Abstract
Previous studies have shown that developmental changes in the structure and function of prefrontal regions can continue throughout childhood and adolescence. Our recent results suggested a role for the left inferior frontal cortex in modulating task-dependent shifts in effective connectivity when adults focus on orthographic versus phonological aspects of presented words. Specifically, the top-down influence of the inferior frontal cortex determined whether incoming word-form information from the fusiform gyrus would have a greater impact on the parietal areas involved in orthographic processing or temporal areas involved in phonological processing. In the current study, we find that children displayed an identical pattern of task-dependent functional activations within this network. In comparison to adults, however, children had significantly weaker top-down modulatory influences emanating from the inferior frontal area. Adult language processing may thus involve greater top-down cognitive control compared to children, resulting in less interference from task-irrelevant information.
Keywords: fMRI, Effective connectivity, Development, Reading, Orthography, Phonology
Introduction
Brain maturation and cognitive development occur concurrently during childhood and adolescence (Casey et al., 2005). While maturation of particular brain regions plays a role in the acquisition of new sensory, motor or cognitive abilities, an important factor in functional brain development is the process of organizing inter-regional interactions (Johnson, 2000). This view is consistent with the notion that complex cognitive functions are subserved by large-scale distributed networks whereby individual components may act as nodal points for integrating and distributing information among other regions in a network (Damasio, 1989; Mesulam, 1998).
One region in which developmental studies have consistently demonstrated late structural changes is the prefrontal cortex (Stuss, 1992; Sowell et al., 2001; Gogtay et al., 2004). Whereas gray matter density in prefrontal regions decreases with age (Gogtay et al., 2004), white matter density and myelination increase with age in these regions (Dobbing and Sands, 1973; Klingberg et al., 1999; Paus et al., 1999). This suggests that there is an increase in connectivity between prefrontal and other regions during late childhood and adolescence (Paus et al., 1999). This age-related increase in structural connectivity is related to developmental improvements in reading (Beaulieu et al., 2005) and memory functions (Sowell et al., 2001). While these results show that development of cognitive abilities is related to changes in structural connectivity, more direct evidence for the importance of inter-regional interaction in development can be provided by examining the relation of cognitive development to functional connectivity. The current study examines developmental changes in the functional interactions among brain regions, and specifically in the interactions between prefrontal and posterior regions.
The left inferior frontal gyrus has an established role in language processing (Cabeza and Nyberg, 2000; Fiez, 1997; Friederici et al., 2003; Poldrack et al., 1999; Price, 2000) and has consistently shown an increase in activation with age in neuroimaging studies on language processing. Both phonological and semantic word generation tasks show increased activation with age in the left inferior frontal cortex (Gaillard et al., 2003; Holland et al., 2001; Schlaggar et al., 2002). Age-related increases in activation in Broadman area 44 of the left inferior frontal gyrus has also been shown in a large sample of children performing a word generation task (332 participants, 5-19 years old) (Schapiro et al., 2004). A similar developmental pattern has been found in other linguistic tasks in different parts of the left inferior frontal cortex. An implicit reading task, in which participants (6-22 years old) were required to detect a visual feature in words and non-words, generated increased activation with age in ventral left inferior frontal gyrus (BA 47) (Turkeltaub et al., 2003). Finally, in a direct comparison of adults and children on phonological and orthographic judgment tasks, greater activation for adults was found in the dorsal part of the left inferior frontal gyrus (BA 9) (Booth et al., 2004).
In addition to language processing, the inferior frontal gyrus has also been implicated in executive functioning tasks requiring cognitive control (Kemmotsu et al., 2005; Miller, 2000; Rowe et al., 2005). In a recent study with adult participants, we suggested that the left inferior frontal gyrus is involved in top-down control over posteriorregions (Bitan et al., 2005). Our findings showed that parietal and temporal regions, which are selectively active during orthographic and phonological judgment tasks, serve as integration zones that respond to both sensory word-form information and top-down modulatory control from the left inferior frontal gyrus. We showed that such modulatory influences from the left inferior frontal gyrus selectively influenced the sensitivity of temporal versus parietal regions to visual word-form information, depending on whether the goal was to engage in phonological or orthographic processing, respectively.
In the current study, we examined the role of the left inferior frontal gyrus in modulating temporal and parietal regions in children. Previous studies have found weaker cognitive control in children compared to adults in tasks that require withholding of inappropriate responses (Bunge et al., 2002; Williams et al., 1999) and suppressing interference from irrelevant stimuli (Bunge et al., 2002; Ridderinkhof et al., 1997; Tipper et al., 1989). We therefore explored whether the inferior frontal cortex would have weaker modulatory control over temporoparietal regions in children engaged in tasks of orthographic and phonological processing. To address this hypothesis, we examined the pattern of effective connectivity (directional influences between brain regions) in an fMRI study in children 9-12 years old. The children performed rhyming and spelling judgment tasks on visually presented words, and their results were compared to our previously published data on adults (Bitan et al., 2005).
Methods
Participants
Fifteen 9- to 12-year-old children (mean 10.9, 7 females) participated in this study. All participants were native English speakers, with no diagnosed neurological/psychiatric disorders or language/reading disabilities. 12 participants were right-handed, and 3 were left-handed. All left-handed participants were left hemisphere dominant for language. The results were compared to previously published data on 12 adults (8 females, all right handed) (Bitan et al., 2005).
Stimuli and procedures
Word judgment tasks
In both the spelling and rhyming tasks, three words were presented visually in a sequential order, and the participant had to determine whether the final word matched either of the two previous words according to a predefined rule. In the spelling task, participants determined whether the final word had the same rime spelling as either of the first two words. The rime included all letters after the first consonant or consonant cluster (Bowey, 1990). In the rhyming task, participants determined whether the final word rhymed with either of the first two words. Participants indicated their judgment by pressing one of two buttons. For both the spelling and rhyming tasks, half of the target trials contained a target word that rhymed and was orthographically similar to one of the preceding two words (e.g., hold-cold). The other half contained a target word that rhymed but was orthographically dissimilar to one of the preceding two words (e.g., hope-soap). In addition, half of the correct trials involved a match to the first stimulus and half involved a match to the second stimulus. 60% of the trials involved a match and 40% involve a non-match. The non-matching trials involved three orthographically different words that were non-rhyming.
Perceptual control task
The experimental set-up and timing for the control blocks was exactly the same as for the word blocks, except the three stimuli were abstract, non-linguistic symbols consisting of straight lines (e.g. \ \ or \ /).
Task procedure
The spelling and the rhyming tasks were each administered in a separate 9-min runs, which was preceded by written instructions to the subject. Each task procedure consisted of 10 blocks of 54 s, in which 5 experimental blocks alternated with 5 control blocks. In each trial, three consecutive stimuli were presented, each stimulus for 800 ms followed by a 200-ms blank interval. Participants had 2000 ms to respond. Each trial lasted a total of 5000 ms. Each block began with a 4-s instruction screen followed by 10 trials.
fMRI acquisition and analysis
Images were acquired using a 1.5-T GE scanner with EPI method. The scanning parameters were TR=3000 ms, TE=40 ms, flip angle=90°, matrix size=64 × 64, field of view=22 cm, slice thickness=4 mm, number of slices=32. These scanning parameters resulted in a 3.437 × 3.437 × 4-mm voxel size. Each task resulted in 180 images. Data analysis was performed using Statistical Parametric Mapping (SPM2) (http://www.fil.ion.ucl.ac.uk/spm). The images were spatially realigned to the first volume to correct for head movements. No individual runs had more than 3 mm of movement. Sinc interpolation was used to minimize timing errors between slices (Henson et al., 1999). The functional images were coregistered with an anatomical image, and normalized to the standard T1 template volume (MNI). The data were then smoothed with a 7-mm isotropic Gaussian kernel.
In order to rule out the possibility that any difference between the groups is due to difference in motion, we calculated GLM analyses of 2 Age groups (children, adults) by 2 Tasks (spelling, rhyming) separately on the x-plane, y-plane, and z-plane motion estimates. These analyses revealed no significant main effects or interactions involving age, indicating that movement was not reliably different between the adults and children for the x-plane (Mc=0.16 ± 0.03; range=0.03 to 0.32 versus Ma=0.17 ± 0.03; range=0.05 to 0.5), y-plane (Mc=0.30 ± 0.04; range=0.11 to 0.74 versus Ma=0.32 ± 0.04; range=0.17 to 0.59), or z-plane (Mc=0.56 ± 0.08; range=0.14 to 1.63 versus Ma=0.46 ± 0.09; range=0.12 to 0.90).
Statistical analyses at the first level were calculated using an epoch-based design, with the two tasks (spelling and rhyming) as conditions of interest. A high pass filter with a cutoff period of 256 s was applied. Group results were obtained using random effects analyses by combining subject-specific summary statistics across the group as implemented in SPM2 (Penny and Holmes, 2003). Group results were then used to choose the regions of interest for the effective connectivity analysis. In order to simplify the network, and due to the strong asymmetry in the activation clusters and well-documented laterality of language processes, only left hemisphere clusters larger than 35 voxels were included. This process resulted in four ROIs in the left hemisphere: fusiform gyrus (FG), inferior frontal gyrus (IFG), intraparietal sulcus (IPS), and lateral temporal cortex (LTC). Two regions were active in both tasks (i.e., FG and IFG), while the other two ROIs were each selectively active in one of the tasks (i.e., the IPS in the spelling task, and the LTC in the rhyming task) (Fig. 1A, Table 1).
Fig. 1.
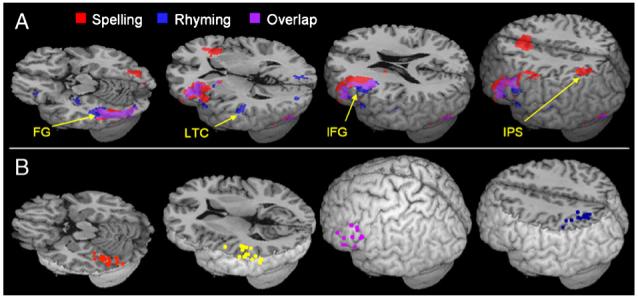
Regions included in the effective connectivity analysis. (A) Group activation for the spelling and rhyming tasks compared to their mean. FG—fusiform gyrus, LTC—lateral temporal cortex, IFG—inferior frontal gyrus, IPS—intraparietal sulcus. (B) Centers of ROIs in individual subjects. Purple—IFG, Orange—FG, Yellow—LTC, Blue—IPS.
Table 1.
Regions of activation in the spelling and rhyming tasks compared to their mean
| Region | BA | H | Voxels | z score | x | y | z |
|---|---|---|---|---|---|---|---|
| Spelling | |||||||
| Fusiform/middle occipital gyrus | 37/18 | L | 347 | 5.95 | -39 | -60 | -18 |
| Inferior/middle frontal gyrus | 45/46/9 | L | 1054 | 5.06 | -45 | 30 | 21 |
| Inferior parietal lobule/precuneus | 40/7 | L | 110 | 5.24 | -33 | -60 | 48 |
| Cingulate gy. | 32 | - | 241 | 4.79 | -3 | 21 | 45 |
| Inferior frontal gyrus/Insula | 47/13 | R | 151 | 4.75 | 36 | 21 | -3 |
| Fusiform/inferior occipital gyrus | 37/19/18 | R | 135 | 4.35 | 39 | -66 | -18 |
| Lentiform nucleus | - | L | 43 | 3.63 | -21 | -3 | 12 |
| Rhyming | |||||||
| Inferior frontal gyrus | 44/45/46 | L | 627 | 5.16 | -51 | 30 | 12 |
| Fusiform gyrus | 37/19 | L | 289 | 5.04 | -42 | -57 | -18 |
| Inferior occipital/lingual gyrus | 17/18 | R | 69 | 5.79 | 27 | -90 | -9 |
| Lentiform nucleus | - | L | 78 | 4.12 | -30 | -18 | -12 |
| Middle temporal gyrus | 22 | L | 36 | 4 | -60 | -33 | 3 |
Note. H: L—left, R—right hemisphere.
Clusters selected as ROIs in the effective connectivity analysis appear in bold.
Effective connectivity
Four regions of interest were specified in the left hemisphere for each individual: FG, IFG, IPS and LTC. Regional responses were summarized as the principal eigenvariates of responses within a sphere centered on the most significant voxel for each subject. For the task-specific regions (LTC and IPS), this was a 6-mm sphere to minimize the number of inactive voxels included. For the task-common regions (IFG and FG), we used a 10-mm sphere in order to include active voxels from both the spelling and rhyming tasks. Subject-specific maxima were defined operationally as the most significant voxel within 28 mm of the group maximum in the appropriate activation map. The coordinates for the task-specific regions, LTC and IPS, were determined by the rhyming and spelling tasks respectively. The subject-specific maxima for the IFG and FG were taken as the average position of the maxima in the rhyming and spelling activation maps. One subject was excluded as there were no significant clusters within 28 mm from the group reference voxel (in LTC). Fig. 1B presents the location of the centers of the individual ROIs.
Effective connectivity analysis was performed using the Dynamic Causal Modeling (DCM) tool in SPM2 (Friston et al., 2003; Penny et al., 2004). In DCM, three sets of parameters are estimated: the direct influence of stimuli on regional activity; the intrinsic or latent connections between regions (i.e., the inter-regional influences in the absence of modulating experimental effects); and the changes in the intrinsic connectivity between regions induced by the experimental design (modulatory effects) (Mechelli et al., 2003). Our analysis adopted a two-stage procedure that is formally identical to the summary statistic approach used in random effects analysis of neuroimaging data. In our analysis, however, the first level (subject-specific) models were the parameters from the DCMs. These parameters were taken to a second (between-subject) level using the random effects approach. Subject-specific DCMs were fully and reciprocally connected (resulting in 12 connections) with modulatory influences from both tasks specified on all the connections. Input stimuli from therhyming and the spelling tasks were modeled as exerting direct effects on the FG. Due to the lack of detailed knowledge of anatomical connectivity in the language system of humans and because reciprocal interconnectivity has been found in other primate brain systems (Bullmore et al., 2000; Mechelli et al., 2002; Chaminade and Fonlupt, 2003), a fully connected and fully modulated model was specified, consistent with our study in adults (Bitan et al., 2005). DCM enables this level of connectivity by using a Bayesian framework in model estimation (Friston et al., 2003).
Since detailed results of the adult group are presented elsewhere (Bitan et al., 2005), we only report results for the children and for the comparison between groups. We restrict our discussion of results to intrinsic connections and modulatory effects significant at a level of p < 0.05 (corrected for 12 comparisons), tested in a 1-sample t-test. To determine whether task-selective regions are involved in integrating task-specific information in children (as previously demonstrated in adults), two types of analyses were done. First, for each selectively active region the modulatory effects were compared in three paired t-tests between converging and diverging connections within task (p < 0.05 corrected for 3 comparisons). Second, for each task the modulatory effects were compared between the influence of FG and IFG on the IPS versus LTC, in two paired t-tests (p < 0.05 corrected for 2 comparisons). To test our main hypothesis, that children and adults differ in the top-down modulation from inferior frontal gyrus to posterior regions, we performed a two-sample t-test within each task comparing the two groups on the modulatory effect from the IFG to the task-selective region (p < 0.05). In order to examine the possibility that the difference in effective connectivity is a result of the difference in behavioral performance between groups, we tested the correlation between performance measures (accuracy and reaction time) and the modulatory effect of each task on the influence from IFG towards each task-selective region. Due to the explicit group structure of age and accuracy, that prevents the usage of partial correlation across groups, the correlation was performed within each group.
Results
In a GLM analysis of behavioral performance within each task, with 2 conditions (words versus control) and 2 age groups, the results show that adults were more accurate than children in both the spelling (Ma=0.98 ± 0.02, range: 0.94-1.0; versus Mc=0.89 ± 0.07, range: 0.78-1.0) and rhyming tasks (Ma=0.97 ± 0.04, range: 0.88-1.0; versus Mc=0.92 ± 0.06, range: 0.78-0.98), (F(1,25)=17.7, 11.8; p < 0.01). In addition, adults have responded faster than children in the spelling (Ma=868 ± 196 ms, range: 575-1190; versus Mc=1529 ± 220 ms, range: 1212-1971) and rhyming tasks (Ma=916 ± 232, range: 595-1282; versus Mc=1416 ± 152 ms, range 1131-1636), (F(1,25)=42.5, 39.7; p < 0.001).
Conventional fMRI analysis
Fig. 1A and Table 1 present the clusters of activation in the spelling and in the rhyming tasks in children. Detail descriptions of activation in adults and group comparisons are presented elsewhere (Bitan et al., 2005 and Booth et al., 2004 respectively). The group maxima of left hemisphere clusters (uncorrected p < 0.001, extent threshold: 35 voxels) were used as reference for choosing the individual ROIs. For the spelling task, these ROIs included the inferior frontal gyrus (BA 45/46/9), the fusiform gyrus (BA 37), and the intraparietal sulcus, including the precuneus and parts of the superior and inferior parietal lobules (BA 7/40). In the rhyming task, these clusters included the inferior frontal gyri (BA 44/46/45), the left fusiform gyrus (BA 37) and the middle temporal gyrus (BA 22). These ROIs were all within 10 mm from those identified in adults (Bitan et al., 2005).
Effective connectivity analysis
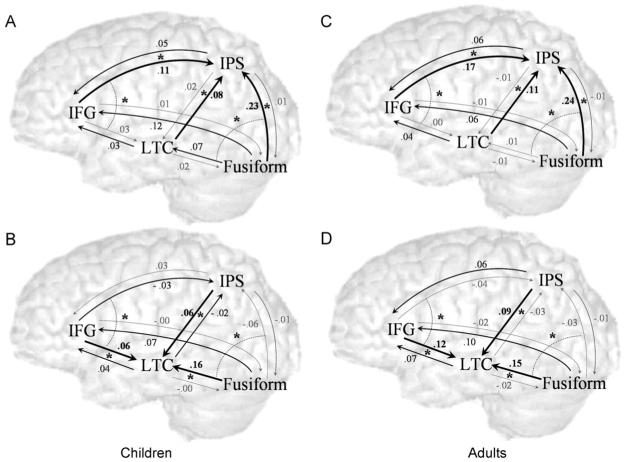
As in adults, all intrinsic connections (i.e., connections that are independent of the task) were significant in children. Figs. 2A and B present the modulatory effects of the spelling and rhyming tasks, respectively, on the influences among brain regions in children. The spelling task (Fig. 2A) elicited significant modulatory effects on all converging influences into IPS, all converging influences into IFG and the influence of FG on LTC. The rhyming task (Fig. 2B) elicited significant modulatory effects on all converging influences into LTC and the converging influences on IFG from LTC and FG. A negative modulatory effect of the rhyming task was found on the converging influences into IPS from IFG and LTC. The pattern of modulatory effects in children stands in striking similarity to the effects in adults (Figs. 2C, D).
Fig. 2.
Modulatory effects of the tasks. Effects of the spelling and rhyming tasks in children (A, B respectively) and in adults (C, D respectively) are presented. The average effects across individuals are displayed, with significant effects in black, and non-significant effect in grey. Pairs of effects that are significantly different are indicated by * (and connected by a dotted arc in distant pairs) with the stronger effect indicated in bold. For example, in panel A, the dotted arc on the right indicates that the fusiform area had a significantly greater influence on the IPS than on the LTC in the spelling task.
The role of task-selective regions in integration was evaluated by examining the modulatory effects of the tasks on connections converging on LTC and IPS versus those diverging from these regions. The effect of the spelling task on converging influences from FG, IFG and LTC onto IPS was stronger than on diverging influences from this region (t(14)=6.4, 3.8, 4.7; p < 0.01 corrected, respectively, Fig. 2A). Similarly, the effect of the rhyming task on converging influences from FG, IFG and IPS onto LTC was significantly stronger than on diverging influences from LTC (t(14)=6.8, 2.6, 5.1; p < 0.05 corrected, respectively, Fig. 2B). These patterns are similar to the findings in adults (Figs. 2C—spelling and d—rhyming).
A further indication for the role of IPS and LTC in integrating task-specific information is provided by the comparison between converging influences into these two regions. Comparisons in adults showed that the spelling task enhanced convergent influences on IPS more than on LTC, while the rhyming task effects showed the opposite pattern (dotted arcs in Figs. 2C and D) (Bitan et al., 2005). These findings were replicated in children, in whom the effect of spelling on influences from FG and IFG onto IPS was stronger than on the influences onto LTC (t(14)=3.8, 4.3; p < 0.01 corrected, respectively), (dotted arcs in Fig. 2A). Conversely, rhyming elicited stronger effects from FG and IFG onto LTC versus the influences onto IPS (t(14)=6.2, 4.0; p < 0.01 corrected, respectively), (dotted arcs in Fig. 2B).
To test our main hypothesis regarding the differences between children and adults in the top-down influence from inferior frontal gyrus to posterior regions, we compared the groups within each task (Fig. 3). The modulatory effect of the spelling task on the influence of IFG on IPS was stronger in adults compared to children (t(25)=1.7, p < 0.05). Similarly, adults showed a greater modulatory effect of the rhyming task on the influence from IFG to LTC (t(25)=2.2, p < 0.05).
Fig. 3.
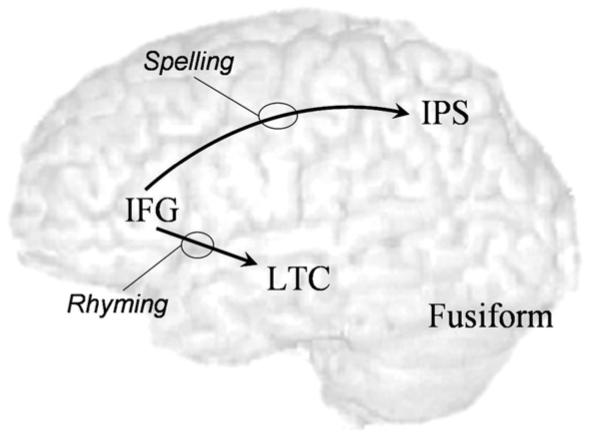
Modulatory effects that are stronger in adults compared to children.
In order to de-confound the effects of age and behavioral performance on effective connectivity, we tested the correlation between performance and effective connectivity within each group. Accuracy and reaction time were separately correlated with the modulatory effect of each task on the influence from IFG towards each task-selective region. Spelling task effects on the influence of IFG on IPS were not correlated with either accuracy (r=0.26, 0.1 in children and adults respectively) or reaction time (r=0.33, 0.06 in children and adults respectively). The effect of the rhyming task on the influence of IFG on LTC was negatively correlated with accuracy in children (r=-0.57, p < 0.05) but not in adults (r=-0.06) and was not correlated with reaction time in either group (r=0.01 children, 0.2 adults). Because the correlation with accuracy in the rhyming task is negative in children (higher accuracy is related to weaker modulatory effects), it cannot account for the difference between groups (adults showed higher accuracy but stronger modulatory effects). These results show that when the effect of age is controlled, variability of performance within group does not account for differences in effective connectivity.
Discussion
The results of the present study, from a group of 9- to 12-year-old children, replicate our results in adults (Bitan et al., 2005) by showing that posterior task-selective regions serve to integrate converging information relevant to a specific task. However, the comparison between groups showed that task-related modulation of connectivity from IFG onto posterior task-selective regions was weaker in children compared to adults. This suggests that adult language processing is characterized by greater top-down cognitive control compared to children.
The children’s performance on the spelling and rhyming tasks in the current study was less accurate and slower compared to adults’. In these tasks, participants are required to base their judgment on either orthographic or phonological information. Both tasks included items that were phonologically similar, but orthographically dissimilar (e.g., hope-soap), so participants had to ignore irrelevant orthography to make a rhyming judgment and irrelevant phonology to make a spelling judgment. Previous studies show that children’s ability to enhance the processing of task-relevant information (Richards, 2003; van der Stelt et al., 1998) and suppress interference from information that is irrelevant to the task is weaker compared to adults (Bunge et al., 2002; Casey et al., 2001, 2002; Tipper et al., 1989; Williams et al., 1999). We suggest that weaker control of the IFG over posterior regions underlies the children’s relative difficulty in performing the tasks in the current study. The results of our previous study with adult subjects (Bitan et al., 2005) suggested that modulatory influences emanating from the left inferior frontal gyrus selectively influence the sensitivity of temporal versus parietal regions to visual word-form information, depending on whether the goal was to engage in phonological or orthographic processing respectively. The weaker top-down modulation from the left inferior frontal gyrus in children presumably results in less selective enhancement of the relevant information, and therefore, less effective suppression of task-irrelevant information. Although we cannot completely rule out the possibility that differences in effective connectivity are the result, rather than the cause of performance differences between groups, our analysis within each group suggests that variability of performance may not account for differences in effective connectivity, therefore supporting our conclusion that the difference in effective connectivity reflects a developmental effect.
Our interpretation for the involvement of the inferior frontal cortex in selective enhancement of task-relevant information is consistent with the role of the prefrontal cortex in cognitive control suggested by studies in primates and humans (Miller, 2000). Primate studies show widespread projections from the prefrontal cortex to cortical and subcortical regions, allowing top-down modulation of posterior regions (Fuster, 1989; Pandya and Barnes, 1987). Single unit recording from the prefrontal cortex in monkeys show that neurons convey information about the specific demands of tasks as opposed to the physical properties of cues (Watanabe, 1992). Human patients with prefrontal lesions have difficulty selecting the most appropriate response for a given situation, and their behavior is captured by salient sensory cues that reflexively elicit strongly associated actions (stimulus bound behavior) (Duncan et al., 1996; Miller, 2000). Studies in healthy human subjects show task-dependent changes in the coupling of prefrontal regions with motor and visual areas (Rowe et al., 2005) as well as with subcortical regions (Lenartowicz and McIntosh, 2005). It has therefore been suggested that activity in the prefrontal cortex can exert a top-down influence by providing an excitatory signal that biases processing in other brain systems towards task-relevant information (Miller, 2000).
Previous studies have suggested that development is characterized by an increase in the effect of top-down control of prefrontal cortex on posterior regions (Brown et al., 2005; Casey et al., 2005; Gilmore and Johnson, 1995; Luna et al., 2001). Indirect evidence for these claims was provided by findings of increased activation with age in prefrontal regions during tasks of executive control. In the Stroop task, increased activation with age (7-22 years old) was found in the left lateral prefrontal cortex in addition to posterior regions (Adleman et al., 2002). In a Go/No-go task, subjects (8-20 years old) showed a positive correlation of activation with age in the left inferior frontal gyrus (Tamm et al., 2002). In a task of oculomotor response suppression, subjects (8-30 years old) showed increased activation with age in prefrontal regions, which correlated with the ability to inhibit prepotent responses (Luna et al., 2001). The results of the current study provide more direct evidence that top-down modulation from prefrontal cortex to posterior regions increases during development and selectively enhances task-relevant information. These results may explain children’s susceptibility to interference from irrelevant information.
Our results may also shed light on the developmental increase in regional specialization of temporoparietal areas for specific cognitive tasks. Previous studies have shown a more diffused and distributed pattern of activation in children compared to focused patterns of activation in adults in both linguistic and perceptual (face and object) tasks (Booth et al., 2004; Brown et al., 2005; Casey et al., 2002; Durston et al., 2005; Gaillard et al., 2000; Hertz-Pannier et al., 1997; Passarotti et al., 2003). ERP studies have also shown that neuronal regions are more broadly tuned in young children compared to adults (de Haan et al., 1998; Neville et al., 1992). It has been suggested that cortical regions initially respond to a wide variety of stimuli, but later during development the same region may only be engaged by a subset of these stimuli. This is analogous to the process of tuning the response properties of single neurons. According to this account, the process of narrowing the response properties facilitates speed of processing for the stimuli or task for which it becomes specialized (Johnson, 2000), and mediates the emergence of greater behavioral expertise and efficiency (Gathers et al., 2004; Gauthier and Nelson, 2001; Passarotti et al., 2003). It has been further suggested that the process of fine-tuning cortical regions results from changing interactions with other brain regions (Johnson, 2001), and specifically with the recruitment of top-down control mechanisms operating from prefrontal regions to posterior regions (Brown et al., 2005; Casey et al., 2005). The results of the current study support these claims. These results further suggest that the increase in top-down modulation from prefrontal to temporoparietal regions could mediate the gradual establishment of regional selectivity in the temporal and parietal regions for phonological and orthographic processing, respectively.
References
- Adleman NE, Menon V, Blasey CM, White CD, Warsofsky IS, Glover GH, Reiss AL. A developmental fMRI study of the Stroop color-word task. NeuroImage. 2002;16:61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- Beaulieu C, Plewes C, Paulson LA, Roy D, Snook L, Concha L, Phillips L. Imaging brain connectivity in children with diverse reading ability. NeuroImage. 2005;25:1266–1271. doi: 10.1016/j.neuroimage.2004.12.053. [DOI] [PubMed] [Google Scholar]
- Bitan T, Booth JR, Choy J, Burman DD, Gitelman DR, Mesulam M-M. Shifts of effective connectivity within a language network during rhyming and spelling. J. Neurosci. 2005;25:5397–5403. doi: 10.1523/JNEUROSCI.0864-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM. Development of brain mechanisms for processing orthographic and phonologic representations. J. Cogn. Neurosci. 2004;16:1234–1249. doi: 10.1162/0898929041920496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowey JA. Orthographic onsets and rimes as functional units of reading. Mem. Cogn. 1990;18:419–427. doi: 10.3758/bf03197130. [DOI] [PubMed] [Google Scholar]
- Brown TT, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL. Developmental changes in human cerebral functional organization for word generation. Cereb. Cortex. 2005;15:275–290. doi: 10.1093/cercor/bhh129. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Horwitz B, Honey G, Brammer M, Williams S, Sharma T. How good is good enough in path analysis of fMRI data? NeuroImage. 2000;11:289–2301. doi: 10.1006/nimg.2000.0544. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J. Cogn. Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Durston S, Fossella JA. Evidence for a mechanistic model of cognitive control. Clin. Neurosci. Res. 2001;1:267–282. [Google Scholar]
- Casey BJ, Thomas KM, Davidson MC, Kunz K, Franzen PL. Dissociating striatal and hippocampal function developmentally with a stimulus-response compatibility task. J. Neurosci. 2002;22:8647–8652. doi: 10.1523/JNEUROSCI.22-19-08647.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Galvan A, Hare TA. Changes in cerebral functional organization during cognitive development. Curr. Opin. Neurobiol. 2005;15:239–244. doi: 10.1016/j.conb.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Chaminade T, Fonlupt P. Changes of effective connectivity between the lateral and medial parts of the prefrontal cortex during a visual task. Eur. J. Neurosci. 2003;18:675–679. doi: 10.1046/j.1460-9568.2003.02787.x. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The brain binds entities and events by multi-regional activation from convergence zones. Neural Comput. 1989;1:123–132. [Google Scholar]
- de Haan M, Oliver A, Johnson MH. Electrophysiological correlations of face processing by adults and 6-month-old infants. J. Cogn. 1998:36–36. [Google Scholar]
- Dobbing J, Sands J. Quantitative growth and development of human brain. Arch. Dis. Child. 1973;48:757–767. doi: 10.1136/adc.48.10.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, Emslie H, Williams P, Johnson R, Freer C. Intelligence and the frontal lobe: the organization of goal-directed behavior. Cogn. Psychol. 1996;30:257–303. doi: 10.1006/cogp.1996.0008. [DOI] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Tottenham N, Spicero J, Galvan A, Fossella JA, Casey BJ. A shift from diffuse to focal cortical activity with development. J. Cogn. Neurosci. 2005:57–58. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Fiez JA. Phonology, semantics, and the role of the left inferior prefrontal cortex. Hum. Brain Mapp. 1997;5:79–83. [PubMed] [Google Scholar]
- Friederici AD, Ruschemeyer SA, Hahne A, Fiebach CJ. The role of left inferior frontal and superior temporal cortex in sentence comprehension: localizing syntactic and semantic processes. Cereb. Cortex. 2003;13:170–177. doi: 10.1093/cercor/13.2.170. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W. Dynamic causal modelling. NeuroImage. 2003;19:1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The Prefrontal Cortex. 1989 [Google Scholar]
- Gaillard WD, Hertz-Pannier L, Mott SH, Barnett AS, LeBihan D, Theodore WH. Functional anatomy of cognitive development—fMRI of verbal fluency in children and adults. Neurology. 2000;54:180–185. doi: 10.1212/wnl.54.1.180. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Sachs BC, Whitnah JR, Ahmad Z, Balsamo LM, Petrella JR, Braniecki SH, McKinney CM, Hunter K, Xu B, Grandin CB. Developmental aspects of language processing: fMRI of verbal fluency in children and adults. Hum. Brain Mapp. 2003;18:176–185. doi: 10.1002/hbm.10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathers AD, Bhatt R, Corbly CR, Farley AB, Joseph JE. Developmental shifts in cortical loci for face and object recognition. NeuroReport. 2004;15:1549–1553. doi: 10.1097/01.wnr.0000133299.84901.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier I, Nelson CA. The development of face expertise. Curr. Opin. Neurobiol. 2001;11:219–224. doi: 10.1016/s0959-4388(00)00200-2. [DOI] [PubMed] [Google Scholar]
- Gilmore RO, Johnson MH. Working-memory in infancy-6-month-olds performance on 2 versions of the oculomotor delayed-response task. J. Exp. Child Psychol. 1995;59:397–418. doi: 10.1006/jecp.1995.1019. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, III, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson R, Buchel C, Josephs O, Friston K. The slice-timing problem in event-related fMRI. NeuroImage. 1999;9:S125. doi: 10.1006/nimg.1999.0498. [DOI] [PubMed] [Google Scholar]
- Hertz-Pannier L, Gaillard WD, Mott SH, Cuenod CA, Bookheimer SY, Weinstein S, Conry J, Papero PH, Schiff SJ, Le Bihan D, Theodore WH. Noninvasive assessment of language dominance in children and adolescents with functional MRI: a preliminary study. Neurology. 1997;48:1003–1012. doi: 10.1212/wnl.48.4.1003. [DOI] [PubMed] [Google Scholar]
- Holland SK, Plante E, Byars AW, Strawsburg RH, Schmithorst VJ, Ball WS. Normal fMRI brain activation patterns in children performing a verb generation task. NeuroImage. 2001;14:837–843. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Functional brain development in infants: elements of an interactive specialization framework. Child Dev. 2000;71:75–81. doi: 10.1111/1467-8624.00120. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Functional brain development in humans. Nat. Rev. Neurosci. 2001;2:475–483. doi: 10.1038/35081509. [DOI] [PubMed] [Google Scholar]
- Kemmotsu N, Villalobos ME, Gaffrey MS, Courchesne E, Muller RA. Activity and functional connectivity of inferior frontal cortex associated with response conflict. Brain Res. Cogn. Brain Res. 2005;24:335–342. doi: 10.1016/j.cogbrainres.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Vaidya CJ, Gabrieli JD, Moseley ME, Hedehus M. Myelination and organization of the frontal white matter in children: a diffusion tensor MRI study. NeuroReport. 1999;10:2817–2821. doi: 10.1097/00001756-199909090-00022. [DOI] [PubMed] [Google Scholar]
- Lenartowicz A, McIntosh AR. The role of anterior cingulate cortex in working memory is shaped by functional connectivity. J. Cogn. Neurosci. 2005;17:1026–1042. doi: 10.1162/0898929054475127. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, Keshavan MS, Genovese CR, Eddy WF, Sweeney JA. Maturation of widely distributed brain function subserves cognitive development. NeuroImage. 2001;13:786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Penny WD, Price CJ, Gitelman DR, Friston KJ. Effective connectivity and intersubject variability: using a multi-subject network to test differences and commonalities. NeuroImage. 2002;17:1459–1469. doi: 10.1006/nimg.2002.1231. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Price CJ, Noppeney U, Friston KJ. A dynamic causal modeling study on category effects: bottom-up or top-down mediation? J. Cogn. Neurosci. 2003;15:925–934. doi: 10.1162/089892903770007317. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. From sensation to cognition. Brain. 1998;121(Pt 6):1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Miller EK. The prefrontal cortex and cognitive control. Nat. Rev., Neurosci. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- Neville H, Mills D, Lawson D. Fractionating language: different neural subsystems with different sensitive periods. Cereb. Cortex. 1992;2:244–258. doi: 10.1093/cercor/2.3.244. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Barnes CL. 1987:41–72. [Google Scholar]
- Passarotti AM, Paul BM, Russiere JR, Buxton RB, Wong EC, Stiles J. The development of face and location processing: an fMRI study. Dev. Sci. 2003;6:100–117. [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Penny WD, Holmes A. Random Effects Analysis. 2003:843–850. [Google Scholar]
- Penny WD, Stephan KE, Mechelli A, Friston KJ. Comparing dynamic causal models. NeuroImage. 2004;22:1157–1172. doi: 10.1016/j.neuroimage.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JDE. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. NeuroImage. 1999;10:15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: contributions from functional neuroimaging. J. Anat. 2000;197(Pt 3):335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JE. Attention affects the recognition of briefly presented visual stimuli in infants: an ERP study. Dev. Sci. 2003;6:312–328. doi: 10.1111/1467-7687.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, vanderMolen MW, Band GPH, Bashore TR. Sources of interference from irrelevant information: a developmental study. J. Exp. Child Psychol. 1997;65:315–341. doi: 10.1006/jecp.1997.2367. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Stephan KE, Friston K, Frackowiak RS, Passingham RE. The prefrontal cortex shows context-specific changes in effective connectivity to motor or visual cortex during the selection of action or colour. Cereb. Cortex. 2005;15:85–95. doi: 10.1093/cercor/bhh111. [DOI] [PubMed] [Google Scholar]
- Schapiro MB, Schmithorst VJ, Wilke M, Byars AW, Strawsburg RH, Holland SK. BOLD fMRI signal increases with age in selected brain regions in children. NeuroReport. 2004;15:2575–2578. doi: 10.1097/00001756-200412030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaggar BL, Brown TT, Lugar HM, Visscher KM, Miezin FM, Petersen SE. Functional neuroanatomical differences between adults and school-age children in the processing of single words. Science. 2002;296:1476–1479. doi: 10.1126/science.1069464. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Delis D, Stiles J, Jernigan TL. Improved memory functioning and frontal lobe maturation between childhood and adolescence: a structural MRI study. J. Int. Neuropsychol. Soc. 2001;7:312–322. doi: 10.1017/s135561770173305x. [DOI] [PubMed] [Google Scholar]
- Stuss DT. Biological and psychological development of executive functions. Brain Cogn. 1992;20:8–23. doi: 10.1016/0278-2626(92)90059-u. [DOI] [PubMed] [Google Scholar]
- Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. J. Am. Acad. Child Adolesc. Psych. 2002;41:1231–1238. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Tipper SP, Bourque TA, Anderson SH, Brehaut JC. Mechanisms of attention—A developmental-study. J. Exp. Child Psychol. 1989;48:353–378. doi: 10.1016/0022-0965(89)90047-7. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF. Development of neural mechanisms for reading. Nat. Neurosci. 2003;6:767–773. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- van der Stelt O, Kok A, Smulders FT, Snel J, Boudewijn Gunning W. Cerebral event-related potentials associated with selective attention to color: developmental changes from childhood to adulthood. Psychophysiology. 1998;35:227–239. doi: 10.1017/s0048577298961303. [DOI] [PubMed] [Google Scholar]
- Watanabe M. Frontal units of the monkey coding the associative significance of visual and auditory stimuli. Exp. Brain Res. 1992;89:233–247. doi: 10.1007/BF00228241. [DOI] [PubMed] [Google Scholar]
- Williams BR, Ponesse JS, Schachar RJ, Logan GD, Tannock R. Development of inhibitory control across the life span. Dev. Psychol. 1999;35:205–213. doi: 10.1037//0012-1649.35.1.205. [DOI] [PubMed] [Google Scholar]