 655074, Greiner Bio-One). The next day, cells were preincubated with testing compounds for 2 h, followed by cell death-inducing agents. At various times thereafter, 20 μl of ATPlite solution was added, and ATP luminescence contents were evaluated using an Analyst™ HT (Molecular Devices Corp.) plate reader with Criterion Host analysis software, applying the luminescence mode.
For flow cytometry, undifferentiated CSM14.1 cells at 104 cells per 400 μl of DMEM containing 2% FBS and antibiotics were cultured in 24-well plates (Greiner Bio One). The next day, cells were preincubated with DMSO or 25 μm of test compounds (same final DMSO volume). After 2 h, cells were treated with cell death-inducing agents, and the plates were returned to culture. After indicated times, all cells were recovered from wells by media transfer and trypsinization, Cells were washed with cold PBS and resuspended in 500 μl of 1× annexin V binding buffer (Biovision 1035-100), including 0.25 mg/ml annexin V FITC (Biovision 1001-1000) and propidium iodide (50 μg/ml). The percentage of annexin V-negative/PI negative cells (live) was determined by flow cytometry, using a FACSort analytical instrument (BD Biosciences).
Immunoblotting—Undifferentiated CSM14.1 cells were seeded at a density of 2 × 105/well in 6-well plates (Falcon). After overnight incubation at 32 °C, cells were treated with 15 μm TG for 2 h (c-Jun, p38 MAPK, eIF2α) or for 8 h (ATF-6 and CHOP), with either DMSO or compounds in DMSO added 2 h previously. Cell extracts were generated by adding lysis buffer containing 50 mm Tris-Cl, pH 7.4, 1% Nonidet P-40, 150 mm NaCl, 1 mm MgCl2, 1 mm Na3VO4, 2.5 mm β-glycerophosphate, 0.5% deoxycholic acid, 0.1% SDS, and protease inhibitor mixture (Roche Applied Science) to collected cells. The extracts were centrifuged, and supernatants were recovered. After protein concentration determination, and boiling in an equal volume of Laemmli gel loading solution, 50 μg of cell extract was analyzed by SDS-PAGE/immunoblotting, using polyvinylidene difluoride membranes (Millipore). Antibodies employed for immunoblotting included the following: anti-GST antibody (produced in our laboratory); anti-HA antibody (Roche Applied Science); anti-CHOP antibody (Sc-7351), anti-c-Jun antibody (Sc-1694), anti-p38 MAPK antibody (Sc-728), anti-ASK1 antibody (Sc-7931), and anti-eIF2α antibody (Sc-11386) (Santa Cruz Biotechnology); anti-phospho c-Jun antibody (9164), anti-phospho p38 MAPK antibody (9216), anti-phospho-eIF2α (3597) antibody, anti-phospho-ASK1 (Ser-967) antibody (3764), anti-phospho-ASK1 (Ser-83) antibody (3761), anti-phospho-ASK1 (Thr-845) antibody (3765), and anti-14-3-3 antibody (9639) (Cell Signaling Technology, Danvers, MA); and anti-ATF-6 antibody (IMG-273) (Imgenex, San Diego). Proteins were detected using various secondary antibodies by Super Signal (West Pico) and chemiluminescence substrate (Pierce) with exposure to x-ray film.
Transfections—293T cells were seeded at a density of 1 × 106 cells/well in 6-well plates. After overnight incubation, cells were transfected with 0.4 μg of pcDNA-HA-ASK1 and cultured for 18 h. Cells were preincubated with 100 μm of test compounds for 2 h prior to culture with or without TG. Alternatively, 293T cells in 6-well plates were transfected with pcDNA-HA-ASK1 (0.5 μg) and pEBG-GST-14-3-3 (0.5 μg). The following day, cells were preincubated with DMSO or 100 μm compounds in DMSO for 2 h before TG (20 μm) treatment.
GST Pulldown and Immunoprecipitation Assays—For binding of ASK1 with 14-3-3, extracts were prepared from transfected cells by adding lysis buffer containing 40 mm Tris-Cl, pH 7.4, 1% Nonidet P-40, 150 mm NaCl, 1 mm MgCl2, 1 mm Na3VO4, 2.5 mm β-glycerophosphate, and protease inhibitor mixture (Roche Applied Science) to collected cells and normalized for total protein content, and GST-14-3-3 protein was recovered using glutathione sulfotransferase 4B-Sepharose beads. Beads were washed and then analyzed by SDS-PAGE/immunoblotting using various antibodies. For analysis of endogenous binding of ASK1 to 14-3-3, lysates were prepared from 100-mm dishes of OVCAR5 and SNB19 cells using the same lysis buffer, to which 1 μg of anti-ASK1 antibody was added for immunoprecipitation, and the resulting immune complexes were analyzed by immunoblotting using anti-14-3-3 antibody.
Primary Cortical Neurons—Primary cortical neurons were prepared from the midbrain of mice. Cerebral cortex was dissected from E16 mouse embryos and was incubated with 0.05% trypsin/EDTA (Invitrogen) for 30 min at 37 °C. After mechanical dissociation by polished Pasteur tube, cortical cells were suspended in neural plating medium as follows: DMEM high glucose (Hyclone) medium containing 12.5% defined FBS (Hyclone), 2 mm glutamine (Invitrogen), 12.5% nutrient mixture/Ham's F-12 (Hyclone), 100 IU of penicillin, and 100 μg/ml streptomycin (Invitrogen) at a density of 5 × 105 cells per well in 6-well plates on sterile coverslips. The medium was replaced with B27-neurobasal medium consisting of neurobasal medium (Hyclone) containing 2% B27 (Invitrogen) and 0.8 mm l-glutamine (Invitrogen) after 24 h. After 14 days of maturation in culture, medium was changed to an alternative neurobasal media (Invitrogen). After 1 day, cells were preincubated with DMSO (0.2%) or 25 μm of compound CID-2891837 for 2 h, prior to treating neurons for 18 h with 5 μm TG. Cells were fixed on glass coverslips using 4% paraformaldehyde and permeabilized with 0.5% Triton X-100 in PBS. After washing in cold PBS, cells were blocked by 5% horse serum in PBS for 1 h, then washed with cold PBS, and incubated with either anti-NeuN monoclonal antibody (MAB377) (Chemicon, Billerica, MA) or anti-MAP2 monoclonal antibody (M4403) (Sigma) with 5% horse serum in PBS. Cells were washed in cold PBS and incubated with Texas Red-conjugated horse anti-mouse IgG (Vector Laboratories) at 1:500 (v/v) dilution in PBS with 2% horse serum for an additional 1 h. Hoechst dye (5 μg/ml) was added for the final 5 min. After washing again, slides were mounted for UV microscopy. Apoptotic neurons were quantified by the percentage of immunostained cells that have both condensed nuclei by Hoechst dye and retracted neurites stained by MAP2 antibody, counting a minimum of >250 cells per sample.
RNAi Experiments—For cell survival assay, HeLa cells were seeded at 1.5 × 103 cells/well in 384-well plates. For efficiency check, cells were seeded at 1 × 106 cells/well in 6-well plates and cultured overnight. The cells were transfected with 10 nm scrambled (siCtrl) or siRNA targeting ASK1 for 48 h using Lipofectamine RNAimax (Invitrogen). The sense sequences of siRNA reagents were siCtrl (5′-GACGCGATCAGAGAGTAAT-3′), siASK1 number 1 (5′-GGTGGCACAAGCAAGTTCT-3′), and siASK1 number 2 (5′-GGTATACATGAGTGGAATT-3′). To check efficiency of RNA interference, ASK1 siRNA-transfected HeLa cells were transfected with pcDNA-HA-ASK1, and 1 day later, cell extracts were prepared, and protein concentration was determined and 50 μg subjected to immunoblotting using anti-HA antibody.
RESULTS
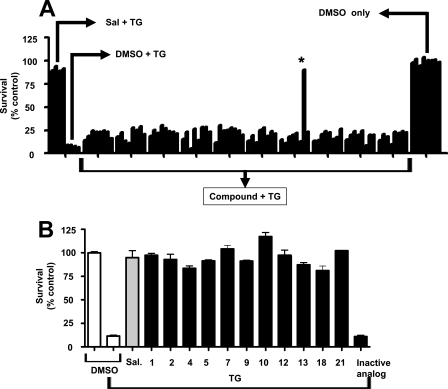
Cell-based Screen for Inhibitors of ER Stress-induced Cell Death—Because cell death linked to ER stress is a prominent feature of several neurological diseases, we focused on developing a primary HTS assay utilizing neuronal cells. CSM14.1 is a rat striatal neuroprogenitor cell line that was established by immortalization using a temperature-sensitive variant of SV40 large T antigen (11). For convenience, and because transient reductions in temperature that might be associated with large screening experiments could restore large T activity, we developed our HTS assay using undifferentiated CSM14.1 cells, with the plan to then confirm hits using differentiated cells. For monitoring cell death, we used a commercially available bioluminescence reagent that determines intracellular ATP content (ATPlite, PerkinElmer Life Sciences). To trigger cell death using a stimulus known to induce ER stress, we selected thapsigargin (TG), a sesquiterpene lactone that irreversibly inhibits the Ca2+-ATPase of the ER (12). As a positive control compound, we employed salubrinal, a compound previously reported to block ER stress-induced cell death (13). Salubrinal (100 μm) significantly protected against TG-induced cell death using undifferentiated CSM14.1 cells and measuring ATP as a surrogate for cell viability (Fig. 1).
FIGURE 1.
Identification of cytoprotective benzodiazepinone by HTS. A, example of HTS screening results is provided. A graphical representation is provided where normalized relative ATP content (y axis) is plotted against well number (1–96 (A1 → H12)) (x axis). Wells A1 to D1 are active compound controls (received salubrinal + TG); wells E1 to H1 are assay negative controls (received DMSO + TG); wells A12 to H12 (column 12) are assay-positive controls (received DMSO without TG). The Z′ for the plate is 0.87. A hit compound is found in well G8 (asterisk). B, 11 benzodiazepinone hit compounds and an inactive analogue (IA-1) (see Table 1) were compared with respect to protection against thapsigargin-induced cell death using undifferentiated CSM14.1 cells. Data represent relative survival measured by ATP content and expressed as % control relative to cells cultured with DMSO and without TG. Sal = salubrinal.
A plate-based HTS assay was established in 96- and 384-well formats, with good assay performance characteristics (Z′ factors = 0.7) (PubChem AID = 1010 and AID = 1222). The NIH library of ∼187,265 compounds was screened at a single concentration of 20 μm (PubChem AID 1222), but no viable hits were identified. We also screened a commercially available library of 50,000 small molecule compounds (ChemBridge) at an average concentration of 15 μg/ml (∼20–50 μm), from which 198 primary hit compounds were identified using the primary HTS assay (work flow for hit evaluation is depicted in supplemental Fig. 1). Of these, 93 compounds were confirmed upon re-testing. Dose-response studies resulted in 30 compounds that displayed appropriate dose-dependent cytoprotection with EC50 values <25 μm. Fresh stocks of the 30 compounds were purchased, and 26 were confirmed to be active, with similar potency to the originally tested material, whereas 4 showed only weak activity. All 26 active compounds were confirmed to be cytoprotective for undifferentiated CSM14.1 cells treated with TG by an orthogonal assay, in which cell viability was assessed by staining of cells with fluorescein-conjugated annexin V.
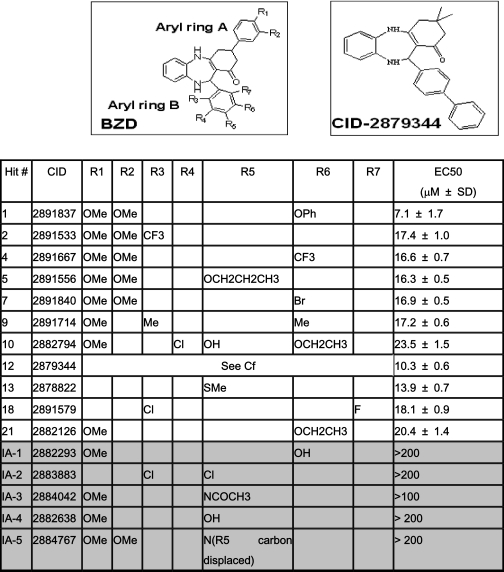
Cheminformatics data analysis revealed that 11 of the 26 confirmed hits constituted a series of 11 structurally related benzodiazepinones (EC50 values ranging from 10 to 25 μm), and thus we focused our efforts on this compound series. Compound CID-2891837 was the most potent (EC50 = 7.1 μm) of the 11 benzodiazepinones (Fig. 1B and Table 1) (PubChem-1247). To confirm the activity of the 11 benzodiazepinone compounds, we tested their efficiency for protecting differentiated neuronal CMS14.1 cells against TG-induced cell death. Although all of them showed cytoprotective activity, 2 of the 11 benzodiazepinone derivatives (CID-2882794, CID-2891579; hits 10 and 18) were less active when tested in differentiated CSM14.1 cells (supplemental Fig. 2), and thus we focused on the remaining nine compounds.
TABLE 1.
Structures of cytoprotective benzodiazepinones Undifferentiated CSM14.1 cells were treated with thapsigargin (15 μm) and with various concentrations of hit compounds (PubChem, pubchem.ncbi.nlm.nih.gov). Cellular ATP levels were measured in triplicate, and data were normalized to calculate the mean (±S.D.) concentration of compounds required to maintain 50% cell survival following treatment with thapsigargin (EC50). Shaded region (bottom) indicates inactive and white (top) represents active compounds. The structure of the benzodiazepinone (BZD) scaffold is presented, indicating seven diversity positions (R1–R7). The structure of compound CID-2879344 is also shown.
To establish that the hit compound series displayed a clear structure-activity relationship, 41 analogues of CID-2891837 were acquired from commercial sources (data not shown). Compounds were tested using TG-treated undifferentiated CSM14.1 cells, measuring viability based on ATP levels, and rank-ordered according to potency. The initial benzodiazepinone pharmacophore has two aryl rings (A and B) with multiple diversity positions (R1–R7) as illustrated in Table 1. Preliminary analysis of structure-activity relationships across the analogues suggests that the benzodiazepinone scaffold tolerates a number of different substituents on either of the pendant aryl rings A and B without loss of cytoprotective activity. The structure-activity relationship is rather flat, with EC50 values of the active analogues (EI-1 to EI-21) ranging from ∼7 to 24 μm. Within the series of active analogues aryl ring A may be unsubstituted, mono-, or di-substituted with methoxy or may be replaced entirely with dimethyl, as in CID-2879344. Any of the positions on aryl ring B (R3–R7) may be substituted to give active analogues, with favored substituents, including methyl, halogen, trifluoromethyl, phenyl, alkoxy, phenoxy, and thiomethyl. Interestingly, however, the inactive analogues (IA-1 to IA-5) are either substituted at the para position (R5) of aryl ring B, contain a polar substituent such as OH (as in IA-1), or aryl ring B is electron-deficient pyridyl (as in IA-5). Although we currently do not have any data on the relative ability of the compounds to enter the cells, lack of cell penetration may in part explain the inactivity of compounds IA-1 to IA-5 (Table 1).
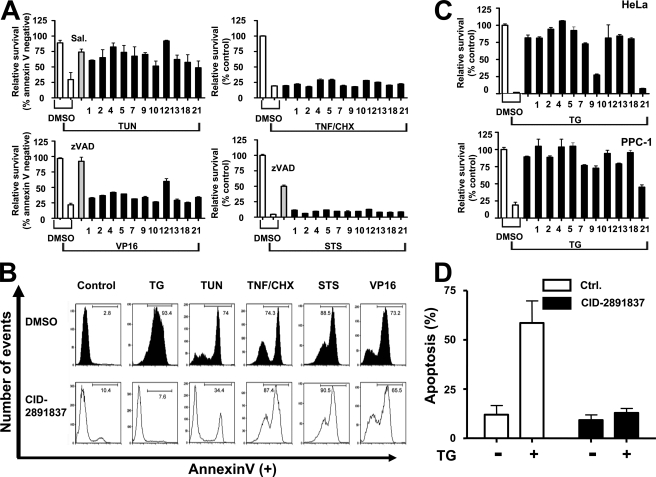
Benzodiazepinone Compounds Selectively Inhibit ER Stress-induced Cell Death—Cell death can be initiated from several locations in cells, besides the ER, and ER stress can be induced by many stimuli, besides TG. To determine whether the benzodiazepinone derivatives are general inhibitors of cell death versus ER-selective or thapsigargin-specific inhibitors, we compared their cytoprotective activity when CSM14.1 cells were challenged with a variety of cell death-inducing stimuli, including tunicamycin (N-linked glycosylation inhibitor and inducer of ER stress), VP16/etoposide (DNA-damaging agent, which activates mitochondrial pathways for cell death), staurosporine (STS) (broad spectrum protein kinase inhibitor), and tumor necrosis factor (TNF) (which activates a plasma membrane death receptor). When tested on undifferentiated CSM14.1 cells, the 11 active benzodiazepinone compounds inhibited tunicamycin-induced cell death, but did not inhibit cell death triggered by VP16, STS, or TNF (Fig. 2A). An example of flow cytometry analysis of FITC-annexin V staining is shown in Fig. 2B, for undifferentiated CSM14.1 cells cultured with DMSO solvent control versus compound CID-2891837, and treated with various cell death stimuli. Thus, the benzodiazepinone compound series appears to be selective for suppression of cell death induced by ER stress. For comparison, salubrinal was tested against the same cell death-inducing reagents. Similar to our hit compounds, salubrinal did not protect against cell death induced by VP16, STS, or TNF/CHX, although it did inhibit cell death induced by the ER stress-inducing agent TG (supplemental Fig. 10).
FIGURE 2.
Benzodiazepinones selectively protect against cell death induced by ER stress. A, undifferentiated CSM14.1 cells were cultured in 96-well plates (for ATP assay) or in 24-well plates (for annexin-V staining/flow cytometry). The next day, cells were treated with DMSO (0.5%) or hit compounds (25 μm compound with 0.5% DMSO final concentrations) for 2 h, followed by treatment of various cell death-inducing reagents, including 15 μm thapsigargin (TG) for 24 h, 10 μg/ml tunicamycin (TUN) for 72 h, 2.5 μm staurosporine (STS) for 24 h, 50 μm VP16 for 48 h, or 30 ng/ml TNF plus 10 μg/ml cycloheximide (CHX) for 24 h. Cellular ATP content was measured for staurosporine and TNF/CHX samples, normalizing data relative to cells treated with DMSO alone and presented as percentage of control (mean ± S.D., n = 3). For measuring cell viability in cultures treated with tunicamycin or VP16, cells were stained with FITC-annexin V and PI and then analyzed by flow cytometry to identify live cells (annexin V-negative plus PI-negative). Assays were performed at least three times and expressed as % control relative to cells culture with DMSO without TG or compounds (mean ± S.D., n = 3). z, benzyloxycarbonyl. B, pathway selectivity of a hit compound (CID-2891837) is demonstrated as an example. Experiments were performed as in A. Cell viability was assessed by annexin-V-FITC staining, followed by fluorescence-activated cell sorter analysis. Data represent relative fluorescence (x axis) versus cell number (y axis). C, benzodiazepinone compounds protect against TG-induced cytotoxicity in HeLa and PPC-1 cells. Data were generated as in A. D, primary cortical neuron cells were preincubated with DMSO or 25 μm compound CID-2891837 in DMSO for 2 h. TG was treated at 5 μm for 18 h prior to determining the percentage of apoptotic neurons (marked by immunostaining) based on Hoechst dye staining (mean ± S.D., n = 3).
To explore the range of cell types in which the benzodiazepinone compounds show cytoprotective activity, we tested seven different cell lines under 10 conditions with respect to cell death induced by TG (supplemental Table 1). For all experiments, compounds were tested at a concentration of 25 μm. In addition to the aforementioned results for undifferentiated and differentiated CSM14.1 rat neuronal cells, the benzodiazepinone compounds showed cytoprotective activity (≥40% survival compared with DMSO control) for HeLa (human cervical carcinoma) (9/11 compounds), PPC1 (human prostate cancer) (10/11 compounds), and SW1 (mouse melanoma) (6/11 compounds) cells (Fig. 2C and data not shown).
In other neuronal cell lines such as differentiated PC12 (rat pheochromocytoma), and differentiated C17.2 (mice neuronal stem cell), the hit compounds showed more variable activity. In differentiated PC12 cells, 2 were active (>40% survival), 4 were partially active (20–40% more survival), and 5 were inactive (supplemental Table 1). In differentiated C17.2 cells, 1 was active (>40% survival), whereas others showed no activity. Moreover, when applied to some types of cells, Jurkat cells (human leukemia), and undifferentiated PC12 cells, compounds showed paradoxical effects, increasing TG-induced cell death. Nevertheless, one of the compounds showed cytoprotective activity against 7 of 10 (70%) of established cell line conditions and 5 showed activity against 5 of 10 (50%) cell line conditions tested (supplemental Table 1).
To extend these studies to primary cells, we examined compound activity against TG-treated primary mouse cortical neurons. After exposure to 5 μm TG for 18 h, primary cortical neuron cells showed retracted neurites and condensed nuclei, typical of apoptosis (data not shown). More than half of primary neurons treated with TG and DMSO (control) became apoptotic within 18 h, whereas neuronal apoptosis was not significantly above background levels (untreated cells) in cultures treated with TG and compound CID-2891837 (Fig. 2D). We conclude therefore that active benzodiazepinones such as CID-2891837 have neuroprotective activity.
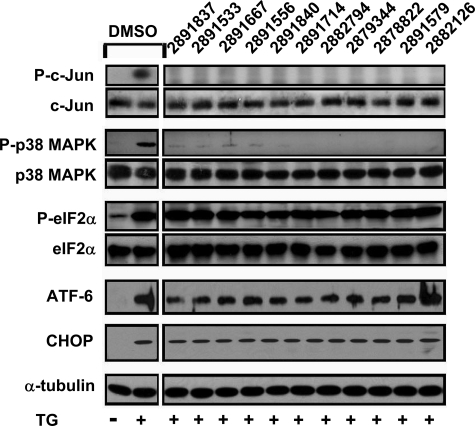
Mapping the Cell Death Pathway Inhibited by Benzodiazepinone Compounds—ER stress induces UPR signal transduction pathways, which are initiated by the ER membrane-associated proteins Ire1α, PERK, and ATF6 (reviewed in Ref. 2). We used immunoblotting methods to assess the impact of active benzodiazepinone derivatives on each of these signaling pathways. Phosphorylation of c-Jun (substrate of JNKs) and p38 MAPK occurs downstream of Ire1α activation. All active compounds suppressed phosphorylation of c-Jun and p38 MAPK in TG-treated CSM14.1 cells (Fig. 3). In contrast, phosphorylation of eIF2α at serine 51 and induction of ATF4 expression (which occur downstream of PERK activation) and proteolytic processing of ATF6 were not modulated by cytoprotective benzodiazepinones (Fig. 3 and data not shown). All three of the major signaling components of the UPR collaborate to induce expression of CHOP, a C/EBP family transcription factor implicated in apoptosis induction in the context of ER stress. In CSM14.1 cells, CHOP protein expression was not inhibited by cytoprotective benzodiazepinones (Fig. 3). Taken together, these data suggest that the cytoprotective benzodiazepinone derivatives selectively inhibit signaling events associated with the branch of the Ire1α pathway that leads to stress kinase activation.
FIGURE 3.
Benzodiazepinones inhibit ER stress-induced JNK and p38 MAPK activation. CSM14.1 cells were cultured with DMSO or with 25 μm hit compounds in DMSO for 2 h, followed by treatment of TG (15 μm). Cell lysates were prepared and analyzed by SDS-PAGE/immunoblotting using antibodies specific for phospho-c-Jun (Ser-73), c-Jun, phospho-p38 MAPK (Thr-180/Tyr-182), p38 MAPK, phospho-eIF2α (Ser-51), eIF2α, ATF6 (showing the cleaved form), CHOP, and α-tubulin. Control samples (left panel) correspond to cells treated with DMSO alone or DMSO plus TG.
We further interrogated the effects of the cytoprotective benzodiazepinone derivatives on the Ire1α pathway. Active benzodiazepinones suppressed TG-induced phosphorylation of c-Jun (at serine 73) and p38 MAPK in cells in a concentration-dependent manner, correlating with cytoprotective activity, with little inhibition of phosphorylation of c-Jun and p38 MAPK observed at 1 μm, partial at 5 μm, and potent (>80% suppression) at 10 μm (supplemental Figs. 3 and 4). However, in vitro kinase assays showed that benzodiazepinone compounds were not direct inhibitors of JNK1, JNK2, or p38 MAPK (supplemental Fig. 6), suggesting they block the Ire1α pathway upstream of these stress-induced kinases. Moreover, active benzodiazepinones did not block cellular phosphorylation of c-Jun or p38 MAPK induced by UV-irradiation or by TNF (supplemental Fig. 5), suggesting that they selectively block stress kinase activation induced by ER stress.
Ire1α possesses both an intrinsic protein kinase activity (believed to initiate stress kinase activation) and an endoribonuclease activity that results in production of XBP-1 protein, a transcription factor (2). However, in assays measuring in vitro kinase activity of Ire1α, the active benzodiazepinone compounds failed to inhibit Ire1 α activity, as measured by in vitro autophosphorylation in kinase assays. Active benzodiazepinones also did not interfere with splicing of XBP-1 mRNA in cells stimulated with TG, as determined by reverse transcriptase-PCR where unspliced and spliced mRNAs were compared (supplemental Figs. 6 and 9). Thus, these compounds do not appear to inhibit Ire1α.
Operating between Ire1α and MAPKs (JNKs/p38 MAPK) are the kinases ASK1 (a MAPKKK) and MKK6 (a MAPKK upstream of p38 MAPK) (6). In vitro kinase assays showed that active benzodiazepinones are not direct inhibitors of ASK1 or MKK6 (supplemental Fig. 6). In addition, the active benzodiazepinone CID-3130510 (MLS-0315763) was tested in a panel of kinase assays (the Ambit KinomeScan) (14) and found to be inactive against all 402 protein kinases tested. Thus, the cytoprotective benzodiazepinones described do not appear to exert their effects by direct inhibition of kinases.
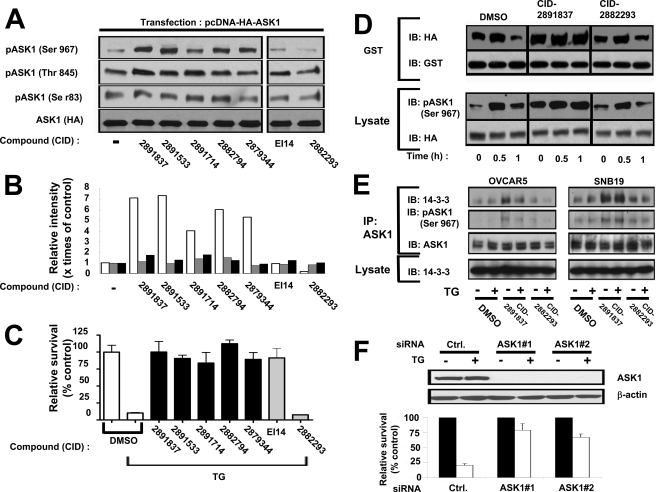
Enhanced Phosphorylation of ASK1 at Serine 967 and Interaction with 14-3-3 Protein in Cells Treated with Cytoprotective Benzodiazepinones—Although cytoprotective benzodiazepinones did not inhibit in vitro kinase activity of ASK1, we observed striking changes in ASK1 phosphorylation in cells treated with these compounds (Fig. 4). ASK1 phosphorylation sites include the following: (a) serine 83, which attenuates activity (15); (b) threonine 845, which enhances activity (16); and (c) serine 967, which reduces activity of ASK1 by promoting its binding to 14-3-3 family proteins (17). By phospho-specific antibody immunoblotting, we observed that cells cultured with active benzodiazepinone derivatives (CID-2891837, CID-2891533, CID-2891714, CID-2882794, and CID-2879344 were specifically tested) increased serine 967 phosphorylation of ASK1, whereas threonine 845 and serine 83 were not modulated (Fig. 4, A and B). This effect of the benzodiazepinone series compounds was specific, in that ASK1 phosphorylation was modulated neither by a structurally related but inactive (noncytoprotective) benzodiazepinone (CID-2882293) nor by another hit compound (EI-14) that inhibits cell death induced by ER stress but that is structurally unrelated (Fig. 4C).
FIGURE 4.
Benzodiazepinone compounds increase Ser-967 phosphorylation of ASK1 and association with 14-3-3. A, phosphorylation of ASK1 at various sites was monitored by phospho-specific antibody immunoblotting. 293T cells were transfected with pcDNA-HA-ASK1, and 1 day later the cells were incubated with DMSO or 100 μm compounds in DMSO for 2 h. Compounds included five active benzodiazepinone hits (CID-2891837, CID-2891533, CID-2891714, CID-2882794, and CID-2879344), an active hit compound representing another chemical class with a different mechanism (EI14), and an inactive benzodiazepinone analogue (CID-2882293). Cell extracts were prepared and subjected to immunoblotting using anti-phospho-ASK1 antibodies specific for Ser-83, Thr-845, or Ser-967 or with anti-HA antibody. B, relative densities of phosphorylated ASK1 bands (gray = Ser-83; black = Thr-845; white = Ser-967) were determined by densitometry mode of ImageJ software and normalized relative to ASK1 protein, calculating intensity relative to cells cultured with DMSO without TG. C, compounds from A were compared with respect to cytoprotective activity against TG-induced killing of undifferentiated CSM14.1 cells. Active benzodiazepinone compounds are represented by black bars, and controls are depicted with gray bars. Relative cell viability was based on measurements of ATP content (mean ± S.D., n = 3). D, 293T cells were transfected with pcDNA-HA-ASK1 and pEBG-GST-14-3-3, and then 1 day later cells were incubated with DMSO, CID-2891837 (active compound) or CID-2882293 (inactive compound) in DMSO for 2 h, before TG (20 μm) treatment for various times as indicated. Cell extracts were prepared, and GST-14-3-3 protein was recovered using glutathione-Sepharose, followed by immunoblotting (IB) analysis using anti-HA antibody to detect HA-ASK1 protein and anti-GST antibody to confirm pulldown of comparable amounts of GST-14-3-3 protein. Aliquots of the same cell lysates (50 μg) were analyzed by immunoblotting using anti-phospho-ASK1(Ser-967) and HA antibodies. E, benzodiazepinone compounds modulate interaction of endogenous ASK1 and 14-3-3 in OVCAR5 and SNB19 cells. Cells (5 × 106) were cultured in 100-mm dishes and incubated overnight, and then compound CID-2891837 (active), CID-2882293 (inactive), or DMSO was added, followed 2 h later by TG (20 μm) treatment for 30 min. Cell extracts were prepared and normalized for total protein content, and endogenous ASK1 protein was immunoprecipitated (IP). Immune complexes were analyzed by immunoblotting using antibodies specific for ASK1, ASK1 (phospho-Ser-967), or 14-3-3. Aliquots of the cell lysates (50 μg) were analyzed directly by immunoblotting using antibodies specific for 14-3-3 to confirm comparable levels of this protein in all samples. F, HeLa cells were transfected with control (Ctrl.) RNAi (scrambled) or two different RNAi reagents targeting ASK1. After 24 h, cells were treated with 15 μm TG for 24 h for analysis of relative cell viability by ATP content (mean ± S.D., n = 3) (bottom). Because of low endogenous levels of ASK1 protein, ASK1 siRNA-transfected cells were transfected with pcDNA-HA-ASK1, and after 24 h, cells were lysed for analysis of HA-ASK1 and β-actin protein levels by immunoblotting (top), to confirm RNAi efficiency.
Examination of the effects of ER stress inducer TG on serine 967 phosphorylation of overexpressed ASK1 showed an initial increase at ∼0.5 h followed by a decline at ∼1 h (Fig. 4D, lower panels, and supplemental Fig. 7). A similar result was obtained when cells were cultured with inactive benzodiazepinone CID-2882293. In contrast, when cells were cultured with active benzodiazepinone CID-2891837, serine 967 phosphorylation was elevated prior to the addition of TG and remained elevated. Thus, active benzodiazepinones disrupt the normal patterns of serine 967 phosphorylation of ASK1.
Because phosphorylation of ASK1 on serine 967 promotes binding to 14-3-3 (17), we tested the effects of active versus inactive compounds on association of ASK1 with 14-3-3. For initial experiments, 293T cells were co-transfected with plasmids encoding HA-tagged ASK1 and GST-tagged 14-3-3 and then treated a day later with or without compounds and TG. Lysates were prepared and subjected to GST pulldown to recover GST-14-3-3 protein, and protein complexes were immunoblotted with anti-HA antibody to detect associated HA-ASK1 protein. In DMSO-treated control cells, TG initially induced an increase at ∼0.5 h followed by a decrease at ∼1 h of HA-ASK1 binding to GST-14-3-3, correlating with the changes in serine 967 phosphorylation (Fig. 4D, upper panels). Similar observations were made using cells treated with inactive benzodiazepinone CID-2882293. In contrast, when cells were cultured with active benzodiazepinone CID-2891837, HA-ASK1 association with GST-14-3-3 was increased, regardless of thapsigargin treatment (Fig. 4D, upper panels).
To extend these studies to endogenous ASK1 and 14-3-3 proteins, we performed experiments using tumor cell lines OVCAR5 and SNB19, which contain higher levels of these proteins compared with 293T cells. Endogenous ASK1 was immunoprecipitated, and bound 14-3-3 was detected by immunoblotting. In these cells, TG had little effect on ASK1 interaction with 14-3-3 but active benzodiazepinone CID-2891837 produced consistent results, causing an increase in the association of endogenous ASK1 with 14-3-3 (Fig. 4E). In contrast, inactive benzodiazepinone CID-2882293 did not increase ASK1 interaction with 14-3-3. When taken together with previous reports that have demonstrated the role of serine 967 of ASK1 in 14-3-3 binding (17), these data suggest that the cytoprotective benzodiazepinone derivatives enhance the binding of ASK1 to 14-3-3 by increasing phosphorylation of serine 967.
If ASK1 is the relevant target of cytoprotective benzodiazepinones, then ablating ASK1 expression should inhibit cell death induced by ER stress inducers such as TG. Indeed, when HeLa cells were transfected using two different RNAi molecules, cell death induced by TG was markedly reduced compared with cells treated with control RNAi reagent (Fig. 4F).
DISCUSSION
The cytoprotective benzodiazepinone series compounds identified here selectively inhibit cell death induced by ER stress, without suppressing other apoptosis pathways. These compounds increase serine 967 phosphorylation of ASK1 and enhance its binding to 14-3-3, correlating with reduced signaling through the Ire1-initiated UPR pathway that results in activation of stress kinases, JNK and p38 MAPK. ASK1 is a multifunctional serine/threonine protein kinase, originally identified as a mitogen-activated protein kinase kinase kinase (MAP3K5) with pro-apoptotic activity (18, 19). ASK1 is reportedly activated by various stress stimuli, including cytokines (TNF) (19, 20), serum deprivation (15), genotoxic chemicals (21), reactive oxygen species (20), and agents that trigger ER stress (6). Cells from ASK1 knock-out mice are resistant to ER stress-induced cell death (6), showing reduced JNK and p38 MAPK activity (7). The activity of ASK1 is regulated by multiple phosphorylation events. Phosphorylation of threonine 845 is necessary for activation of ASK1 in the context of cellular stress, whereas phosphorylation of serine 83 and serine 967 is known to reduce activity. Phosphorylation on serine 967 is required for binding of ASK1 to 14-3-3, reducing ASK1 signaling (17). Additional regulators of ASK1 include the following: (i) AKT, which negatively regulates ASK1 by phosphorylation on serine 83 (15); (ii) thioredoxin, which inhibits ASK1 activity in a redox-dependent manner (22); and (iii) probably TRAF family ubiquitin-protein isopeptide ligases, which induce lysine 63-linked polyubiquitination of target proteins via UBC13 (23).
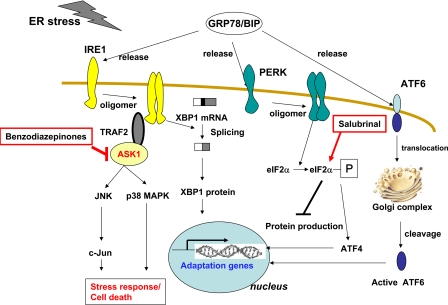
Although we showed physical evidence of compound acting mechanism, the detailed mechanism(s) by which the cytoprotective benzodiazepine compounds described herein modulate ASK1 phosphorylation and activity remain to be elucidated (Fig. 5). The increases in serine 967 phosphorylation induced by these compounds could be due to either inhibition of a protein phosphatase or activation of a serine 967 protein kinase. With respect to phosphatases, calcineurin (PPase3, formerly PPase2B) has been reported to dephosphorylate serine 967 of ASK1 (24). However, we did not observe inhibition of calcineurin by compound CID-2891837 using an in vitro ASK1 dephosphorylation assay (data not shown), implying that calcineurin is not a direct target of cytoprotective benzodiazepinones. Interestingly, the cytoprotective compound salubrinal inhibits ER stress-induced cell death by disrupting a protein phosphatase complex that includes GADD34 and protein phosphatase 1 (PPase-1), thus increasing steady-state levels of serine 51 phosphorylation on phospho-eIF2α, without inhibiting the active site of PPase-1 (Fig. 5). It therefore remains possible that our benzodiazepinones could somehow interfere with calcineurin-mediated dephosphorylation in cells by affecting protein interactions that target this phase onto ASK1. Alternatively, whereas Lys-63-linked ubiquitination and interaction with thioredoxin represent additional potential mechanisms for controlling ASK1 activity, we failed to observe specific effects of active benzodiazepinone compounds on these regulatory events (data not shown).
FIGURE 5.
Illustration showing site of action of cytoprotective benzodiazepinone compounds in comparison with salubrinal in modulating UPR pathways. The active benzodiazepinones, such as prototype compound CID-2891837, interfere with ASK1 by inhibiting dephosphorylation of Ser-967 residue, leading to increased binding with 14-3-3. Inactive ASK1 cannot propagate stress signals to JNK and p38 MAPK. Salubrinal suppresses dephosphorylation of eIF2α.
Benzodiazepines and related compounds have a long history in medicine. First identified in the 1950s, the prototypical benzodiazepines such as chlordiazepoxide (Librium) and diazepam (Valium) were widely employed as anxiolytic and sedative medications. In the 1970s it was shown that these compounds exert their anxiolytic and sedative effects by modulating the γ-aminobutyric acid, type A subtype of ligand-gated ion channels (25). Since then several other molecular targets for benzodiazepines have been identified, including G-protein-coupled receptors (GPCRs) (26–29), proteases (30, 31), and proteins involved in cell proliferation (32, 33). Among the targets of benzodiazepines of relevance to cell death regulation is the peripheral benzodiazepine receptor (PBR), also known as the “transporter protein of mammalian cells” (TSPO), a putative channel-like protein (34). The benzodiazepine Ro-54864, for example, is known to bind TSPO, modulating cell death mechanisms (35), variably promoting either cell survival or cell death, depending on cellular context (36, 37). Although we cannot exclude a role for cytoprotective benzodiazepinones in regulating PBR/TSPO activity, PBR/TSPO is located in the membranes of mitochondria (34), not ER membranes. Furthermore, the cytoprotective benzodiazepinones described here do not block cell death induced via mitochondria-driven stimuli, such as VP16 and STS. Thus, we do not favor PBR as a relevant target of these compounds in terms of their ER stress modulating activity. With respect to GPCRs, β-arrestin 2, a protein involved in GPCR desensitization, has been reported to activate ASK1 (38). Also, the ligand of another GPCR, angiotensin II, has been reported to signal through a ASK1-dependent mechanism in the context of cardiac hypertrophy in mice (39). Thus, cytoprotective benzodiazepinone compounds theoretically could indirectly modulate serine 967 phosphorylation of ASK1 by binding to and regulating the signaling of GPCRs. Differential expression of these hypothetical GPCR might then explain why these compounds displayed cytoprotective activity in some types of cells but not others.
Although the benzodiazepinone compounds identified here showed cytoprotective activity for 7 of 10 cell lines tested and for primary cultured neurons, some cell lines showed paradoxical enhancement of killing induced by ER stress. Interestingly, in these cells, which included human Jurkat T-cell leukemia and PC12 rat pheochromocytoma cells, UPR pathway signaling appeared to be regulated differently. Specifically, in Jurkat and PC12 cells, stress kinases JNK and p38 MAPK were not activated by ER stress inducer TG, and exposure to TG caused a reduction rather than increase in phosphorylation of c-Jun and p38 MAPK (supplemental Fig. 8). In this regard, JNK and p38 MAPK can manifest either pro-apoptotic or anti-apoptotic phenotypes, depending on cellular context (40–43), and gene ablation studies in mice suggest that requirements for JNK family kinases are highly variable among different types of cells (44–46). Also, whereas p38 MAPK was identified as a mediator of apoptosis induced by TNF (47), transforming growth factor-β (48), and reactive oxygen species (49), other studies have suggested an anti-apoptotic role for p38 MAPK in cell death induced by Fas ligand and UV irradiation (50), as well as TNF-induced killing of adipocytes (47), illustrating the importance of cell type and variations in the cell death stimulus applied in defining the phenotype of this protein kinase with respect to cell death regulation.
UPR signaling events linked to cell death also probably vary among cell types and contexts, but many studies point to the Ire1 pathway as a principal mediator, in contrast to the PERK- and ATF6-initiated pathways that are more often associated with cell survival (4, 5). Furthermore, within the Ire1 pathway are two signaling branches that bifurcate from Ire1 to ASK1 versus XBP-1, with XBP-1 often associated with cytoprotection and ASK1 associated with cell death induction. Thus, selectively blocking the Ire1-initiated pathway that leads to ASK1 activation should allow the PERK- and ATF6-initiated UPR signal transduction pathways to provide compensatory functions that help preserve cell survival, whereas avoiding the ASK1-mediated events that tend to promote cell death. Downstream targets of JNK and p38 MAPK relevant to cell death include anti-apoptotic protein Bcl-2, which is inhibited by JNK-mediated phosphorylation, and pro-apoptotic protein Bim, which is activated by JNK-mediated phosphorylation. Also, p38 MAPK mediates stimulatory phosphorylation of CHOP, enhancing its transcriptional activity (51). In this regard, CHOP has been shown to induce expression of the gene encoding Bim while repressing expression of the gene encoding Bcl-2 (8, 10). Indeed, we observed that siRNA targeting Bim protects HeLa cells against thapsigargin-induced cell death,3 similar to siRNA targeting ASK-1, suggesting that ASK1 and Bim contribute to ER stress signals that cause cell death.
Increasing evidence implicates ASK1 in diseases associated with ER stress (6, 52, 53). For example, ASK1 activity is implicated in neuronal cell death in models of Huntington disease and amyotrophic lateral sclerosis (6, 53), as well as in myocardial cell death and cardiac injury in rodent models of myocardial infarction and heart failure (52, 54). The benzodiazepinone compounds reported here provide a starting point for generating potentially therapeutically useful small molecule modulators that suppress ASK1 activity through an indirect mechanism impacting serine 967 phosphorylation, rather than attacking the active site of the kinase. Moreover, these compounds provide research tools for elaborating the circumstances during which the ASK1-activating pathway triggered by ER stress contributes to cell death and for eventually revealing the protein targets that bind these compounds and that are responsible for controlling ASK1 phosphorylation and activity. Thus, future studies of the cytoprotective benzodiazepinones reported here may reveal novel targets for drug discovery and provide new insights into the cellular mechanisms that regulate ASK1 in the context of UPR signaling.
Supplementary Material
Acknowledgments
We thank T. Siegfried and M. Hanaii for manuscript preparation and Michael Hedrick and the staff of the Burnham Center for Chemical Genomics for technical assistance and helpful discussions.
This work was supported, in whole or in part, by National Institutes of Health Grants HG003916, DA024887, and AG15393. This work was also supported by Department of Defense Grant W81XWH-07-1-0569. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Table 1 and Figs. 1–10.
Footnotes
The abbreviations used are: ER, endoplasmic reticulum; UPR, unfolded protein response; HTS, high throughput screening; TNF, tumor necrosis factor; siRNA, small interfering RNA; CHX, cycloheximide; GST, glutathione S-transferase; TG, thapsigargin; PBS, phosphate-buffered saline; FBS, fetal bovine serum; DMEM, Dulbecco's modified Eagle's medium; RNAi, RNA interference; HA, hemagglutinin; FITC, fluorescein isothiocyanate; STS, staurosporine; GPCR, G-protein-coupled receptor; TSPO, transporter protein of mammalian cells; PBR, peripheral benzodiazepine receptor; PPase, protein phosphatase; MAPK, mitogen-activated protein kinase; ASK1, apoptotic signal kinase-1; PI, propidium iodide.
W. S. Chih, K. Inki, and J. C. Reed, unpublished data.
References
- 1.Marciniak, S. J., and Ron, D. (2006) Physiol. Rev. 86 1133-1149 [DOI] [PubMed] [Google Scholar]
- 2.Xu, C., Bailly-Maitre, B., and Reed, J. C. (2005) J. Clin. Investig. 115 2656-2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindholm, D., Wootz, H., and Korhonen, L. (2006) Cell Death Differ. 13 385-392 [DOI] [PubMed] [Google Scholar]
- 4.Yoshida, H. (2007) FEBS J. 274 630-658 [DOI] [PubMed] [Google Scholar]
- 5.Kim, I.-K., Xu, W., and Reed, J. C. (2008) Nat. Drug Discovery Rev. 7 1013-1030 [DOI] [PubMed] [Google Scholar]
- 6.Nishitoh, H., Matsuzawa, A., Tobiume, K., Saegusa, K., Takeda, K., Inoue, K., Hori, S., Kakizuka, A., and Ichijo, H. (2002) Genes Dev. 16 1345-1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tobiume, K., Matsuzawa, A., Takahashi, T., Nishitoh, H., Morita, K., Takeda, K., Minowa, O., Miyazono, K., Noda, T., and Ichijo, H. (2001) EMBO Rep. 2 222-228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puthalakath, H., O'Reilly, L. A., Gunn, P., Lee, L., Kelly, P. N., Huntington, N. D., Hughes, P. D., Michalak, E. M., McKimm-Breschkin, J., Motoyama, N., Gotoh, T., Akira, S., Bouillet, P., and Strasser, A. (2007) Cell 129 1337-1349 [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto, K., Ichijo, H., and Korsmeyer, S. J. (1999) Mol. Cell. Biol. 19 8469-8478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCullough, K. D., Martindale, J. L., Klotz, L. O., Aw, T. Y., and Holbrook, N. J. (2001) Mol. Cell. Biol. 21 1249-1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haas, S. J., and Wree, A. (2002) J. Anat. 201 61-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsukamoto, A., and Kaneko, Y. (1993) Cell Biol. Int. 17 969-970 [DOI] [PubMed] [Google Scholar]
- 13.Boyce, M., Bryant, K. F., Jousse, C., Long, K., Harding, H. P., Scheuner, D., Kaufman, R. J., Ma, D., Coen, D. M., Ron, D., and Yuan, J. (2005) Science 307 935-939 [DOI] [PubMed] [Google Scholar]
- 14.Fabian, M. A., Biggs, W. H., III, Treiber, D. K., Atteridge, C. E., Azimioara, M. D., Benedetti, M. G., Carter, T. A., Ciceri, P., Edeen, P. T., Floyd, M., Ford, J. M., Galvin, M., Gerlach, J. L., Grotzfed, R. M., Herrgard, S., Insko, D. E., Insko, M. A., Lai, A. G., Lelias, J. M., Mehta, S. A., Milanov, Z. V., Velasco, A. M., Wodicka, L. M., Patel, H. K., Zarrinkar, P. P., and Lockhart, D. J. (2005) Nat. Biotechnol. 23 329-336 [DOI] [PubMed] [Google Scholar]
- 15.Kim, A. H., Khursigara, G., Sun, X., Franke, T. F., and Chao, M. V. (2001) Mol. Cell. Biol. 21 893-901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tobiume, K., Saitoh, M., and Ichijo, H. (2002) J. Cell. Physiol. 191 95-104 [DOI] [PubMed] [Google Scholar]
- 17.Zhang, L., Chen, J., and Fu, H. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 8511-8515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tobiume, K., Inage, T., Takeda, K., Enomoto, S., Miyazono, K., and Ichijo, H. (1997) Biochem. Biophys. Res. Commun. 239 905-910 [DOI] [PubMed] [Google Scholar]
- 19.Ichijo, H., Nishida, E., Irie, K., ten Dijke, P., Saitoh, M., Moriguchi, T., Takagi, M., Matsumoto, K., Miyazono, K., and Gotoh, Y. (1997) Science 275 90-94 [DOI] [PubMed] [Google Scholar]
- 20.Gotoh, Y., and Cooper, J. A. (1998) J. Biol. Chem. 273 17477-17482 [DOI] [PubMed] [Google Scholar]
- 21.Chen, Z., Seimiya, H., Naito, M., Mashima, T., Kizaki, A., Dan, S., Imaizumi, M., Ichijo, H., Miyazono, K., and Tsuruo, T. (1999) Oncogene 18 173-180 [DOI] [PubMed] [Google Scholar]
- 22.Saitoh, M., Nishitoh, H., Fujii, M., Takeda, K., Tobiume, K., Sawada, Y., Kawabata, M., Miyazono, K., and Ichijo, H. (1998) EMBO J. 17 2596-2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noguchi, T., Takeda, K., Matsuzawa, A., Saegusa, K., Nakano, H., Gohda, J., Inoue, J., and Ichijo, H. (2005) J. Biol. Chem. 280 37033-37040 [DOI] [PubMed] [Google Scholar]
- 24.Liu, Q., Wilkins, B. J., Lee, Y. J., Ichijo, H., and Molkentin, J. D. (2006) Mol. Cell. Biol. 26 3785-3797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tallman, J. F., and Gallager, D. W. (1985) Annu. Rev. Neurosci. 8 21-44 [DOI] [PubMed] [Google Scholar]
- 26.Gao, F., Sexton, P. M., Christopoulos, A., and Miller, L. J. (2008) Bioorg. Med. Chem. Lett. 18 4401-4404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang, S. C., Zhang, L., Chiang, H. C., Wank, S. A., Maton, P. N., Gardner, J. D., and Jensen, R. T. (1989) Am. J. Physiol. 257 G169-G174 [DOI] [PubMed] [Google Scholar]
- 28.Anzini, M., Canullo, L., Braile, C., Cappelli, A., Gallelli, A., Vomero, S., Menziani, M. C., De Benedetti, P. G., Rizzo, M., Collina, S., Azzolina, O., Sbacchi, M., Ghelardini, C., and Galeotti, N. (2003) J. Med. Chem. 46 3853-3864 [DOI] [PubMed] [Google Scholar]
- 29.Cheng, M. F., and Fang, J. M. (2004) J. Comb. Chem. 6 99-104 [DOI] [PubMed] [Google Scholar]
- 30.Churcher, I., Williams, S., Kerrad, S., Harrison, T., Castro, J. L., Shearman, M. S., Lewis, H. D., Clarke, E. E., Wrigley, J. D., Beher, D., Tang, Y. S., and Liu, W. (2003) J. Med. Chem. 46 2275-2278 [DOI] [PubMed] [Google Scholar]
- 31.Nelson, F. C., Delos Santos, E., Levin, J. I., Chen, J. M., Skotnicki, J. S., DiJoseph, J. F., Sharr, M. A., Sung, A., Killar, L. M., Cowling, R., Jin, G., Roth, C. E., and Albright, J. D. (2002) Bioorg. Med. Chem. Lett. 12 2867-2870 [DOI] [PubMed] [Google Scholar]
- 32.Blatt, N. B., Bednarski, J. J., Warner, R. E., Leonetti, F., Johnson, K. M., Boitano, A., Yung, R., Richardson, B. C., Johnson, K. J., Ellman, J. A., Opipari, A. W., Jr., and Glick, G. D. (2002) J. Clin. Investig. 110 1123-1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sundberg, T. B., Ney, G. M., Subramanian, C., Opipari, A. W., Jr., and Glick, G. D. (2006) Cancer Res. 66 1775-1782 [DOI] [PubMed] [Google Scholar]
- 34.Papadopoulos, V., Baraldi, M., Guilarte, T. R., Knudsen, T. B., Lacapere, J. J., Lindemann, P., Norenberg, M. D., Nutt, D., Weizman, A., Zhang, M. R., and Gavish, M. (2006) Trends Pharmacol. Sci. 27 402-409 [DOI] [PubMed] [Google Scholar]
- 35.Chelli, B., Salvetti, A., Da Pozzo, E., Rechichi, M., Spinetti, F., Rossi, L., Costa, B., Lena, A., Rainaldi, G., Scatena, F., Vanacore, R., Gremigni, V., and Martini, C. (2008) J. Cell. Biochem. 105 712-723 [DOI] [PubMed] [Google Scholar]
- 36.Mills, C., Makwana, M., Wallace, A., Benn, S., Schmidt, H., Tegeder, I., Costigan, M., Brown, R. H., Jr., Raivich, G., and Woolf, C. J. (2008) Eur. J. Neurosci. 27 937-946 [DOI] [PubMed] [Google Scholar]
- 37.Decaudin, D., Castedo, M., Nemati, F., Beurdeley-Thomas, A., De Pinieux, G., Caron, A., Pouillart, P., Wijdenes, J., Rouillard, D., Kroemer, G., and Poupon, M. F. (2002) Cancer Res. 62 1388-1393 [PubMed] [Google Scholar]
- 38.McDonald, P. H., Chow, C. W., Miller, W. E., Laporte, S. A., Field, M. E., Lin, F. T., Davis, R. J., and Lefkowitz, R. J. (2000) Science 290 1574-1577 [DOI] [PubMed] [Google Scholar]
- 39.Izumiya, Y., Kim, S., Izumi, Y., Yoshida, K., Yoshiyama, M., Matsuzawa, A., Ichijo, H., and Iwao, H. (2003) Circ. Res. 93 874-883 [DOI] [PubMed] [Google Scholar]
- 40.Liu, J., and Lin, A. (2005) Cell Res. 15 36-42 [DOI] [PubMed] [Google Scholar]
- 41.Xia, Z., Dickens, M., Raingeaud, J., Davis, R. J., and Greenberg, M. E. (1995) Science 270 1326-1331 [DOI] [PubMed] [Google Scholar]
- 42.Ikeda, F., Matsubara, T., Tsurukai, T., Hata, K., Nishimura, R., and Yoneda, T. (2008) J. Bone Miner. Res. 23 907-914 [DOI] [PubMed] [Google Scholar]
- 43.Kuan, C. Y., Yang, D. D., Samanta Roy, D. R., Davis, R. J., Rakic, P., and Flavell, R. A. (1999) Neuron 22 667-676 [DOI] [PubMed] [Google Scholar]
- 44.Sabapathy, K., Jochum, W., Hochedlinger, K., Chang, L., Karin, M., and Wagner, E. F. (1999) Mech. Dev. 89 115-124 [DOI] [PubMed] [Google Scholar]
- 45.Sabapathy, K., Hu, Y., Kallunki, T., Schreiber, M., David, J. P., Jochum, W., Wagner, E. F., and Karin, M. (1999) Curr. Biol. 9 116-125 [DOI] [PubMed] [Google Scholar]
- 46.Sabapathy, K., Kallunki, T., David, J. P., Graef, I., Karin, M., and Wagner, E. F. (2001) J. Exp. Med. 193 317-328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valladares, A., Alvarez, A. M., Ventura, J. J., Roncero, C., Benito, M., and Porras, A. (2000) Endocrinology 141 4383-4395 [DOI] [PubMed] [Google Scholar]
- 48.Edlund, S., Bu, S., Schuster, N., Aspenstrom, P., Heuchel, R., Heldin, N. E., ten Dijke, P., Heldin, C. H., and Landstrom, M. (2003) Mol. Biol. Cell 14 529-544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhuang, S., Demirs, J. T., and Kochevar, I. E. (2000) J. Biol. Chem. 275 25939-25948 [DOI] [PubMed] [Google Scholar]
- 50.Nemoto, S., Xiang, J., Huang, S., and Lin, A. (1998) J. Biol. Chem. 273 16415-16420 [DOI] [PubMed] [Google Scholar]
- 51.Wang, X. Z., and Ron, D. (1996) Science 272 1347-1349 [DOI] [PubMed] [Google Scholar]
- 52.Yamaguchi, O., Higuchi, Y., Hirotani, S., Kashiwase, K., Nakayama, H., Hikoso, S., Takeda, T., Watanabe, T., Asahi, M., Taniike, M., Matsumura, Y., Tsujimoto, I., Hongo, K., Kusakari, Y., Kurihara, S., et al. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 15883-15888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nishitoh, H., Kadowaki, H., Nagai, A., Maruyama, T., Yokota, T., Fukutomi, H., Noguchi, T., Matsuzawa, A., Takeda, K., and Ichijo, H. (2008) Genes Dev. 22 1451-1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Okada, K., Minamino, T., Tsukamoto, Y., Liao, Y., Tsukamoto, O., Takashima, S., Hirata, A., Fujita, M., Nagamachi, Y., Nakatani, T., Yutani, C., Ozawa, K., Ogawa, S., Tomoile, H., Hori, M., and Kitakaze, M. (2004) Circulation 110 705-712 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.