Abstract
Side chain oxysterols exert cholesterol homeostatic effects by suppression of sterol regulatory element-binding protein maturation and promoting degradation of hydroxymethylglutaryl-CoA reductase. To examine whether oxysterol-membrane interactions contribute to the regulation of cellular cholesterol homeostasis, we synthesized the enantiomer of 25-hydroxycholesterol. Using this unique oxysterol probe, we provide evidence that oxysterol regulation of cholesterol homeostatic responses is not mediated by enantiospecific oxysterol-protein interactions. We show that side chain oxysterols, but not steroid ring-modified oxysterols, exhibit membrane expansion behavior in phospholipid monolayers and bilayers in vitro. This behavior is non-enantiospecific and is abrogated by increasing the saturation of phospholipid acyl chain constituents. Moreover, we extend these findings into cultured cells by showing that exposure to saturated fatty acids at concentrations that lead to endoplasmic reticulum membrane phospholipid remodeling inhibits oxysterol activity. These studies implicate oxysterol-membrane interactions in acute regulation of sterol homeostatic responses and provide new insights into the mechanism through which oxysterols regulate cellular cholesterol balance.
Cellular cholesterol requirements are met through de novo cholesterol synthesis and uptake of lipoprotein cholesterol. These homeostatic responses are regulated at multiple steps through a negative feedback loop that responds to elevations in cellular cholesterol. Central to this pathway is the sterol regulatory element-binding protein (SREBP)3 family of transcription factors, which directly activates expression of genes involved in the synthesis and uptake of cholesterol and lipogenesis. In the endoplasmic reticulum (ER), cholesterol modulates SREBP processing by binding to the sterol-sensing domain of SREBP cleavage-activating protein (SCAP) and inducing conformational change in SCAP (1). Cholesterol also promotes interaction between SCAP and the ER retention proteins, Insig-1 and Insig-2, which in turn prevents movement of the SCAP-SREBP complex to the Golgi and maturation of the SREBP transcription factors (1, 2).
Cholesterol homeostasis is governed not only by its end product, cholesterol, but also by oxygenated derivatives of cholesterol, known as oxysterols. Side chain oxysterols, such as 24-, 25-, and 27-hydroxycholesterol (HC), are generated enzymatically in vivo and are important physiological regulators of cholesterol homeostasis. These side chain oxysterols contribute to the maintenance of cellular cholesterol balance by serving as endogenous ligands for the liver X receptors (LXRs), which activate cholesterol elimination and efflux pathways (3), and through suppression of SREBP proteolysis. Unlike cholesterol, the side chain oxygenated sterols 25-HC and 27-HC do not induce conformational changes in SCAP and are unable to bind to the sterol-sensing domain of SCAP (1, 4). Rather, 25-HC and 27-HC have been shown to bind to the Insig proteins, promoting interaction between SCAP and Insig proteins (5) and retention of the SCAP-SREBP complex in the ER. Binding of side chain oxysterols to Insig proteins also promotes rapid proteolytic inactivation of HMG-CoA reductase (HMGR), the rate-limiting enzyme in cholesterol biosynthesis (6). Synthesis of side chain oxysterols is required for rapid inactivation of HMGR in response to excess cellular cholesterol (7).
Side chain oxysterols also contribute to cholesterol homeostasis by promoting the transfer of cholesterol to ER membranes (8, 9). The rapid influx of cholesterol into the ER suppresses SREBP processing, possibly through the modulation of SCAP conformation, and stimulates acyl-CoA:cholesterol O-acyltransferase activity. The activation of acyl-CoA:cholesterol O-acyltransferase prevents cholesterol cytotoxicity by promoting esterification of ER-associated free cholesterol and storage of cholesterol esters in neutral lipid droplets. Although the mechanism of oxysterol-mediated transfer of cholesterol into the ER is not well understood, 25-HC, like known membrane-intercalating agents (e.g. n-octanol and 1,2-dioctanoyl-sn-glycerol), has been shown to increase susceptibility of membrane cholesterol to cyclodextrin extraction and interaction with cholesterol oxidase (10). The effect of oxysterols on ER cholesterol levels may reflect their ability to compete with cholesterol for association with membrane lipids (e.g. in the plasma membrane (PM)), thereby increasing the availability of membrane cholesterol for translocation into the ER (10).
In model membrane systems, oxysterols affect the formation of liquid-ordered phases and alter phospholipid packing (11). The differential membrane properties of specific oxysterols are dependent on the chemical nature and location of the oxidative modification of the sterol, as well as the phospholipid composition of the membranes (11). In the present study, we examined whether such oxysterol-membrane interactions contribute to the regulation of cholesterol homeostasis in vivo. We used a unique oxysterol probe, the enantiomer of 25-HC (ent-25-HC), to deconvolute membrane effects of oxysterols from effects of oxysterol-protein interactions. We provide evidence that oxysterols contribute to the regulation of cholesterol homeostatic responses through non-stereospecific interactions. We show that the behavior of side chain oxysterols in phospholipid monolayers and bilayers in vitro is non-enantiospecific and is modulated both in vitro and in cells by the degree of saturation of phospholipid acyl chain constituents. Our findings support a role for direct oxysterol-membrane interactions in regulating cellular cholesterol homeostasis.
MATERIALS AND METHODS
Cell Culture and Chemicals—CHO-K1 cells (American Type Culture Collection) were cultured in 1:1 Dulbecco's modified Eagle's medium:Ham's F-12 with 5% (v/v) fetal bovine serum (Sigma), 2 mm glutamine, 50 units/ml penicillin, and 50 μg/ml streptomycin. Cells were maintained in monolayer culture at 37 °C with 5% CO2. For esterification and luciferase assays, cells were incubated in medium containing Dulbecco's modified Eagle's medium and 5% lipoprotein-deficient serum (LPDS) (CoCalico Biologicals). Oxysterols were obtained from Steraloids (25-HC, 7α-HC, 7β-HC), Sigma (7-ketocholesterol (7-KC)), and Research Plus (27-HC) and were provided to cells dissolved in ethanol (final concentration of 0.01% in the medium). As enantiomeric steroids are not naturally available, ent-25-HC was prepared by total steroid synthesis (stepwise construction of ring system and side chain) as described (12).
Luciferase Reporter Assays—For the quantification of SREBP-dependent gene expression, transfection assays with Chinese hamster ovary (CHO) cells were performed as described (8). For these experiments, cells were transfected either with pLDLp-588luc, driven by the human low density lipoprotien receptor promoter, or with pSynSRE (gift from T. Osborne), a minimal promoter driven by tandem sterol-regulatory element (SRE) sequences (13-15). For measurement of LXR-dependent gene expression, cells were transfected with a reporter containing 990 bp of the human ABCA1 promoter linked to a luciferase reporter construct (gift from S. Santamarina-Fojo), pLXRα (gift from D. Mangelsdorf), and pTK-Renilla. Transfections were performed as described (8). Oxysterols were provided to cells dissolved in ethanol (final concentration of 0.01% in the medium).
HMGR Degradation Assay—CHO cells were transfected with an HMGR-green fluorescent protein expression construct (gift from R. Simoni and T. Y. Chang) harboring a D766N point mutation in the catalytic domain, and clonal lines were isolated. For the quantification of HMGR degradation, HMGR-green fluorescent protein-expressing cell lines were cultured overnight in LPDS medium containing 20 μm compactin and 50 μm mevalonate and treated with oxysterols as described, and green fluorescent protein fluorescence was measured in triplicate by flow cytometry.
Cholesterol Esterification Assays—Cells were incubated in LPDS medium in the presence and absence of fatty acids for 24 h. The following day, cells were pulsed at 4 °C for 10 min with [3H]cholesterol (PerkinElmer Life Sciences) methyl-β-cyclodextrin (CD; Sigma) (16), rinsed with phosphate-buffered saline, and cells were incubated in the presence and absence of oxysterols and/or fatty acids for the indicated times. Lipids were extracted, separated by TLC, and analyzed as described previously (17).
Monolayer Studies—Surface pressure-molecular area isotherms (see Fig. 3) were determined using a Langmuir-type surface balance as described previously (18). The monolayer behaviors of nat- and ent-25-hydroxycholesterol alone and in mixtures with various lipids (see Fig. 4) were examined using a Langmuir film balance (KSV).
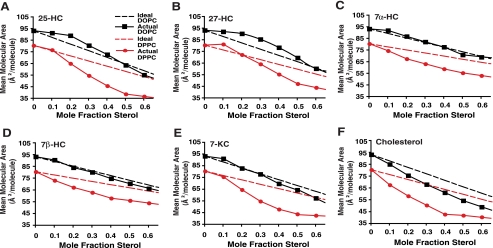
FIGURE 3.
Surface pressure-molecular area isotherms for mixed monolayers of oxysterols and DOPC or DPPC. DOPC and DPPC monolayer mixtures were prepared containing 25-HC (A), 27-HC (B), 7α-HC (C), 7β-HC (D), 7-KC (E), and cholesterol (F). Molecular areas are plotted versus the mole fraction of sterol at a constant surface pressure of 5 millinewtons/m (solid lines). Dashed lines represent the theoretical ideal (non-interacting) mixture of the pure components. Increased mean molecular area versus ideal isotherm indicates membrane expansion effects of sterols, whereas decreased mean molecular area versus ideal isotherm indicates membrane-condensing effects of sterols.
FIGURE 4.
Surface pressure-molecular area isotherms for mixed monolayers of nat- and ent-25-HC with DOPC (A and D), POPC (B and E), and DPPC (C and F). Molecular areas for monolayers containing nat-25-HC (A-C) and ent-25-HC (D-F) are plotted versus the mole fraction of sterol at a constant surface pressure of 5 millinewtons/m. Dashed lines represent the theoretical ideal (non-interacting) mixture of the pure components. Membrane expansion effects of oxysterols are abrogated with increasing phospholipid acyl chain saturation. For each experiment, each set of conditions was performed with three to six replicates. Values represent means ± S.E.
Preparation of Carboxyfluorescein (CF)-loaded Liposomes—Unilamellar liposomes were prepared as described (19). Liposomes with a mean diameter of 207 ± 12.5 nm S.D., as measured by light scattering with a Beckman-Coulter N5 submicron particle size analyzer, were used for CF dequenching and surface plasmon resonance (SPR) experiments.
Surface Plasmon Resonance Spectroscopy—The incorporation of oxysterols into dioleoylphosphatidylcholine (DOPC)/dioleoylphosphatidic acid (70:30) liposomes was determined by SPR spectroscopy. Liposomes (3 ng of phospholipid) were immobilized on an L1 chip and the indicated oxysterol at 1 μm was injected into the chamber at a volume replacement rate of two times/s. Liposome lipid and oxysterol accumulating in the liposome bilayer were determined by the SPR signal and used to calculate the mol % of oxysterol.
Fluorescence Dequenching Assay Spectroscopy—Liposomes were prepared as described above. The fluorescence dequenching assay was performed as described previously (20). Briefly, 3 ng of liposome lipid containing 20 mm CF was placed in a 500-μl spectrofluorometric cuvette, and the indicated concentration of oxysterol (1-50 μm) was added to the solution. Fluorescence was monitored at 520 nm and normalized to the total dequenching following the addition of 1% Triton X-100. Where indicated, 5 mm CD was added to extract oxysterol from liposomes. This concentration of CD did not quench CF fluorescence in solution or after solubilization of liposomes with Triton X-100. Values are normalized to the fluorescence measured following Triton X-100 addition at the end of each time series and corrected for solvent effects.
Subcellular Fractionation and Western Blot Analysis—Subcellular fractionation of CHO cells, isolation of ER-enriched microsomes, and Western blotting were performed as described (21).
Cholesterol Mass Measurements—Lipids were extracted from total cellular and ER-enriched fractions using the Bligh and Dyer method, and cholesterol mass was determined as described previously by gas chromatography-mass spectrometry (8).
Lipid Determination by Electrospray Ionization-Mass Spectrometry—Cell lysates were prepared and analyzed by electrospray ionization-mass spectrometry as described previously (22).
Statistics—All results are expressed as the means ± S.E. The statistical significance of differences in mean values was determined by single factor analysis of variance unless otherwise specified. Data shown are representative of at least two similar experiments.
RESULTS
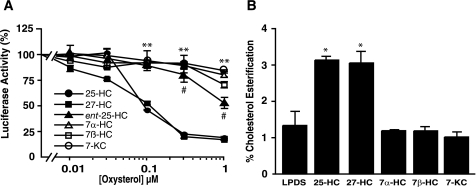
Effect of Oxysterols on Regulating Cholesterol Movement into ER Membranes—To investigate the molecular mechanism of oxysterol regulation of cholesterol homeostasis, we first characterized structure-activity relationships of a panel of biologically active oxysterols. Using a sterol regulatory element-containing reporter construct as an indicator of the status of SREBP maturation, we examined the ability of both side chain oxysterols (25-HC and 27-HC) and steroid B ring-modified oxysterols (7α-HC, 7β-HC, and 7-KC) to suppress SREBP-dependent gene expression (Fig. 1A). We found that the EC50 values for suppression of sterol-regulated gene expression by 25-HC and 27-HC are 0.17 and 0.20 μm, respectively, and that these side chain oxysterols were ∼20-fold more potent than 7α-HC, 7β-HC, and 7-KC. Similar structure-activity relationships for the oxysterols were observed in the trafficking of cholesterol to the ER, as assessed by cholesterol esterification assays. The rate of oxysterol-induced transfer of PM cholesterol into ER membranes correlated directly with the ability of specific oxysterols to suppress SREBP-mediated gene expression (Fig. 1B). These dichotomous effects of the side chain and steroid ring-modified oxysterols closely mirror the specificity for oxysterol binding to Insig-2 (5). However, the non-stoichiometric Insig-oxysterol binding suggests that oxysterols may exert their effect on SREBP-dependent gene expression through additional mechanisms (5).
FIGURE 1.
Oxysterol regulation of ER cholesterol homeostasis. A, effect of oxysterols on sterol-regulated gene expression using a human low density lipoprotein receptor-luciferase reporter construct. **, p < 0.01 for 25-HC and 27-HC versus 7α-HC, 7β-HC, or 7-KC treatment; #, p < 0.01 for ent-25-HC treatment versus 7α-HC, 7β-HC, and 7-KC treatments. B, effect of oxysterols on PM-to-ER cholesterol movement. CHO cells were cultured in LPDS medium for 24 h. Cells were pulse-labeled with [3H]cholesterol-CD complexes for 10 min at 4 °C, and then incubated with 1 μm oxysterols in LPDS medium at 37 °C for 5 h. Cells were harvested and incorporated [3H]cholesterol into the cholesteryl esters determined. Assay was performed in triplicate and is presented as the percent esterification of total cell-associated radiolabel. Data are presented as means ± S.E. *, p < 0.05 for 25-HC and 27-HC treatments versus vehicle alone.
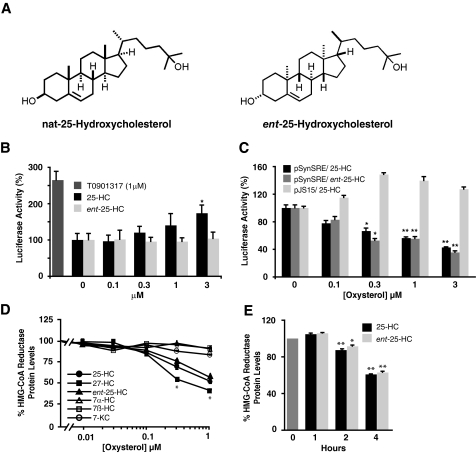
To further delineate the mechanism of action of oxysterols, we synthesized ent-25-HC (Fig. 2A) (12). Whereas enantiomeric steroid pairs have been shown to have identical effects on membrane lipids, steroid-protein interactions are in general enantiospecific (23). Oxysterol-protein interactions are similarly enantioselective, as evidenced by the ability of natural 25-HC (nat-25-HC), but not ent-25-HC, to ligand LXR and activate target gene expression (Fig. 2B). In the SREBP reporter assay, the EC50 for ent-25-HC for suppression of sterol-regulated gene expression was 6-fold lower than the EC50 for nat-25-HC, although ent-25-HC was still significantly more potent than the steroid ring-modified oxysterols (Fig. 1A). When the assay was performed using a reporter construct driven by a minimal promoter regulated by tandem SRE sequences (15) instead of the human low density lipoprotein receptor promoter, differences between nat- and ent-25-HC were no longer apparent (Fig. 2C), suggesting that oxysterols may additionally modulate expression from the low density lipoprotein receptor promoter through non-SRE sequences and that such a mechanism is likely introducing an enantioselective step into regulation of reporter expression. The recent identification of novel negative LXR response elements in the promoters of SREBP-2 target genes provides a plausible explanation for the apparent partial enantioselectivity (24). Reporter activity was dependent on the SRE sequences because the introduction of point mutations into the SREs abrogated the effects of the oxysterols (Fig. 2C). To further clarify the mechanism by which oxysterols acutely regulate cholesterol homeostasis, we compared the effect of the oxysterols on degradation of HMGR, an independent Insig-mediated sterol homeostatic response (6). Similar to the SREBP reporter assay, the EC50 values were significantly lower for 25-HC and 27-HC than for the ring-modified oxysterols (Fig. 2D). Moreover, consistent with the SRE-minimal promoter assay (Fig. 2C), nat- and ent-25-HC were equally effective in promoting the HMGR degradation, and degradation of the protein was detected within 2 h post-incubation (Fig. 2E). Together, our findings demonstrate that 25-HC likely modulates acute sterol homeostatic responses through non-enantiospecific interactions.
FIGURE 2.
Effect of oxysterols on sterol homeostatic responses. A, chemical structures of nat- and ent-25-HC. B, enantioselectivity of 25-HC-dependent activation of LXR target gene expression. Cells were transfected with the ABCA1-luciferase reporter construct and treated for 24 h with nat- or ent-25-HC. T0901317 (10 μm) served as a control for LXR activation. *, p < 0.05 for nat-25-HC versus ent-25-HC treatment. C, effect of oxysterols on sterol-dependent gene expression. Cells were transfected with either pSynSRE or pJS15, a control harboring a point mutation in the SRE sequences that abolishes sterol-regulated activity. *, p < 0.05 for nat- and ent-25-HC versus vehicle treatment; **, p < 0.01 for nat- and ent-25-HC versus vehicle treatment. D, effect of oxysterols on degradation of HMGR protein. *, p < 0.05 for nat-25-HC, ent-25-HC, and 27-HC versus 7α-HC, 7β-HC, or 7-KC treatment. E, time course for degradation of HMGR by 1 μm 25-HC. *, p < 0.05 for t = 2 h versus t = 0 h;**, p < 0.01 for t = 2 or 4 h versus t = 0 h.
Effect of Oxysterols on Defined Monolayer and Bilayer Membranes—We modeled the interaction of oxysterols with biological membranes by first studying the monolayer behavior of both the side chain and steroid ring-modified oxysterols in various phospholipid mixtures. The Langmuir film balance is widely used for the characterization of sterol-phospholipid interaction (18, 25). In these experiments, the molecular areas of the oxysterols in the membranes were examined as a function of the sterol mole fraction at a constant surface pressure (5 millinewtons/m). We found that mixtures of 25-HC or 27-HC with DOPC (only monounsaturated 18:1 acyl chains) were expanded (i.e. increased mean molecular areas) relative to the ideal (non-interacting) mixture of components (Fig. 3, A and B). The positive deviations from ideal behavior, which indicate an expansion effect, covered a considerable range (0 < XHC < 0.5) and peaked at XHC of 0.2-0.3 (Fig. 3, black lines). This response contrasts with the behavior of 7α-HC with DOPC, which was similar to the theoretical ideal mixture of the pure components (Fig. 3C), and with mixtures of 7β-HC and 7-KC with DOPC, which exhibited mean molecular areas slightly less than the theoretical ideal when sterol mole fractions exceeded 0.3 (Fig. 3, D and E). The latter observations suggest that 7β-HC and 7-KC exert only a slight condensing or membrane-ordering effect in DOPC monolayers, in contrast to the more substantial condensing effect of cholesterol (Fig. 3F) (18).
To assess the role of acyl chain saturation in the regulation of phospholipid-oxysterol lateral interactions, we investigated the monolayer behavior of the side chain oxysterols mixed with phospholipids containing increasingly saturated acyl chains. Compared with mixtures involving DOPC, oxysterols mixed with palmitoyloleoylphosphatidylcholine (POPC; saturated 16:0 and monounsaturated 18:1 acyl chains) or with dipalmitoylphosphatidylcholine (DPPC; only saturated 16:0 acyl chains) displayed diminished expansion effects that became progressively more negative as the saturation state of the phosphatidylcholine (PC) acyl chains increased: DOPC ≥ POPC > DPPC (Fig. 3, red lines, and supplemental Fig. 1). To determine the stereospecificity of the oxysterol-phospholipid interaction, we examined the monolayer behavior of the nat- and ent-25-HC enantiomeric pair in DOPC, POPC, and DPPC phospholipid mixtures. We found that the membrane activity of ent-25-HC was identical to that of nat-25-HC (Fig. 4). These findings demonstrate that the oxysterol-membrane interaction responsible for the expansion effect of 25-HC in the DOPC and POPC mixtures and for the abrogation effect of the saturated acyl chains is non-enantioselective.
We next investigated the membrane behavior of oxysterols in DOPC/dioleoylphosphatidic acid-containing liposomes (i.e. membrane bilayers). For these studies, we employed a fluorescence dequenching assay that has been extensively used to study pore activation and swelling of liposomes (26). In these experiments, CF dye is contained in the liposome at quenching concentrations, and the fluorescence increases when the dye is released or when the internal volume of the liposome is increased, diluting the dye concentration. The addition of 25-HC led to a dose-dependent increase in fractional fluorescence dequenching (Fig. 5A). The dequenching effects of 25-HC exhibited a clear dose response over a broad range of concentrations (1-70 μm) (Fig. 5B). Similar fluorescence dequenching was observed following incubation with 27-HC, but was much less apparent with the steroid ring-modified oxysterols, 7α-HC, 7β-HC, and 7-KC (Fig. 5C). The more pronounced effect of the side chain oxysterols as compared with ring-modified oxysterols was not due to greater accumulation of these oxysterols in the liposome membranes, as less 25-HC was incorporated into the liposomes than, for example, 7-KC over the time course of the dequenching assay (XHC at 300 s, 0.033 for 27-HC, 0.083 for 25-HC, 0.31 for 7α-HC, 0.28 for 7β-HC, and 0.20 for 7-KC; p < 0.05) (Fig. 5D). Indeed, when dequenching assay data are normalized for the extent of oxysterol incorporation, then 27-HC, which has the lowest rate of incorporation into liposomes, exhibits nearly identical membrane expansion properties as 25-HC. To exclude the possibility that the dequenching effect of 25-HC resulted from physical disruption of liposomes and carboxyfluorescein release, we performed the assay by first incubating the liposomes with oxysterol, followed by the addition of excess CD, an avid sterol acceptor (Fig. 5E). Because CD was able to completely reverse the fluorescence dequenching induced by 25-HC, the increase in fluorescence is consistent with membrane expansion caused by a reversible oxysterol-membrane interaction, rather than through liposome disruption and release of the fluorophore. Importantly, the specificity of the sterol-membrane expansion for side chain versus steroid ring-modified oxysterols in the liposome studies mirrors the differential effect of the oxysterols in membrane monolayers in vitro and the cell-based structure-activity relationships with respect to sterol-regulated gene expression.
FIGURE 5.
Effects of oxysterols on unilamellar liposomes. A, oxysterol-induced dequenching of CF-containing liposomes. Liposomes containing 20 mm CF were maintained at room temperature followed by the addition of 0-50 μm 25-HC to the solution. Fluorescence was normalized to the total dequenching, following the addition of 1% Triton X-100. B, concentration dependence of 25-HC on fluorescence dequenching in liposomes. A dequenching curve was fitted to a linear function using standard least-squares methods (95% confidence limits are plotted). C, comparison of the effect of oxysterols (50 μm) on fluorescence dequenching of liposomes. Oxysterol dequenching was normalized to complete dequenching by Triton X-100 and is corrected for solvent effects on the fluorescence base line. Data shown are means ± S.E. *, p < 0.05 for 27-HC versus 7β-HC; **, p < 0.01 for 25-HC versus 7α-HC, 7β-HC, or 7-KC treatment and 27-HC versus 7α-HC or 7-KC treatment. Data shown are the mean of three experiments. D, comparison of the incorporation of 25-HC and 7-KC in DOPC/dioleoylphosphatidic acid (70:30) liposomes as determined by SPR spectroscopy. The liposome lipid and the oxysterol in the liposomes were determined by the SPR signal and used to calculate the mol % sterol in the liposome bilayer. Data were acquired at a rate of 10 data points/s and plotted as one data point/s. E, inhibition of oxysterol-induced fluorescence dequenching by CD. CF-containing liposomes were monitored for fluorescence and maintained at room temperature. At time 0, 0 μm (black line), 7.3 μm (blue line), or 50 μm (red line) 25-HC was added to the liposomes. At 300 s, 5 μm CD was added (black arrow) to the 25-HC liposome mixture, and subsequent fluorescence changes were plotted. Values are normalized to fluorescence measured following Triton X-100 addition at the end of each time series. Data shown are the mean of three experiments.
Modulation of Oxysterol Effects on Sterol-regulated Gene Expression by Fatty Acid Supplementation—To extend our observations of the effect of oxysterols on model membranes to a cell-based model, we studied the effect of fatty acid supplementation on sterol-regulated gene expression in CHO cells. We supplemented media with free fatty acid-bovine serum albumin complexes such that the fatty acid concentrations were below that which activates apoptotic lipotoxic pathways (data not shown). Incubation with the saturated fatty acid palmitate (16:0), but not the monounsaturated fatty acid oleate (18:1), increased the stability of HMGR (Fig. 6A). Palmitate treatment relieved 25-HC-mediated suppression of HMGR degradation and attenuated the suppressive effects of either nat- or ent-25-HC in a non-enantioselective manner (Fig. 6B). Palmitate supplementation similarly modulated SREBP-dependent gene expression and blunted the effects of both nat- and ent-25-HC on sterol-regulated gene expression (data not shown). Together, these findings suggest that the effect of fatty acid exposure on acute regulation of sterol homeostatic response is more strongly modulated by the membrane environment than by direct sterol-protein interactions.
FIGURE 6.
Fatty acid modulation of oxysterol-regulated HMGR degradation. A, effect of palmitate and oleate treatment on HMGR degradation. *, p < 0.05 for palmitate treatment versus vehicle alone. B, non-enantioselective effects of fatty acids on oxysterol suppression (1 μm) of HMGR degradation. *, p < 0.05 for palmitate plus either nat-25-HC or ent-25-HC treatment versus nat-25-HC or ent-25-HC alone, respectively.
We and others have demonstrated that side chain oxysterols increase ER cholesterol levels (Fig. 1B) (8, 27). This elevation in ER membrane cholesterol content has been shown to modulate SCAP conformation and its binding to Insig, leading to the activation of sterol-dependent gene expression (1, 2). Accordingly, palmitate supplementation might attenuate 25-HC suppression of sterol-dependent gene expression by interfering with the transfer of PM cholesterol into ER membranes. We therefore directly examined the effect of palmitate supplementation on the rate of PM-to-ER cholesterol transfer by pulse labeling CHO cells with [3H]cholesterol-CD complexes and measuring the rate of incorporation of [3H]cholesterol into cholesteryl esters. Cholesterol esterification in CHO cells is mediated by acyl-CoA:cholesterol O-acyltransferase, an exclusively ER-localized enzyme, and is used to monitor the arrival of free cholesterol into the ER compartment. We found that overnight incubation of CHO cells in palmitate had no effect on the rate of cholesterol esterification (supplemental Fig. 2A), indicating that palmitate supplementation does not affect the basal rate of PM cholesterol movement into the ER. We confirmed these findings by directly measuring the effect of palmitate on the size of the ER cholesterol pool. For these studies, CHO cells were incubated in the presence and absence of palmitate, followed by isolation of membrane fractions. The crude ER fractions were enriched in rough ER and smooth ER markers and depleted in mitochondrial and PM markers (supplemental Fig. 2B). Quantification of cholesterol mass in the total cell homogenates and the ER fractions demonstrated that ∼1.0% of total cellular cholesterol was associated with the ER fraction, in close agreement with previous experimentally determined values (28). In accordance with the cholesterol esterification assay results, we found that palmitate supplementation did not affect the partitioning of cholesterol into ER membranes (supplemental Fig. 2C). Together with the reporter assays, these studies provide support that the action of 25-HC on the regulation of sterol-dependent gene expression is palmitate-sensitive. Moreover, the mechanism through which palmitate exerts its effect involves a non-enantioselective step, such as through modulation of oxysterol interaction with the membrane environment.
Effect of Palmitate Supplementation on ER Phospholipid Composition—On the basis of our studies of the behavior of side chain oxysterols with model membranes in vitro, we hypothesized that palmitate supplementation and membrane phospholipid remodeling might attenuate oxysterol-membrane effects in a cell culture model. We further reasoned that ER membranes are a likely site of oxysterol action, given the established ER localization of the sterol-sensing machinery and its high representation (85%) of unsaturated acyl chains (21), which promote oxysterol membrane-disordering activity. To test this hypothesis, CHO cells were incubated in the presence and absence of 50 μm palmitate, followed by isolation of crude ER fractions (supplemental Fig. 2B). Lipid species in the ER fractions were quantified using electrospray ionization tandem mass spectrometry. Palmitate supplementation was characterized by significant increases in PC, phosphatidylglycerol, and phosphatidylinositol species containing 16:0 acyl chains (Fig. 7). In particular, 16:1-16:0 PC, a phospholipid species that accounts for ∼20% of total ER membrane lipid constituents, was elevated 3.2-fold in palmitate-treated cells. By contrast, these 16:0-containing PC species were not elevated in ER membranes of oleate-treated cells (data not shown). Similarly, incorporation of 16:0 acyl chains was significantly increased for phosphatidylglycerol and phosphatidylinositol species. These findings provide a plausible and provocative mechanism through which fatty acid supplementation could modulate oxysterol-membrane interactions in vivo.
FIGURE 7.
Phospholipid analysis of ER membrane in palmitate-treated cells. CHO cells were cultured for 24 h in LPDS medium in the presence and absence of 50 μm palmitate. ER-enriched fractions were prepared, and lipids were extracted. Electrospray ionization-mass spectrometry was performed, and lipid species were analyzed. Data are shown for PC (A), phosphatidylglycerol (PG) (B), and phosphatidylinositol (PI) (C) content of the ER membranes. Values are means ± S.E. *, p < 0.05 for palmitate treatment versus LPDS.
DISCUSSION
In this study, we investigated the molecular mechanism through which side chain oxysterols exert their cholesterol homeostatic effects. Using the unique oxysterol probe ent-25-HC, we have provided evidence that the oxysterol suppression of cholesterol homeostatic responses in cells is not mediated by stereospecific oxysterol-protein interactions. In model membrane systems, we have demonstrated that side chain oxysterols, but not steroid ring-modified oxysterols, exhibit membrane-disordering behavior in phospholipid monolayers and bilayers and that this oxysterol-mediated effect is non-enantiospecific. Moreover, the membrane-disordering behavior of side chain oxysterols is abrogated by increasing the saturation of phospholipid acyl chains. Treatment of cells with palmitate, a saturated fatty acid, leads to the remodeling of ER membrane phospholipids and similarly inhibits the activity of oxysterols. Our studies provide new insight into the molecular basis for fatty acid modulation of SREBP-dependent gene expression and provide the first evidence that non-enantiospecific oxysterol-membrane interactions contribute to acute regulation of cholesterol homeostasis.
Sterol enantiomers provide a powerful approach to deconvolute the membrane effects of sterols from the effects of sterol-protein interactions. Biophysical studies examining the enantioselectivity of cholesterollipid interactions demonstrate that mixed sterol-lipid monolayers containing nat- and ent-cholesterol exhibit identical lateral compressional behavior (25). Similarly, nat- or ent-cholesterol have identical effects on sphingomyelin-containing multilamellar vesicles with respect to temperature, cooperativity, enthalpy of gel/liquid-crystalline phase transition, and x-ray diffraction patterns (23). On the other hand, nat- and ent-cholesterol differ with respect to their effect on proteins that interact directly with cholesterol. The cholesterol-binding bacterial toxins Vibrio cholerae cytolysin and streptococcal streptolysin O both require enantiospecific recognition of cholesterol to function (23). ent-cholesterol is also differentially oxidized by cholesterol oxidase (25), is both a poor allosteric activator and substrate for acyl-CoA:cholesterol O-acyltransferase (29), and has been used to demonstrate stereospecificity for ABCG5 and ABCG8 sterol transport activity (30). Taken together, these findings suggest that sterol-lipid interactions depend on the unique physical properties of cholesterol, whereas interaction with sterol-binding proteins or enzymes that metabolize cholesterol appears to be enantioselective, requiring a precise three-dimensional structure.
In the present study, we used ent-25-HC to investigate the molecular mechanism of the action of side chain oxysterols. Unlike diastereomers that differ in configuration at one or several chiral centers, nat- and ent-25-HC are mirror images of each other that differ in configuration at all eight chiral centers. ent-25-HC is unique as an oxysterol probe in that it has the identical chemical composition, bonding pattern, and relative configuration as nat-25-HC (12). Because the enantiomeric pair share identical chemical and physical properties, these enantiomers, like nat- and ent-cholesterol, can be distinguished only by their opposite absolute configuration, such as by optical rotation or by interaction with another chiral molecule. The finding that both nat- and ent-25-HC are able to promote rapid degradation of HMGR and to suppress SREBP-dependent gene expression provides compelling evidence for the contribution of non-enantiospecific interactions in the acute regulation of cellular cholesterol balance by side chain oxysterols. These results implicate oxysterol-membrane interactions in mediating sterol homeostatic responses, although the potential contribution of oxysterol-Insig complexes cannot be excluded in the absence of the direct demonstration that the oxysterol-protein binding is enantioselective.
In light of previous studies demonstrating lack of enantioselectivity for sterol-membrane interactions (25), we reasoned that examination of the behavior of oxysterols in model membrane systems would provide insight into their mechanism of action. Unlike cholesterol, which has a strong ordering effect on membranes, oxysterols have been shown to promote or inhibit the formation of a liquid-ordered phase depending on the chemical structure of the oxysterol and phospholipid composition of the membranes (11). Our studies in phospholipid monolayers indicate that side chain oxysterols, in contrast to steroid ring-modified oxysterols, increase the mean molecular area of oxysterol-unsaturated phospholipid mixtures, consistent with the known membrane-disordering effects of these oxysterols mixed with POPC (31). Similarly, we have shown that incubation with 25-HC or 27-HC induces liposome swelling, a likely consequence of increased permeability of the phospholipid bilayer caused by the membrane-disordering effects specific to the side chain oxysterols. In these studies, the membrane expansion effects of 27-HC were detected at oxysterol:phospholipid ratios as low as 3 mol %, raising the possibility that the membrane effects observed in vitro could occur in specific oxysterol-enriched domains in vivo. The differential membrane properties of the side chain versus ring-modified oxysterols closely correlate with the ability of these sterols to mediate a host of sterol homeostatic responses, including ER retention of Insig-SCAP-SREBP complexes (5), mobilization of lysosomal cholesterol storage in Niemann-Pick C1-deficient cells (8, 32), and degradation of HMGR (6). Although side chain oxysterols may act through specific oxysterol-binding domains, such as those identified for Insig and NPC1 proteins (5, 33), the lack of common sequence motifs or structural similarities among these domains raises the possibility that side chain oxysterols alternatively may contribute to the regulation of cellular cholesterol homeostasis through modulation of the properties of the lipid environment in which these integral membrane proteins are embedded.
On the basis of the behavior of side chain oxysterols in model membranes, we propose a model for oxysterol-phospholipid interactions (Fig. 8). In contrast to the steroid ring structure of cholesterol, which has a moderate condensing or ordering effect on DOPC monolayers and assumes an orientation parallel to the acyl chains (Fig. 8, A and C) (18), our data suggest that in DOPC mixtures the steroid nucleus of 25-HC may not penetrate into the membrane as deeply as cholesterol. Rather, 25-HC may insert into the membrane in a tilted orientation, such that both the 3β- and 25-hydroxyl groups are able to interact with the polar headgroups of the phospholipid constituents (Fig. 8, B and D). This model is supported by recent atomistic simulations of phospholipid interactions with various sterols (34) and by small-angle x-ray diffraction experiments demonstrating that in POPC phospholipid bilayers 25-HC does not intercalate into the membrane hydrocarbon core (35).
FIGURE 8.
Model of cholesterol- and side chain oxysterol-phospholipid interaction. Lipid membranes consisting of POPC and cholesterol or side chain oxysterols (e.g. 25-HC) were constructed using VMD Version 1.86 (Beckman Institute for Advanced Science and Technology). A and C show cholesterol in condensed packing with three phospholipids in horizontal and vertical views. B and D show similar views for 25-HC associated with three phospholipids where the alkane chains are in the more usual non-condensed conformation. This configuration promotes the membrane expansion observed upon the addition of 25-HC to phospholipid monolayers and bilayers. Increasing the saturation of phospholipid acyl chains (e.g. DPPC) promotes less tilted and deeper insertion of side chain oxysterols into membranes, thereby abrogating their membrane-expansion effects.
Our studies indicate that a key determinant of the membrane behavior of side chain oxysterols is the phospholipid acyl chain composition. In model membrane systems, increasing the saturation of phospholipid acyl chains results in a progressive decrease in the mean molecular areas for 25-HC and 27-HC, presumably by promoting insertion of the oxysterols into membranes in a less tilted orientation and facilitating condensed membrane packing. Likewise, we have provided evidence that the degree of saturation of phospholipid acyl chains may influence the cholesterol homeostatic activity of side chain oxysterols in a cell culture model. We have demonstrated that exposure of cells to non-toxic levels of palmitate inhibits the suppression of SREBP-dependent gene expression and HMGR degradation by 25-HC and that palmitate exerts its effect through a non-enantiospecific mechanism. The effect of palmitate supplementation does not appear to be mediated by alterations in the cholesterol content of the ER membrane, but rather through remodeling of ER membrane phospholipids. Analysis of the ER lipidome in palmitate-treated cells demonstrated increased incorporation of palmitate into several phospholipid species, including 2-3-fold elevations of 16:1-16:0, 16:0-18:2, and 16:0-18:1 PC. As these PC species represent ∼45% of total ER membrane phospholipid in palmitate-supplemented CHO cells (data not shown), their increased representation in the predominantly unsaturated lipid environment of the ER membrane would be expected to favor a condensed packing configuration for membrane-associated oxysterols.
Previous studies have shown that fatty acids, in concert with cholesterol and side chain oxysterols, contribute to the overall regulation of SREBP-dependent gene expression. Treatment of cultured cells with oleate or polyunsaturated fatty acids has been shown to inhibit SREBP processing and to decrease transcription of sterol-regulated genes (13-15). By contrast, our studies demonstrate that palmitate treatment enhances SREBP processing. Although several mechanisms have been proposed to explain the effect of fatty acids on the transcription of sterol-regulated genes, including changes in the ER regulatory cholesterol pool or in the levels of intermediates of sphingomyelin metabolism (14, 15), our findings suggest that fatty acids may primarily exert their effect through incorporation into ER membrane phospholipids and, in turn, modulation of the activity of side chain oxysterols. Such membrane remodeling might have profound effects not only on membrane function, but also in mediating protein-protein, protein-membrane, or sterol-protein interactions.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants HL67773 (to D. S. O. and D. F. C.), HL83762 (to D. S. O.), GM47969 (to D. F. C.), DK54268 (to J. E. S.), HL49180 (to H. L. B.), GM45928 (to R. E. B.), and P30 DK56341 and by Training Grant 5T32HL007275-29 (to E. J. W. and N. D.). This work was also supported by the Hormel Foundation (to H. L. B. and R. E. B.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2.
Footnotes
The abbreviations used are: SREBP, sterol regulatory element-binding protein; SRE, sterol regulatory element; HMGR, hydroxymethylglutaryl-CoA reductase; HC, hydroxycholesterol; ent-25-HC, enantiomer of 25-Hydroxycholesterol; nat-25-HC, natural 25-Hydroxycholesterol; ER, endoplasmic reticulum; SCAP, SREBP cleavage-activating protein; LXR, liver X receptor; PM, plasma membrane; CHO, Chinese hamster ovary; LPDS, lipoprotein-deficient bovine serum; CF, carboxyfluorescein; SPR, surface plasmon resonance; POPC, palmitoyloleoylphosphatidylcholine; DPPC, dipalmitoylphosphatidylcholine; DOPC, dioleoylphosphatidylcholine; PC, phosphatidylcholine; CD, methyl β-cyclodextrin; 7-KC, 7-ketocholesterol.
References
- 1.Radhakrishnan, A., Sun, L. P., Kwon, H. J., Brown, M. S., and Goldstein, J. L. (2004) Mol. Cell 15 259-268 [DOI] [PubMed] [Google Scholar]
- 2.Adams, C. M., Reitz, J., De Brabander, J. K., Feramisco, J. D., Li, L., Brown, M. S., and Goldstein, J. L. (2004) J. Biol. Chem. 279 52772-52780 [DOI] [PubMed] [Google Scholar]
- 3.Chawla, A., Repa, J. J., Evans, R. M., and Mangelsdorf, D. J. (2001) Science 294 1866-1870 [DOI] [PubMed] [Google Scholar]
- 4.Brown, A. J., Sun, L., Feramisco, J. D., Brown, M. S., and Goldstein, J. L. (2002) Mol. Cell 10 237-245 [DOI] [PubMed] [Google Scholar]
- 5.Radhakrishnan, A., Ikeda, Y., Kwon, H. J., Brown, M. S., and Goldstein, J. L. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 6511-6518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song, B. L., Javitt, N. B., and DeBose-Boyd, R. A. (2005) Cell Metab. 1 179-189 [DOI] [PubMed] [Google Scholar]
- 7.Lange, Y., Ory, D. S., Ye, J., Lanier, M. H., Hsu, F. F., and Steck, T. L. (2008) J. Biol. Chem. 283 1445-1455 [DOI] [PubMed] [Google Scholar]
- 8.Frolov, A., Zielinski, S. E., Crowley, J. R., Dudley-Rucker, N., Schaffer, J. E., and Ory, D. S. (2003) J. Biol. Chem. 278 25517-25525 [DOI] [PubMed] [Google Scholar]
- 9.Lange, Y. (1994) J. Biol. Chem. 269 3411-3414 [PubMed] [Google Scholar]
- 10.Lange, Y., Ye, J., and Steck, T. L. (2004) Proc. Natl. Acad. Sci. U. S. A. 101 11664-11667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Massey, J. B., and Pownall, H. J. (2006) Biochemistry 45 10747-10758 [DOI] [PubMed] [Google Scholar]
- 12.Westover, E. J., and Covey, D. F. (2006) Steroids 71 484-488 [DOI] [PubMed] [Google Scholar]
- 13.Hannah, V. C., Ou, J., Luong, A., Goldstein, J. L., and Brown, M. S. (2001) J. Biol. Chem. 276 4365-4372 [DOI] [PubMed] [Google Scholar]
- 14.Worgall, T. S., Johnson, R. A., Seo, T., Gierens, H., and Deckelbaum, R. J. (2002) J. Biol. Chem. 277 3878-3885 [DOI] [PubMed] [Google Scholar]
- 15.Worgall, T. S., Sturley, S. L., Seo, T., Osborne, T. F., and Deckelbaum, R. J. (1998) J. Biol. Chem. 273 25537-25540 [DOI] [PubMed] [Google Scholar]
- 16.Cruz, J. C., Sugii, S., Yu, C., and Chang, T. Y. (2000) J. Biol. Chem. 275 4013-4021 [DOI] [PubMed] [Google Scholar]
- 17.Millard, E., Gale, S., Dudley, N., Zhang, J., Schaffer, J., and Ory, D. (2005) J. Biol. Chem. 280 28581-28590 [DOI] [PubMed] [Google Scholar]
- 18.Smaby, J. M., Momsen, M. M., Brockman, H. L., and Brown, R. E. (1997) Biophys. J. 73 1492-1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szoka, F., Jr., and Papahadjopoulos, D. (1978) Proc. Natl. Acad. Sci. U. S. A. 75 4194-4198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rex, S., and Schwarz, G. (1998) Biochemistry 37 2336-2345 [DOI] [PubMed] [Google Scholar]
- 21.Borradaile, N. M., Han, X., Harp, J. D., Gale, S. E., Ory, D. S., and Schaffer, J. E. (2006) J. Lipid Res. 47 2726-2737 [DOI] [PubMed] [Google Scholar]
- 22.Gale, S. E., Frolov, A., Han, X., Bickel, P. E., Cao, L., Bowcock, A., Schaffer, J. E., and Ory, D. S. (2006) J. Biol. Chem. 281 11082-11089 [DOI] [PubMed] [Google Scholar]
- 23.Westover, E. J., and Covey, D. F. (2004) J. Membr. Biol. 202 61-72 [DOI] [PubMed] [Google Scholar]
- 24.Wang, Y., Rogers, P. M., Su, C., Varga, G., Stayrook, K. R., and Burris, T. P. (2008) J. Biol. Chem. 283 26332-26339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Westover, E. J., Covey, D. F., Brockman, H. L., Brown, R. E., and Pike, L. J. (2003) J. Biol. Chem. 278 51125-51133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathai, J. C., Tristram-Nagle, S., Nagle, J. F., and Zeidel, M. L. (2008) J. Gen. Physiol. 131 69-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lange, Y., Ye, J., Rigney, M., and Steck, T. L. (1999) J. Lipid Res. 40 2264-2270 [PubMed] [Google Scholar]
- 28.Lange, Y., and Steck, T. L. (1997) J. Biol. Chem. 272 13103-13108 [DOI] [PubMed] [Google Scholar]
- 29.Liu, J., Chang, C. C., Westover, E. J., Covey, D. F., and Chang, T. Y. (2005) Biochem. J. 391 389-397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang, J., Sun, F., Zhang, D. W., Ma, Y., Xu, F., Belani, J. D., Cohen, J. C., Hobbs, H. H., and Xie, X. S. (2006) J. Biol. Chem. 281 27894-27904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kauffman, J. M., Westerman, P. W., and Carey, M. C. (2000) J. Lipid Res. 41 991-1003 [PubMed] [Google Scholar]
- 32.Lange, Y., Ye, J., Rigney, M., and Steck, T. (2000) J. Biol. Chem. 275 17468-17475 [DOI] [PubMed] [Google Scholar]
- 33.Infante, R. E., Abi-Mosleh, L., Radhakrishnan, A., Dale, J. D., Brown, M. S., and Goldstein, J. L. (2008) J. Biol. Chem. 283 1052-1063 [DOI] [PubMed] [Google Scholar]
- 34.Aittoniemi, J., Rog, T., Niemela, P., Pasenkiewicz-Gierula, M., Karttunen, M., and Vattulainen, I. (2006) J. Phys. Chem. B 110 25562-25564 [DOI] [PubMed] [Google Scholar]
- 35.Phillips, J. E., Geng, Y. J., and Mason, R. P. (2001) Atherosclerosis 159 125-135 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.