Abstract
Activation of the p53 tumor suppressor by cellular stress leads to variable responses ranging from growth inhibition to apoptosis. TIGAR is a novel p53-inducible gene that inhibits glycolysis by reducing cellular levels of fructose-2,6-bisphosphate, an activator of glycolysis and inhibitor of gluconeogenesis. Here we describe structural and biochemical studies of TIGAR from Danio rerio. The overall structure forms a histidine phosphatase fold with a phosphate molecule coordinated to the catalytic histidine residue and a second phosphate molecule in a position not observed in other phosphatases. The recombinant human and zebra fish enzymes hydrolyze fructose-2,6-bisphosphate as well as fructose-1,6-bisphosphate but not fructose 6-phosphate in vitro. The TIGAR active site is open and positively charged, consistent with its enzymatic function as bisphosphatase. The closest related structures are the bacterial broad specificity phosphatase PhoE and the fructose-2,6-bisphosphatase domain of the bifunctional 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase. The structural comparison shows that TIGAR combines an accessible active site as observed in PhoE with a charged substrate-binding pocket as seen in the fructose-2,6-bisphosphatase domain of the bifunctional enzyme.
The p53 tumor suppressor protein is a stress-induced transcription factor that controls various cellular response mechanisms including cell cycle arrest and apoptosis (1, 2). As part of a complex signaling network that includes positive and negative feedback loops, the p53 pathway determines cell death or survival after its activation by various forms of cellular damage (3). TIGAR (TP53-induced glycolysis and apoptosis regulator) is a recently identified p53-regulated gene that was found to lower fructose-2,6-bisphosphate (Fru-2,6-P2)2 levels in cells. TIGAR expression resulted in down-regulation of glycolysis, reduction of intracellular levels of reactive oxygen species, and protection from apoptosis (4).
Fru-2,6-P2 is a key regulator of cellular metabolism that activates glycolysis and inhibits gluconeogenesis. Fru-2,6-P2 is found in almost all eukaryotes but is absent from prokaryotes (5). Other functions for this molecule in addition to its allosteric regulation of glycolysis and gluconeogenesis have been identified (6). The structure and function of the bifunctional 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (FBPase-2) enzymes responsible for Fru-2,6-P2 synthesis and removal within the cell have been studied for more than 20 years (7). The transcription of a hypoxia-inducible isoform (PFKFB3) is controlled by hypoxia-inducible factor 1 (8), and this isoform is frequently found in cancer cells (9). Thus, synthesis and removal of Fru-2,6-P2 by PFKFB3 and TIGAR is controlled by hypoxia-inducible factor 1 and p53, emphasizing the importance of metabolic regulation for tumor suppression (10).
Histidine phosphatases share a strictly conserved catalytic core centered at a histidine residue that is phosphorylated during the reaction. Two arginine residues and a second histidine form a structurally conserved phosphate-binding pocket that coordinates the substrate phosphate during catalysis. Several metabolic enzymes, such as cofactor-dependent phosphoglycerate mutase, bisphosphoglycerate mutase, and the fructose-2,6-bisphosphatase domains of 6-phosphofructo-2-kinase/FBPase-2 belong to the larger branch 1 of the histidine phosphatase superfamily (11).
To study the structural and biochemical properties of this enzyme, we purified recombinant human TIGAR and homologs from mouse, rat, and zebrafish (sequence- and structure-based alignments are shown in supplemental Fig. S1). We first obtained crystals from the recombinant Danio rerio homolog after limited proteolysis and determined the structure of the full-length protein based on the initial experiments.
EXPERIMENTAL PROCEDURES
Protein Expression, Purification, and Crystallization—The full-length TIGAR homolog from D. rerio (GenBank™ BC045897, zgc: 56074) was cloned into the pET26b vector (Novagen) and overexpressed in Escherichia coli strain BL21Star (Invitrogen) at 293 K. Bacterial cells were lysed by ultrasonification on ice in a buffer containing 20 mm Tris, pH 8.5, 200 mm NaCl, 5 mm β-mercaptoethanol, 0.1% Triton X-100, and 5% glycerol. Soluble C-terminally hexahistidine-tagged TIGAR was bound to nickel-agarose affinity resin (Qiagen), washed with a buffer containing 20 mm Tris, pH 8.5, 200 mm NaCl, and 10 mm imidazole, and eluted with a buffer containing 20 mm Tris, pH 8.8, 250 mm NaCl, and 150 mm imidazole. The protein was further purified with anion exchange chromatography, using a linear gradient of 10 mm to 1 m NaCl concentration and size exclusion chromatography at pH 8.5 and 200 mm NaCl. Purified TIGAR was concentrated to 20 mg/ml in a buffer containing 20 mm Tris, pH 8.5, and 10 mm NaCl. Full-length TIGAR from Homo sapiens (GenBank™ BC012340) and fructose-1,6-bis-phosphatase from Thermus thermophilus (GenBank™ YP_144712) were generated following similar protocols as described above.
For limited proteolytic digest, trypsin (MP Biomedicals) was added to the purified protein in a 1:100 (w/w) ratio and incubated at 277 K for 6 h. 4-(2-Aminoethyl)benzenesulfonylfluoride (Calbiochem) was added to a final concentration of 2 mm to stop the reaction. The stable core fragment was then purified by size exclusion chromatography at pH 8.5 and 200 mm NaCl and concentrated to 20 mg/ml in a buffer containing 20 mm Tris, pH 8.5, and 10 mm NaCl. For the production of selenomethionyl protein, the expression construct was transformed into B834(DE3) cells (Novagen). The bacterial growth was carried out in defined LeMaster medium (12), and the protein was purified and digested using the same protocols as for the wild type protein.
Crystals of the tryptic core fragment were obtained with the vapor diffusion method. 1 μl of protein was mixed with 1 μl of a solution containing 1 m potassium sodium tartrate and 100 mm MES, pH 6.5. The crystals in space group P65 grew in 3 days to a maximum size of 100 × 150 × 80 μm at 293 K. To obtain crystals for the full-length protein, 1 μl of protein was mixed with 1 μl of a solution containing 1 m sodium phosphate at a pH of 8.5. Crystal clusters in space group C2 grew in 3-5 days to a maximum size of 300 × 300 × 600 μm at 293 K. A piece of a larger crystal with a size of 80 × 80 × 10 μm was used for data collection. To obtain crystals for the H11A mutant form, 1 μl of protein was mixed with 1 μl of a solution containing 3% polyethylene glycol 8000, 100 mm calcium acetate, and 100 mm imidazole, pH 7.5. Crystals in space group P65 grew in 2-5 days to a maximum size of 200 × 50 × 50 μm at 293 K. The crystals were flash frozen in liquid nitrogen from their mother liquor supplemented with 25% ethylene glycol.
Data Collection, Structure Determination, and Refinement—Diffraction data were collected on a MAR CCD detector at the X4C beamline of the National Synchrotron Light Source in Brookhaven at a wavelength of 0.979 Å at 100 K. For the structure determination of the tryptic core fragment, a selenomethionyl single wavelength anomalous dispersion data set was collected. Diffraction images were processed and scaled with the HKL2000 package (13). The data processing statistics are summarized in Table 1. The locations of 13 selenium atoms were determined with the program Solve (14), and an initial model was built with ARP/wARP (15). The structures of the full-length protein and the H11A mutant crystal form were solved by molecular replacement with the programs COMO (16) and Phaser (17) with the tryptic core structure as a search model. Missing parts of the initial models were built manually into σA-weighted Fo-Fc difference density maps with the program Coot (18). The models were refined with Refmac (19), and the CCP4 (20) package was used for subsequent calculations. Electron density was in general well defined with the exception of the loop region between residues 150 and 160. The final model of full-length TIGAR contains residues 1-250 in molecule A, whereas residues 149-159 in molecule B were omitted. The same loop region between residues 150 and 159 was disordered in the final model of the H11A mutant. The final model of the tryptic core fragment structure included the following regions: residues 1-14, 28-92, 122-153, and 158-250 in molecule A and residues 2-14, 28-92, 119-148, and 160-250 in molecule B.
TABLE 1.
Data collection and refinement statistics
| Core fragment | Full length | H11A mutant | |
|---|---|---|---|
| Data collectiona | |||
| Space group | P65 | C2 | P65 |
| Cell dimensions | |||
| a, b, c (Å) | 136.7, 136.7, 66.8 | 127.7, 115.5, 61.3 | 91.4, 91.4, 155.9 |
| α, β, γ (°) | 90, 90, 120 | 90, 104.66, 90 | 90, 90, 120 |
| Resolution (Å)b | 20-2.0 (2.05-2.0) | 30-2.0 (2.05-2.0) | 30-2.1 (2.15-2.1) |
| Rmerge | 0.058 (0.439) | 0.076 (0.425) | 0.079 (0.446) |
| I/σI | 27.0 (3.8) | 14.3 (1.8) | 16.8 (2.4) |
| Completeness (%) | 100 (100) | 95.3 (66.3) | 99.2 (97.1) |
| Redundancy | 5.8 (5.8) | 3.0 (2.2) | 3.8 (3.5) |
| Refinement | |||
| No. reflections | 44,941 (3,396) | 53,351 (3,487) | 40,383 (3,038) |
| Rwork/Rfree | 0.170/0.209 (0.263/0.288) | 0.171/0.213 (0.243/0.273) | 0.192/0.234 (0.231/0.276) |
| No. atoms | |||
| Protein | 3,194 | 3,826 | 3,743 |
| Ligand/ion | 2 | 22 | 10 |
| Water | 483 | 698 | 330 |
| B-factors | |||
| Protein | 26.91 | 25.53 | 29.07 |
| Ligand/ion | 24.12 | 35.97 | 42.24 |
| Water | 45.23 | 41.72 | 39.30 |
| Root mean square deviations | |||
| Bond lengths (Å) | 0.012 | 0.012 | 0.012 |
| Bond angles (°) | 1.16 | 1.14 | 1.25 |
One crystal was used for each data set.
The values in parentheses are for the highest resolution shell.
The Ramachandran statistics calculated with Procheck (21) are (most favored/additionally allowed/generously allowed/disallowed) 91.4/8.4/0.3/0.0% for the tryptic core fragment, 92.7/7.3/0.0/0.0% for the full-length structure, and 90.1/9.2/0.7/0.0% for the H11A mutant structure. The structure refinement statistics are summarized in Table 1. The figures were generated using Pymol.
Synthesis of Fructose-2,6-bisphosphate—Fuctose-2,6-bisphosphate was synthesized from fructose-1,6-bisphosphate (Sigma-Aldrich) as described by van Schaftingen et al. (22). The sodium salt of fructose-1,6-bisphosphate was converted to the pyridinium salt on a Dowex 50 (200-400 mesh, H+ form; Acros Organics) column that had been neutralized with pyridine. 12 ml of a 0.2 m solution of the pyridinum salt of fructose-1,6-bisphosphate were mixed with 1 ml of triethylamine, 40 ml of pyridine, and 4.8 g of dicyclohexylcarbodiimide (Fluka) in 20 ml of pyridine. The mixture was stirred at room temperature for 24 h. The reaction was stopped by the addition of 80 ml of water and then extracted with 200 ml of ether. The aqueous phase was extracted with 200 ml of ether four more times. The aqueous phase was rotated in an evaporator for 30 min to remove traces of ether. 0.2 volume of 2.5 m NaOH were added, and the aqueous solution was incubated for 30 min at 37 °C. After incubation, glycine was added to a final concentration of 20 mm. The solution was rapidly cooled, and the pH was adjusted to 9.4 by adding concentrated HCl. 10 mg of T. thermophilus fructose-1,6-bisphosphatase and MnCl2 to a final concentration of 0.5 mm were added, and the mixture was incubated overnight at room temperature. The mixture was then diluted with water to reduce the sodium concentration below 100 mm. Fructose bisphosphates were separated by anion exchange chromatography using a linear gradient of 0 mm to 400 m NaCl. Fractions containing sugar derivatives were detected with the phenol-sulfuric acid method described by Masuko et al. (23). Fractions containing the compound that could be hydrolyzed by human or zebrafish TIGAR but not by fructose-1,6-bisphosphatase were collected, lyophilized, and dissolved in D2O. The compound was confirmed as fructose-2,6-bisphosphate by 13C NMR (24).
Phosphatase Activity Assay—N-terminally histidine-tagged proteins were used for all activity assays to exclude a potential interference of the affinity tags with enzymatic activity. The protein concentrations were determined by Bradford assay. Phosphatase activity was assayed by measuring p-nitrophenol release for p-nitrophenylphosphate (Fluka) as a substrate or by measuring phosphate release for other phosphoesters. All of the assays were carried out at room temperature in 96-well plates using a Molecular Devices SpectraMax M5 microplate reader. For the p-nitrophenylphosphate assay, each reaction contained 95 μl of different concentrations of p-nitrophenylphosphate in 50 mm Tris buffer, pH 7.3, and the reactions were started by the addition of 2 μl of enzyme at a concentration of 20 mg/ml. The change of A405 was monitored over a period of 5 min. Inorganic phosphate release was measured following the method described by Tedokon et al. (25). The reactions were started by adding 95 μl of reaction mixture into wells that contained 5 μl of substrates with varying concentrations. The reaction mixture contained 200 mm sucrose (EMD), 6 mm NAD+ (Sigma-Aldrich), 500 units/liter sucrose phosphorylase (Sigma-Aldrich), 2000 units/liter phosphoglucomutase (Sigma-Aldrich), 5000 units/liter glucose-6-phosphate dehydrogenase (Sigma-Aldrich), 3 mm MgCl2.6H2O (Acros Organics), and 0.1 g/liter bovine serum albumin (New England Biolabs), and 25 μg of enzyme. Control reactions were performed without the addition of enzyme to the reaction mixture. The change of A340 was monitored over a period of 5 min. At least three replicates were performed for each concentration. The reaction rates and standard errors were determined in Excel. The kinetic parameters were determined with SigmaPlot.
Accession Codes—Protein Data Bank coordinates and structure factors have been deposited with accession codes 3E9C, 3E9D, and 3E9E for the core fragment structure, the full-length structure, and the H11A mutant form, respectively.
RESULTS
Structure Determination and Overall Structure of TIGAR—We determined the crystal structure of TIGAR from D. rerio (257 residues) by single-wavelength anomalous dispersion methods in space group P65 initially from crystals obtained after partial trypsin digestion of the recombinant protein. The crystallographic R/Rfree factors for this structure are 0.170 and 0.209. There are two molecules in the asymmetric unit, and inspection of the interface region between the two monomers revealed the presence of an unexpected disulfide bond. Following this observation, we repeated crystallization experiments with the full-length protein in the absence of reducing agents and obtained crystals from different conditions than the tryptic fragment crystals. We solved the full-length structure in space group C2 to 2.0-Å resolution by molecular replacement using the tryptic core structure as model. The crystallographic R/Rfree factors for the full-length structure are 0.171 and 0.213. The final model of the full-length protein consists of residues 1-250 for molecule A and residues 1-251 for molecule B. The full-length protein contains 257 residues, and no electron density was observed for the C-terminal residues and the C-terminal hexahistidine tag. Electron density was well defined for both molecules with the exception of residues 149-159 in the loop region between helix α5 and strand β4 in molecule B. These residues were not included in the model. The crystals of the full-length protein were obtained with sodium phosphate as a precipitant, and there are two phosphate molecules bound in the active site in this structure. To determine a substrate-bound structure, we generated an H11A site-directed mutant form of the zebrafish homolog. We were able to obtain another crystal form for the mutant protein from phosphate-free crystallization conditions. We solved the structure of this crystal form by molecular replacement in space group P65 to 2.1 Å resolution. Experiments to trap a substrate-bound complex by soaking crystals with fructose-1,6-bisphosphate (Fru-1,6-P2) or Fru-2,6-P2 were not successful. Instead of the bisphosphates, we observed a single phosphate molecule bound in the active site of the H11A mutant structure. The data collection and refinement statistics for the three data sets are given in Table 1.
As expected from sequence homology, the overall TIGAR structure contains a histidine-phosphatase fold (Fig. 1A). An α/β/α sandwich forms the core with a central six-stranded mixed β-sheet that is flanked by two helices on each side. Two inserted regions (between β1 and α2 and between β3 and α7) provide a short helix α1 and helices α5 and α6 that together enclose the active site region. The TIGAR structure contains an additional longer inserted region between α7 and β4 that folds as a long loop to partially cover the central β-sheet. This loop insertion is not observed in other histidine phosphatases. A comparison of the trypsin-stable fragment structure with the full-length structure shows that the two inserted regions defining one side of the active site and the protruding part of the long loop have been cleaved and are either completely absent or disordered (Fig. 1B). The structure of the core fragment is virtually identical with the full-length structure with a root mean square deviation of 0.38 Å for 201 Cα atoms.
FIGURE 1.
Overall structure of TIGAR. A, ribbon diagram of the full-length structure. Two bound phosphate molecules and residues in the active site are shown as sticks. B, structure of the tryptic core fragment in the same orientation. The positions of structural elements missing after trypsin digest are indicated with arrows. C, symmetric dimer formation between two monomers in the asymmetric unit of the full-length structure. D, close-up view illustrating the hydrogen-bonding network between the two molecules and the coordination of the two metal ions. E, dimer formation in the H11A mutant structure. Molecule A (colored in blue and cyan) is shown in the same orientation as in C. F, close-up view of the interface region illustrating the substantially different orientation of molecule B (orange) compared with the full-length structure in D.
Dimer Formation—Estimates of protein size from size exclusion chromatography indicated that the recombinant TIGAR proteins from human, mouse, and zebrafish are monomeric in solution. We were therefore surprised when we found that the two monomers in the asymmetric unit form a symmetric dimer via a β-strand interaction between both strands β5. A disulfide bond between Cys201 of each monomer coordinates this protein-protein interaction. In the structures of the trypsin fragment and the wild type protein, the additional coordination of two metal ions in the interface region further stabilizes the interface (Fig. 1, C and D). We modeled these metal ions as potassium, which was required to obtain crystals for both protein samples and could not be replaced by sodium, calcium, or magnesium. In the final model, the potassium temperature factors are consistent with the average temperature factor for protein atoms, and the average metal-oxygen distance is comparable with values found in the metalloprotein data base (26). Both metal ions are in symmetric positions and are coordinated in a somewhat distorted octahedral geometry by five main chain oxygen atoms and a solvent water molecule. Additional hydrogen bonds between the side chains of Glu157 in chain A and Arg238 in chain B and a symmetric interaction between both side chains Gln199 and Gln240 contribute to the dimer interface.
H11A mutant crystals were obtained in the absence of metal ions. The disulfide bond is retained in this crystal form, but there are no additional ions coordinated in the interface. The orientation of the two monomers with respect to each other differs significantly from the first two crystal forms. One of the two molecules rotates by almost 180 degrees, and the β-strand interaction between the two monomers is absent in this crystal form (Fig. 1, E and F). Together with the observation that Cys201 is not conserved in other TIGAR homologs, these results indicate that dimer formation is unlikely to be of physiologic relevance.
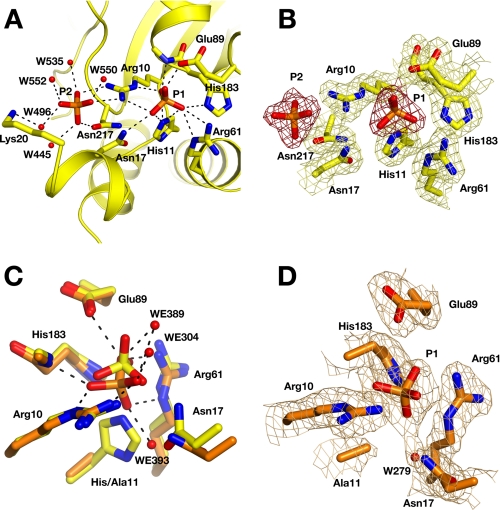
Phosphate Coordination in the Active Site—We observed two phosphate ions bound in the active site of the full-length TIGAR structure (Fig. 2, A and B). The first phosphate molecule coordinates to the catalytic residues His11, His183, Arg10, Arg61, and Glu89. The second phosphate molecule is located closer to the surface of the active site cavity at a distance of 7.5 Å from the first phosphate and coordinates to Arg10, Asn217, and several solvent water molecules. The position of this second phosphate molecule could indicate the substrate orientation of a bound fructose bisphosphate molecule. Substrate docking calculations with the program Dock6 (27) indeed indicate that both Fru-1,6-P2 and Fru-2,6-P2 can be placed in the active site in an orientation analogous to the position of the two phosphate molecules. However, the TIGAR active site is comparatively open, and other substrate binding orientations can also be found. In addition, the position of the second phosphate molecule is occupied by protein residues in structurally related phosphatases, and partial closure of the active site by residues in the disordered C-terminal region might prevent substrate binding in the second phosphate-binding site (further discussed below).
FIGURE 2.
Phosphate binding in the wild type and H11A mutant active site. A, ribbon diagram for the active site of wild type TIGAR. Residues engaged in hydrogen bonds with the two phosphate molecules are shown as sticks. Solvent water molecules are shown as red spheres. B, final σA-weighted 2Fo-Fc electron density for the active site region in the full-length wild type structure contoured at the 1 σ level. C, comparison of phosphate position in wild type (yellow) and H11A mutant (orange). Solvent water molecules are shown for the mutant structure. D, final σA-weighted 2Fo-Fc electron density for the H11A mutant structure.
Mutation of His11 to alanine does not abrogate phosphate binding in the active site. The phosphate ion coordinated in the mutant structure is slightly shifted toward the position of the absent His11 side chain. It interacts with an additional solvent water molecule (WE393), which partly occupies the position of the absent histidine side chain. The conformation of all other active site residues remains unchanged (Fig. 2, C and D). The second phosphate-binding site is not occupied in the mutant structure. The concentration of phosphate ions was substantially lower in this experiment, because phosphate ions were presumably only generated by hydrolysis of fructose bisphosphates during substrate soaking of the crystals. The lack of phosphate binding to the second binding site is consistent with the weaker coordination of the second phosphate group seen in the wild type structure.
Catalytic Activity—To confirm the catalytic activity, we performed enzymatic assays with the recombinant human and zebrafish enzymes. Both enzymes are active with Fru-2,6-P2 and with Fru-1,6-P2 (Table 2 and supplemental Fig. S2), but we did not detect any activity with fructose 6-phosphate or ribose 5-phosphate. In histidine phosphatases, catalysis generally proceeds via a covalent histidine-phosphate intermediate (11). Nevertheless, the H11A mutant form retained some catalytic activity toward both bisphosphate substrates (14% of kcat for Fru-2,6-P2 and 32% for Fru-1,6-P2; further discussed below).
TABLE 2.
Kinetics of human and zebrafish TIGAR
|
F16BP
|
F26BP
|
|||||
|---|---|---|---|---|---|---|
| Tigar_hs | Tigar_dr | Tigar_dr_H11A | Tigar_hs | Tigar_dr | Tigar_dr_H11A | |
| Kcat (min−1) | 4.23 | 4.59 | 1.47 | 4.35 | 4.28 | 0.59 |
| Km (mm) | 0.43 | 0.31 | 0.3340 | 0.1064 | 0.0930 | 0.1534 |
| kcat/Km (min−1 mm−1) | 9.84 | 14.81 | 4.40 | 40.88 | 46.02 | 3.85 |
DISCUSSION
A data base search with the SSM algorithm (28) showed that the TIGAR structure is most closely related to the bacterial broad specificity phosphatase PhoE (29), followed by phosphoglycerate mutases from Mycobacterium tuberculosis (30) and Saccharomyces cerevisiae (31). The structural comparison with the PhoE phosphatase (Protein Data Bank code 1H2E) (32) shows that the core phosphatase fold is well conserved between both enzymes (supplemental Fig. S1 and Fig. 3A). The two structures can be superimposed with a root mean square deviation of 1.2 Å for 173 Cα atoms. In TIGAR, the β-strands β6 and β7 extend longer at the remote side of the central β-sheet, and the long loop insertion between α7 and β4 is not observed in PhoE. More significantly, however, the inserted regions defining the active site are substantially different between both enzymes. In PhoE, two helices occupy the position of the TIGAR helix α6, and the following loop region is longer and forms an additional short helical turn. Conversely, the loop region between helix α8 and β6 is longer in TIGAR and contains an additional helix (α9) that is not present in PhoE.
In the active site, the orientations of key catalytic residues and the position of the first phosphate molecule are nearly identical between both enzymes (Fig. 3B). However, the C-terminal residue Val208 occupies the position of the second phosphate coordinated in the TIGAR structure, and the C-terminal carboxylate group engages in hydrogen bonds to Arg9 that are equivalent to the phosphate coordination to Arg10. In a comparison of the catalytic activity, PhoE is most active with 3-phosphoglycerate and hydrophobic compounds such as p-nitrophenylphosphate and α-naphtyl-phosphate (33). This substrate preference is consistent with a large number of hydrophobic residues present in the active site in PhoE. Rigden et al. (29) proposed that the combination of a broad and more open active site with the low electrostatic charge in the active site determines the higher activity of PhoE with larger and hydrophobic substrates such as α-naphtylphosphate. Similar to PhoE, the TIGAR active site is open and easily accessible from the solvent region. However, the active site pocket is significantly more positively charged compared with PhoE, which is consistent with its catalytic activity as bisphosphatase.
FIGURE 3.
Structural comparison with the PhoE phosphatase and with fructose-2,6-bisphosphatase. A, least squares superposition of TIGAR with PhoE (Protein Data Bank code 1H2E). TIGAR is colored yellow, and PhoE is colored cyan. The positions of significant structural differences are indicated with arrows. B, comparison of phosphate binding in TIGAR and PhoE. C, least squares superposition of TIGAR (yellow) with FBPase-2 (green; Protein Data Bank code 2BIF). D, comparison of phosphate binding in TIGAR and FBPase-2.
A substantial rearrangement of the C-terminal region was described in PhoE. In the apo-enzyme, the last five residues were disordered, but in the phosphate-bound structure, these residues fold back to the active site where the C-terminal Val208 coordinates to the active site Arg9 (32). In TIGAR, the last six residues are also disordered, raising the question whether a similar disorder-order transition might occur in the presence of substrates. The TIGAR C-terminal tail is longer by one residue, which would prevent the coordination of the C-terminal carboxylate group in the same orientation as in PhoE. In addition, a simple homology model of the TIGAR C-terminal residues based on the PhoE structure reveals that interacting residues in other regions are not conserved between the two enzymes. In PhoE, Tyr177 forms a hydrogen bond to Glu207 that stabilizes the C-terminal loop conformation, and Tyr175 interacts with the C-terminal carboxylate group. Both tyrosine residues are not conserved in TIGAR. Furthermore, the predicted orientation of Lys247 (Val299 in PhoE) would likely clash with the side chain of Val253 (Val205 in PhoE). Nevertheless, it is conceivable that the TIGAR C-terminal tail region can adopt an orientation that blocks the binding position of the second phosphate molecule in an analogous manner, as has been seen in PhoE.
To better understand the structural determinants for substrate recognition, we compared the TIGAR structure with the functionally closest related fructose-2,6-bisphosphatase domain of the bifunctional 6-phosphofructo-2-kinase/FBPase-2 (Protein Data Bank code 2BIF) (34). The comparison reveals similar differences as observed with PhoE (Fig. 3C). Again, the core phosphatase fold is well conserved, and strands β6 and β7 are longer in the TIGAR structure. In the active site region, the TIGAR helix α6 is replaced by two separate helices, and the loop region including helix α9 is not present in FBPase-2. More significantly, the C-terminal region of FBPase-2 contains 25 additional residues that are not present in either TIGAR or PhoE. These C-terminal residues form two extended loop regions that enclose the active site. The access to the FBPase-2 active site is therefore more restricted, which likely contributes to the high specificity of this enzyme for Fru-2,6-P2.
In the active site, the phosphate molecule is slightly shifted in FBPase-2 together with a shift of the catalytic histidine His256 (Fig. 3D). Similar to PhoE, the binding site for the second phosphate molecule is occupied by protein residues from the C-terminal tail. In FBPase-2, Val441 and Thr443 are located in this region, and Thr443 coordinates to Arg255 (Arg10 in TIGAR). The comparison of interactions with fructose 6-phosphate bound in the FBPase-2 active site shows that only some phosphate-coordinating residues are conserved. Arg395 (Arg188 in TIGAR) and Lys354 (Arg115) are located at conserved positions in TIGAR but are oriented toward the region that is closed by the C-terminal extension in FBPase-2. Other interacting residues, such as Arg350 and Tyr365, are not conserved in TIGAR, although Arg90 could conceivably substitute for these residues. Overall, the Fru-2,6-P2 binding site is not fully conserved between both enzymes, and at least two different orientations for Fru-2,6-P2 are therefore conceivable in TIGAR. The 6-phosphate group could be placed either as observed in the FBPase-2 structure or as indicated by the position of the second phosphate molecule bound in the TIGAR structure.
The comparison with FBPase-2 reveals similarities regarding the mechanism of bisphosphate hydrolysis. The TIGAR H11A mutant enzyme retains a considerable amount of catalytic activity given the key functional role of the catalytic histidine. Catalysis in the rat liver FBPase-2 has been shown to proceed via a phosphoenzyme intermediate and mutation of the catalytic histidine results in loss of activity in this enzyme (35). However, a more recent study found that the H258A mutant form of the rat liver FBPase-2 is an active bisphosphatase that catalyzes hydrolysis without formation of a covalent intermediate (36). In addition, residual catalytic activity for the H256A mutant has been reported for rat testis FBPase-2, which retains about 17% catalytic activity (37). Because of the formation of a phospho-histidine intermediate in the wild type enzymes, the hydrolysis reaction in the mutant enzymes has to occur by a different mechanism. For the rat testis FBPase-2, Yuen et al. (38) proposed an alternative catalytic mechanism involving the direct hydrolysis of Fru-2,6-P2 by water. The positions of the phosphate and solvent water molecules that we observed in the TIGAR H11A mutant structure would be consistent with such a reaction mechanism. Although the second phosphate of the substrate may be oriented differently in TIGAR, the phosphate-binding interactions in the active site are well conserved between both enzymes. Together, these observations suggest that, despite the differences between the substrate binding sites, the same active site geometry and likely the same catalytic mechanism operate in TIGAR and FBPase-2.
During the preparation of this manuscript, the structure of human TIGAR was deposited in the Protein Data Bank by the Center for Eukaryotic Structural Genomics with Protein Data Bank code 3DCY. The human TIGAR structure is highly similar to the zebrafish structure with an overall root mean square deviation of 1.0 Å for 215 Cα atoms. The intermolecular disulfide bond is not conserved in the human structure, supporting our conclusion that the enzyme is likely to function as a monomer in solution. The conformations of active site residues and the phosphate molecule bound in the active site are virtually identical in both structures. Only two residues close to the active site differ between both structures. Lys100, which extends into the active site in the zebrafish structure, is replaced with Leu100 in the human structure, and the side chain of Arg188 is oriented toward the active site in the human structure where it might interact with a substrate phosphate group.
In summary, we show that the TIGAR structure forms a histidine phosphatase fold that is closest related to the PhoE phosphatase. Secondary structure elements surrounding the active site region in the TIGAR structure are distinct from PhoE and from the functionally related FBPase-2. The TIGAR active site is readily accessible and positively charged, consistent with the observed catalytic activity with both Fru-2,6-P2 and Fru-1,6-P2. Given its enzymatic activity for both fructose bisphosphates, expression of TIGAR inhibits cellular glycolysis not only by removing the allosteric regulator Fru-2,6-P2 but also by removing the glycolytic intermediate Fru-1,6-P2. Considering the structural similarity of the active site to the PhoE phosphatase, the enzyme may even act on additional substrate molecules. Further studies will therefore be required to better define this potential broader substrate specificity of TIGAR and the implications for glycolysis and apoptosis regulation.
Supplementary Material
Acknowledgments
We thank John Schwanof and Randy Abramowitz for access to the X4C beamline at the National Synchrotron Light Source and Hasan Demirci for help with data collection.
The atomic coordinates and structure factors (codes 3E9C, 3E9D, and 3E9E) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
This work was supported, in whole or in part, by National Institutes of Health Grant P20RR15578. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
Footnotes
The abbreviations used are: Fru-2,6-P2, fructose-2,6-bisphosphate; Fru-1,6-P2, fructose-1,6-bisphosphate; FBPase-2, fructose-2,6-bisphosphatase; MES, 4-morpholineethanesulfonic acid.
References
- 1.Laptenko, O., and Prives, C. (2006) Cell Death Differ. 13 951-961 [DOI] [PubMed] [Google Scholar]
- 2.Vogelstein, B., and Kinzler, K. W. (2004) Nat. Med. 10 789-799 [DOI] [PubMed] [Google Scholar]
- 3.Harris, S. L., and Levine, A. J. (2005) Oncogene 24 2899-2908 [DOI] [PubMed] [Google Scholar]
- 4.Bensaad, K., Tsuruta, A., Selak, M. A., Vidal, M. N. C., Nakano, K., Bartrons, R., Gottlieb, E., and Vousden, K. H. (2006) Cell 126 107-120 [DOI] [PubMed] [Google Scholar]
- 5.Michels, P. A. M., and Rigden, D. J. (2006) IUBMB Life 58 133-141 [DOI] [PubMed] [Google Scholar]
- 6.Wu, C. D., Khan, S. A., Peng, L. J., and Lange, A. J. (2006) Adv. Enz. Regul. 46 72-88 [DOI] [PubMed] [Google Scholar]
- 7.Rider, M. H., Bertrand, L., Vertommen, D., Michels, P. A., Rousseau, G. G., and Hue, L. (2004) Biochem. J. 381 561-579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minchenko, A., Leshchinsky, I., Opentanova, I., Sang, N. L., Srinivas, V., Armstead, V., and Caro, J. (2002) J. Biol. Chem. 277 6183-6187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chesney, J., Mitchell, R., Benigni, F., Bacher, M., Spiegel, L., Al-Abed, Y., Han, J. H., Metz, C., and Bucala, R. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 3047-3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartrons, R., and Caro, J. (2007) J. Bioenerg. Biomembr. 39 223-229 [DOI] [PubMed] [Google Scholar]
- 11.Rigden, D. J. (2008) Biochem. J. 409 333-348 [DOI] [PubMed] [Google Scholar]
- 12.Hendrickson, W. A., Horton, J. R., and LeMaster, D. M. (1990) EMBO J. 9 1665-1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Otwinowski, Z., and Minor, W. (1997) Methods Enzymol. 276 307-326 [DOI] [PubMed] [Google Scholar]
- 14.Terwilliger, T. C., and Berendzen, J. (1999) Acta Crystallogr. Sect. D Biol. Crystallogr. 55 849-861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen, S. X., Morris, R. J., Fernandez, F. J., Ben Jelloul, M., Kakaris, M., Parthasarathy, V., Lamzin, V. S., Kleywegt, G. J., and Perrakis, A. (2004) Acta Crystallogr. Sect. D Biol. Crystallogr. 60 2222-2229 [DOI] [PubMed] [Google Scholar]
- 16.Jogl, G., Tao, X., Xu, Y. W., and Tong, L. (2001) Acta Crystallogr. Sect. D Biol. Crystallogr. 57 1127-1134 [DOI] [PubMed] [Google Scholar]
- 17.McCoy, A. J., Grosse-Kunstleve, R. W., Storoni, L. C., and Read, R. J. (2005) Acta Crystallogr. Sect. D Biol. Crystallogr. 61 458-464 [DOI] [PubMed] [Google Scholar]
- 18.Emsley, P., and Cowtan, K. (2004) Acta Crystallogr. Sect. D Biol. Crystallogr. 60 2126-2132 [DOI] [PubMed] [Google Scholar]
- 19.Murshudov, G. N., Vagin, A. A., and Dodson, E. J. (1997) Acta Crystallogr. Sect. D Biol. Crystallogr. 53 240-255 [DOI] [PubMed] [Google Scholar]
- 20.Bailey, S. (1994) Acta Crystallogr. D. Biol. Crystallogr. 50 760-763 [DOI] [PubMed] [Google Scholar]
- 21.Laskowski, R. A., MacArthur, M. W., Moss, D. S., and Thornton, J. M. (1993) J Appl Crystallogr. 26 283-291 [Google Scholar]
- 22.van Schaftingen, E., and Hers, H. G. (1981) Eur. J. Biochem. 117 319-323 [DOI] [PubMed] [Google Scholar]
- 23.Masuko, T., Minami, A., Iwasaki, N., Majima, T., Nishimura, S. I., and Lee, Y. C. (2005) Anal. Biochem. 339 69-72 [DOI] [PubMed] [Google Scholar]
- 24.Pilkis, S. J., Elmaghrabi, M. R., Cumming, D. A., Pilkis, J., and Claus, T. H. (1982) Methods Enzymol. 89 101-107 [Google Scholar]
- 25.Tedokon, M., Suzuki, K., Kayamori, Y., Fujita, S., and Katayama, Y. (1992) Clin. Chem. 38 512-515 [PubMed] [Google Scholar]
- 26.Castagnetto, J. M., Hennessy, S. W., Roberts, V. A., Getzoff, E. D., Tainer, J. A., and Pique, M. E. (2002) Nucleic Acids Res. 30 379-382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moustakas, D. T., Lang, P. T., Pegg, S., Pettersen, E., Kuntz, I. D., Brooijmans, N., and Rizzo, R. C. (2006) J. Comput.-Aided Mol. Des. 20 601-619 [DOI] [PubMed] [Google Scholar]
- 28.Krissinel, E., and Henrick, K. (2004) Acta Crystallogr. Sect. D Biol. Crystallogr. 60 2256-2268 [DOI] [PubMed] [Google Scholar]
- 29.Rigden, D. J., Mello, L. V., Setlow, P., and Jedrzejas, M. J. (2002) J. Mol. Biol. 315 1129-1143 [DOI] [PubMed] [Google Scholar]
- 30.Muller, P., Sawaya, M. R., Pashkov, I., Chan, S., Nguyen, C., Wu, Y., Perry, L. J., and Eisenberg, D. (2005) Acta Crystallogr. Sect. D Biol. Crystallogr. 61 309-315 [DOI] [PubMed] [Google Scholar]
- 31.Rigden, D. J., Walter, R. A., Phillips, S. E. V., and Fothergill-Gilmore, L. A. (1999) J. Mol. Biol. 289 691-699 [DOI] [PubMed] [Google Scholar]
- 32.Rigden, D. J., Littlejohn, J. E., Henderson, K., and Jedrzejas, M. J. (2003) J. Mol. Biol. 325 411-420 [DOI] [PubMed] [Google Scholar]
- 33.Rigden, D. J., Bagyan, I., Lamani, E., Setlow, P., and Jedrzejas, M. J. (2001) Protein Sci. 10 1835-1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hasemann, C. A., Istvan, E. S., Uyeda, K., and Deisenhofer, J. (1996) Structure 4 1017-1029 [DOI] [PubMed] [Google Scholar]
- 35.Tauler, A., Lin, K., and Pilkis, S. J. (1990) J. Biol. Chem. 265 15617-15622 [PubMed] [Google Scholar]
- 36.Okar, D. A., Wu, C. D., and Lange, A. J. (2004) Adv. Enzyme Regul. 44 123-154 [DOI] [PubMed] [Google Scholar]
- 37.Mizuguchi, H., Cook, P. F., Tai, C. H., Hasemann, C. A., and Uyeda, K. (1999) J. Biol. Chem. 274 2166-2175 [DOI] [PubMed] [Google Scholar]
- 38.Yuen, M. H., Mizuguchi, H., Lee, Y. H., Cook, P. F., Uyeda, K., and Hasemann, C. A. (1999) J. Biol. Chem. 274 2176-2184 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.