Summary
Background
Conserved intraflagellar transport (IFT) particle proteins and IFT-associated motors are needed to assemble most eukaryotic cilia and flagella. Proteins in an IFT-A subcomplex are generally required for dynein-driven retrograde IFT, from the ciliary tip to the base. We describe novel structural and functional roles for IFT-A proteins in chordotonal organs, insect mechanosensory organs with cilia that are both sensory and motile.
Results
The reduced mechanoreceptor potential A (rempA) locus of Drosophila encodes the IFT-A component IFT140. Chordotonal cilia are shortened in rempA mutants and an IFT-B protein accumulates in the mutant cilia, consistent with a defect in retrograde IFT. A functional REMPA-YFP fusion protein concentrates at the site of the ciliary dilation (CD), a highly structured axonemal inclusion of hitherto unknown composition and function. The CD is absent in rempA mutants, and REMPA-YFP is undetectable in the absence of another IFT-A protein, IFT122. In a mutant lacking the IFT dynein motor, the CD is disorganized and REMPA-YFP is mislocalized. A TRPV ion channel, required to generate sensory potentials and regulate ciliary motility, is normally localized in the cilia, proximal to the CD. This channel spreads into the distal part of the cilia in dynein mutants, and is undetectable in rempA mutants.
Conclusions
IFT-A proteins are located at and required by the ciliary dilation, which separates chordotonal cilia into functionally distinct zones. A requirement for IFT140 in stable TRPV channel expression also suggests that IFT-A proteins may mediate preciliary transport of some membrane proteins.
Background
Eukaryotic cilia and flagella exemplify both molecular conservation and functional diversity. Their axonemal cytoskeleton, a radially symmetric array of nine microtubule doublets, is one of the most distinctive structures in the eukaryotic cell, and a large set of ciliary proteins is conserved across evolutionarily distant eukaryotes. Cilia are best known as propulsive motors, but many function as sensory probes and show diverse structural variations on the canonical, axonemal form; some cilia combine sensory functions with motility. We are interested in how the conserved molecular mechanisms for ciliary assembly are adapted to construct specialized sensory cilia and to perform sensory functions.
Among the conserved ciliary proteins are parts of an intraflagellar transport (IFT) mechanism, which is required to extend a cilium from the cell surface (reviews: [1–3]). In Chlamydomonas flagella, IFT is seen as the processive movement of discrete particles along the axoneme [4]. Anterograde transport, towards the tip of the cilium, depends on kinesin-2, and retrograde transport is driven by an isoform of cytoplasmic dynein [5–8]. Uninterrupted particle movement in each direction indicates that the two motor activities are tightly regulated, and that the IFT particles are reconfigured at the base and tip of the cilium to load and unload cargoes and switch motor activities.
The component proteins of Chlamydomonas IFT particles associate in A and B subcomplexes [9, 10]. Mutants lacking an IFT-B protein, in Chlamydomonas and in many other species, lack IFT and typically have very short or no cilia. In contrast, IFT-A proteins may be specifically involved in retrograde transport. Chlamydomonas mutants specifically defective in retrograde IFT show reductions in IFT-A proteins [11], although the specific mutated loci are unknown. Nematode [12, 13] and Tetrahymena [14] mutants lacking IFT-A proteins have shortened or swollen cilia that accumulate material including IFT-B proteins; silencing IFT-A gene expression in trypanosomes [15] gives a similar phenotype. Other IFT-associated proteins are also implicated in retrograde IFT [13, 16].
This study focuses on IFT-A proteins in chordotonal organs, ciliated mechanosensory organs in insects and crustaceans [17]. A scolopidium, the chordotonal functional unit, includes one to three sensory neurons and several specialized support cells, one of which constructs the scolopale, a fusiform, membrane-lined cavity. Each neuronal sensory process inserted at the base of the scolopale extends a cilium to its apex, where its tip is attached to an extracellular cap. The cap is connected, either directly or via another support cell, to the cuticle; a pull on the cap stretches the cilia and stimulates the neurons. Johnston’s organ, an auditory chordotonal organ in the antenna, includes over two hundred scolopidia which detect and transduce airborne vibrations from nearby sound sources [17–19]. Drosophila mutations affecting antennal sound-evoked potentials have identified gene products implicated in chordotonal differentiation and mechanotransduction [20]. These include two TRP superfamily ion channels. The TRPV subunits Nanchung (NAN) and Inactive (IAV) form a channel which is located in chordotonal cilia and is required to generate sound-evoked potentials [21, 22]. Mutants lacking NOMPC, the TRPN channel that transduces touch in bristles [23], also have reduced sound-evoked potentials [20]. The TRPN and TRPV channels also regulate a mechanical activity that increases antennal sensitivity to low-intensity stimuli [24].
Assembly of Drosophila sensory cilia requires anterograde IFT: mutants for the IFT-B protein IFT88/NOMPB [25] or a kinesin-2 subunit [26] lack cilia and all mechanosensory responses. Here we report that the reduced mechanoreceptor potential A (rempA) locus encodes the Drosophila homolog of IFT140, a component of the IFT-A subcomplex. Cilia in rempA mutants are shortened and accumulate IFT88, consistent with a defect in retrograde IFT. The wild type REMPA protein is localized to the ciliary dilation (CD), a characteristic, highly structured feature of chordotonal cilia. The effects of other IFT mutants on REMPA expression and localization, and of rempA and dynein mutants on ion channel expression and localization, indicate that the CD is an IFT-A-dependent structure which divides the chordotonal cilium into functionally distinct zones.
Results
rempA encodes IFT140
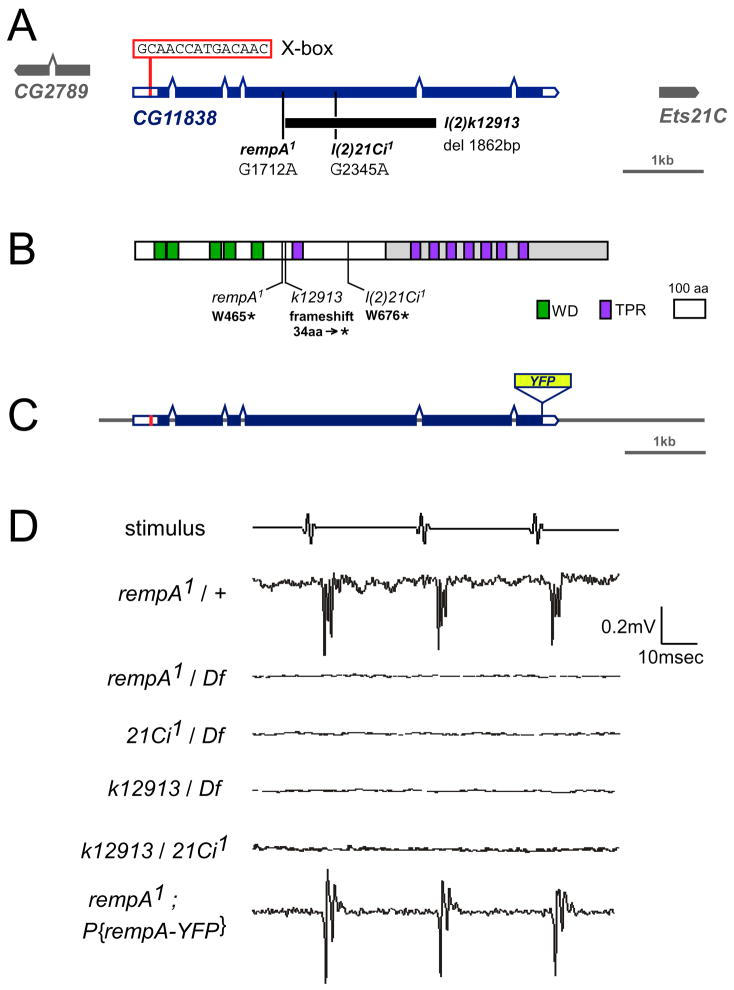
rempA1 mutants show moderate to severe uncoordination and reduced bristle mechanoreceptor potentials [27], and lack antennal sound-evoked potentials [20] (Figure 1). rempA was mapped to cytogenetic interval 21C (details in supplemental figure S1). Two other semilethal mutations in this interval, l(2)21Ci1 and l(2)k12913, fail to complement the behavioral and viability defects of rempA1, although l(2)21Ci1 homozygotes or l(2)21Ci1/rempA1 trans-heterozgotes show less severe uncoordination than rempA1 homozygotes or hemizygotes. The genomic sequence corresponding to 21C includes the predicted gene CG11838/oseg3, which encodes the Drosophila IFT140 homolog and was also identified as a ciliary protein by comparative genomics [28]. All three rempA mutations disrupt the coding region of CG11838: single-base nonsense mutations in rempA1 and l(2)21Ci1, and a 3kb deletion and frameshift in l(2)k12913 (Figure 1). An 8kbp genomic DNA fragment containing CG11838 rescued the viability, behavioral, and electrophysiological phenotypes of rempA mutants, confirming its identity with CG11838/oseg3.
Figure 1. rempA encodes the IFT-A protein IFT140.
A, Genomic region including CG11838/rempA, showing the deletion in l(2)k12913 (bar) and the positions of two nonsense mutations. Boxes, exons; filled boxes: coding region. The X box (Rfx transcription factor binding site) may not be functional [51]. B., Predicted protein structure and mutant changes. Domains were predicted by the REP repeat prediction algorithm [52]; gray shading indicates the more conserved region. The deletion in l(2)k12913 causes a frameshift and premature termination. C, Genomic rescue construct, showing the C-terminal insertion of the yellow fluorescent protein (YFP) coding region. D., Compound potentials recorded from the antennal nerve in response to a pulsed sound stimulus. Each trace is the averaged response to ten trials. rempA1/rempA+ heterozygotes show a wild-type response; but homozygotes, hemizygotes and trans-heterozygotes for rempA alleles lack sound-evoked potentials. A single insertion of the rescue fusion construct restores the response.
A single cDNA clone (IP14838 in the Berkeley Drosophila Genome Project database) matches the longer (CG11838-PB) of two predicted open reading frames, and encodes a 1503 amino-acid polypeptide with 33% and 27% overall sequence identity to the mammalian and Chlamydomonas IFT140 homologs respectively. The protein has an “oseg” domain architecture [28], which includes up to 7 WD repeats in the N-terminal half and a C-terminal part with alpha-helical tetratricopeptide repeats (TPR). The C-terminal, alpha-helical half is the more conserved, with 48% amino-acid identity to mammalian IFT140; all three mutations are predicted to remove this part of the protein.
rempA mutants have short cilia that accumulate IFT-B proteins
We visualized chordotonal neurons and cilia with cytoplasmic red fluorescent protein (RFP) (Figure 2). In wild type larval scolopidia, the cilia extend through the scolopales and into the distal extracellular caps, but in rempA mutants, the RFP-labeled cilia end within the scolopales, at about half the wild type length. Electron microscopy of the adult antennal chordotonal organ confirmed that the cilia are indeed present but truncated: transverse sections of mutant scolopidia show ciliary profiles at proximal, but not distal levels (Figure 2; compare to supplemental Figure S2). Thus, chordotonal cilia are present in rempA mutants, but are shortened and mostly disconnected from the dendritic cap. The cap-cilium connection is required for transduction [29], so its disconnection in rempA mutants accounts for their complete loss of antennal chordotonal responses [20].
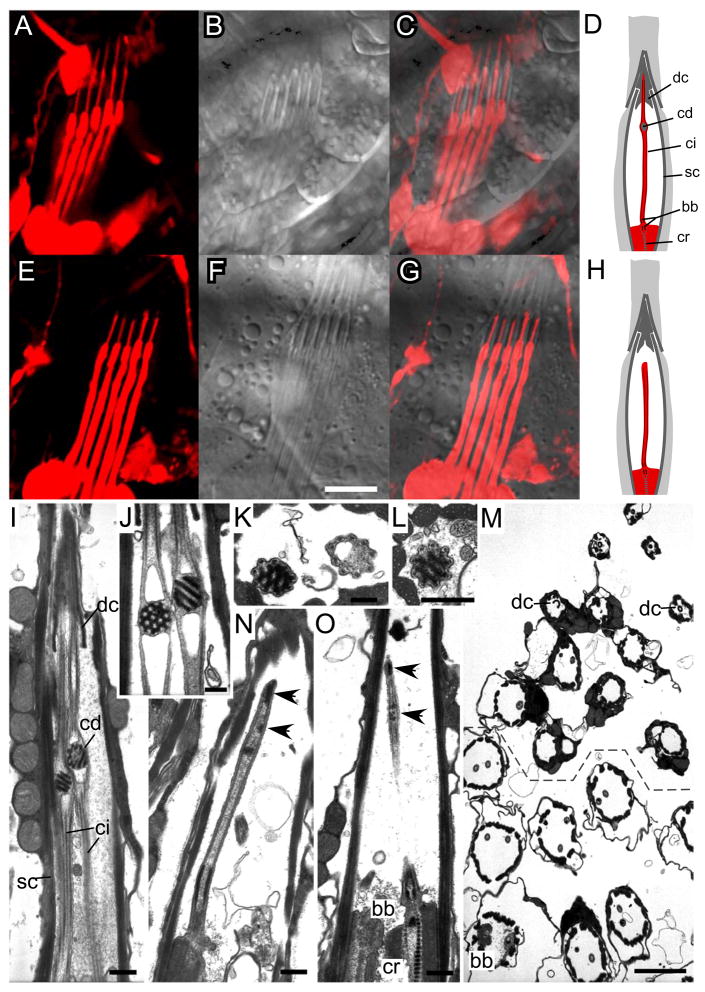
Figure 2. Disrupted ciliary morphology in rempA mutants.
A–C and G–F, Confocal fluorescence (A, E), DIC (B, F) and merged (C, G) images of a pentascolopidial chordotonal organ in a larva expressing red fluorescent protein (RFP) in neurons. D, H, schematics of single scolopidia. Wild-type cilia (A–D, rempA1/+ heterozygote) extend through the scolopale into a dendritic cap; mutant cilia (E–H, rempA1 homozygote) end within the scolopale cavity. Scale bar, 5μm. I–O; electron micrographs of wild type (I–L) and rempA mutant (M–O) antennal scolopidia. I–L, Longitudinal (I, J) and transverse (K, L) sections of wild type scolopidia, showing the subterminal ciliary dilations (cd) proximal to the dendritic cap. M, transverse section of scolopidia in a rempA1 mutant. Moving from the bottom toward the top of the image, scolopidia are sectioned at progressively more apical levels, from basal body (bb) to dendritic cap (dc). In scolopidia above the dashed line, one or both ciliary profiles are absent; no ciliary dilations are observed in any section. N, O, longitudinal sections of scolopidia in rempA1(N) and rempAk12913 (O) mutants. No ciliary dilations are observed; arrowheads indicate disorganized electron-dense material in the lumen of the axoneme. Other abbreviations: ci, cilium, cr, ciliary rootlet, sc, scolopale rods. Scale bars, 0.5μm (I–L, N, O); 2 μm (M).
Chordotonal cilia in Drosophila and other insects share a distinctive feature, the ciliary dilation (CD). This is an electron-dense inclusion within the axoneme, at about 2/3 the length of the cilium, proximal to the dendritic cap (Figure 2, supplemental figure S2). In Drosophila the electron-dense material appears as a hexagonal lattice or as parallel tubes at an oblique angle to the ciliary axis, depending on the plane of section (Figure 2). The microtubule doublets bend outward at this site but otherwise continue without interruption past the inclusion. Axonemal dynein arms are present in the proximal zone, but not distal to the CD [17], (supplemental figure S2). The CD is absent in rempA mutants; indeed, mutant cilia end at about the position where the dilation is normally located (Figure 2).
Caenorhabditis IFT140 (che-11) mutants [30], have distended sensory cilia [12], which lack retrograde IFT and accumulate IFT-B proteins [13]. We examined the distribution of the IFT-B protein NOMPB (IFT88) [25] in Drosophila IFT-A mutants. A transgene expressing NOMPB-GFP [25] was introduced into rempA and oseg1 (IFT122) [28] strains. In wild type and heterozygote controls, NOMPB-GFP is distributed along the cilia [25], and low levels are present in the cell body. In rempA and oseg1 mutants, it is present in the truncated cilia at higher levels than in wild type (Figure 3). Thus neither IFT140 nor IFT122 is required for transport of IFT88 into the cilium, but both are probably required for its retrograde transport back to the cell body.
Figure 3. An IFT-B protein accumulates in IFT-A mutant cilia.

Confocal projections of larval abdominal ch organs (A, B) and pupal antennal (C–E) ch organs in rempA1/+ heterozygotes (A, C), rempA1 homozygotes (B, D) and an oseg1EP3616 homozygote (E), all expressing functional, GFP-tagged NOMPB/IFT88 (green). Neurons are counterstained with MAb22C10 (red), which detects MAP1B/Futsch throughout all neurons, except for their cilia. IFT88 is distributed along wild type cilia (extent indicated by bracket in A), but accumulates at or near the tips of the truncated cilia in the mutants. Scale bar (C–E): 10μm.
In contrast to their defective sensory cilia, rempA mutant males had motile, apparently normal sperm flagella (not shown), indicating that IFT-A proteins, like IFT-B and heterotrimeric kinesin [25] [26], are not needed in Drosophila spermatogenesis.
IFT140/REMPA localizes at the ciliary dilation in chordotonal organs
To view the distribution of IFT140 in sensory neurons, we examined ciliated sense organs in embryos, larvae and pupae expressing a functional REMPA-YFP fusion protein from the rescuing transgene (Figure 1). In mechanosensory bristles and campaniform sensilla, a single spot of YFP was associated with each sensillum (Supplemental figure S3), coincident with staining by the 21A6 monoclonal antibody and proximal to the dendritic sheath protein NOMPA [29]. This places REMPA in the connecting cilium, just proximal to the outer segment, consistent with a previous report of IFT140 and OSEG1/IFT122 localization in bristle neurons [28].
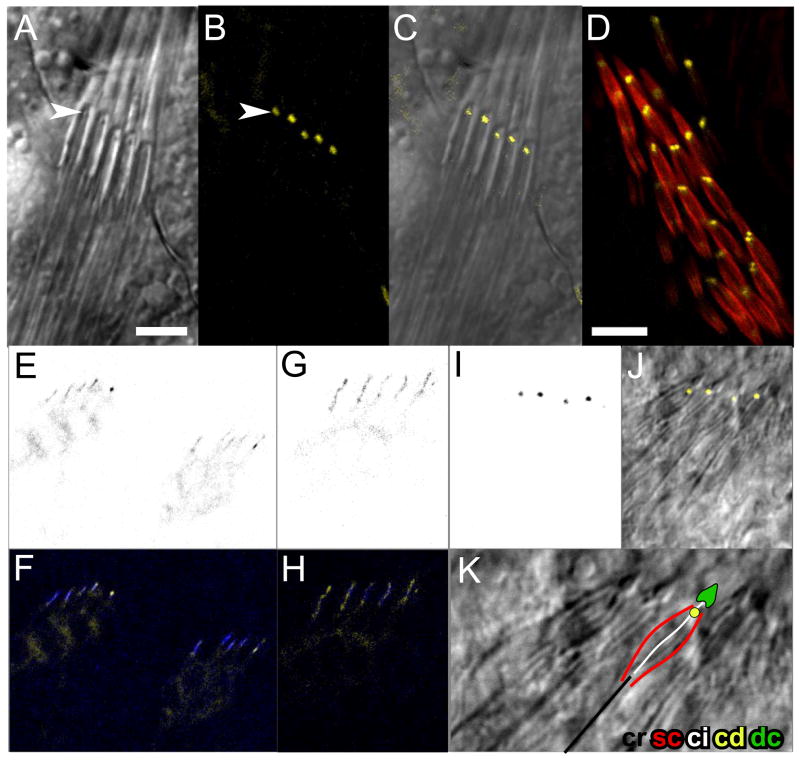
In the longer cilia of larval chordotonal organs, REMPA-YFP appeared at a single, well-defined focus in each scolopale, at the position of the ciliary dilation (Figure 4A–C). As these organs differentiate [31], REMPA-YFP initially appears diffusely in the neuronal cytoplasm and along the growing cilium, but ultimately concentrates at the ciliary focus and disappears from other locations (Figure 4 E–J). A similar progression during differentiation of pupal chordotonal neurons (Figure 5A) culminates in paired foci, in accordance with the paired cilia in adult scolopidia (Figures 4D and 5C). If the cilium is missing, as in Klp64D mutants which lack an IFT-associated kinesin-2 subunit, the REMPA focus is also absent, and a reduced amount of protein accumulates near the basal body (Supplemental Figure S4). In hypomorphic Klp64D mutants, which retain cilia, the REMPA-YFP foci are present but are mislocated within the dendritic caps (supplemental figure S4), as previously observed for the ciliary dilations [26].
Figure 4. REMPA localizes to the ciliary dilation in chordotonal organs.
A–C, DIC (A), confocal fluorescence (B) and merged (C) images of an abdominal chordotonal organ in a 3rd instar larva expressing the P{rempA-YFP} rescue construct. The YFP signal is concentrated at the site of the ciliary dilation (arrowheads in A and B). D, femoral chordotonal organ in a pupa expressing P{rempA-YFP}, showing paired REMPA-YFP foci corresponding to the paired cilia and ciliary dilations in adult scolopidia. Scolopale rods stained with rhodamine-phalloidin (red). E–K, REMPA-YFP expression in differentiating embryonic chordotonal organs. E–H, inverted YFP fluorescence images (E, G) and merged fluorescence images counterstained with MAb 21A6 (F, H) showing expression of REMPA-YFP in the cell body at embryonic stage 15 (E, F; two organs shown) and concentration in the cilium at stage 16 (G, H). I–K, stage 17 organ, in inverted YFP fluorescence (I), and merged with DIC (J). K is an enlarged detail from J with DIC only, overlaid with a schematic of a single scolopidium. Abbreviations as in Figure 2. Scalebars: 5 μm (A–C); 10 μm (D).
Figure 5. REMPA requires IFT122 for expression and IFT dynein for normal localization.
A–D, Differentiating antennal chordotonal organs in oseg1EP3616/+ heterozygotes (A, C) and oseg1EP3616 homozygotes (B, D) with a REMPA-YFP transgene. A and B show an earlier stage, indicated by the parallel arrangement of the scolopales (stained with phalloidin, red) and the distribution of YFP along cilia in A; In C and D the YFP signal resolves to foci and the apical ends of the scolopidia are drawn together. No REMPA-YFP signal was detectable in any stage in the oseg1 mutants. E, F, Antennal chordotonal organs in a btv5P1/+ heterozygote and a btv5P1 homozygote, expressing REMPA-YFP and counterstained with MAb21A6 (blue) and phalloidin (red). G, schematic interpretation of E and F; with the elongated dendritic tips in the antennal scolopidia; only one of the two cilia in a scolopale is drawn. In the heterozygote, the paired REMPA-YFP foci are located just distal to the major zone of 21A6 staining. In btv mutants, most 21A6 staining is shifted to the base of the scolopale, and the YFP signal is redistributed along the cilium and throughout the length of the tubular dendritic cap. Scalebars = 10μm.
The 21A6 antigen is the agrin/perlecan-related protein Eyes Shut (EYS) [32] or Spacemaker (SPAM) [33], which is secreted into the scolopale space where it has a mechanoprotective role under osmotic stress [34]. It was described as being located at the ciliary dilation [32], but 21A6 staining in differentiated chordotonal organs is distinct from and proximal to the REMPA-YFP focus (Figure 5E), consistent with the normal ultrastructure of the ciliary dilations in an eys mutant [32] [34].
IFT122 is essential for IFT 140 expression, and the btv dynein for IFT140 localization
The Chlamydomonas IFT-A subcomplex includes six polypeptides [3]. The amino acid sequences of three of these – IFT140, IFT139 and IFT122 – are published, and the nematode ifta-1 gene product, which is required for retrograde IFT, may also be an IFT-A component [13]. All four proteins are widely conserved in ciliated eukaryotes. (Unusually, the genomes of Drosophila melanogaster and closely related species lack IFT139, although it is present in more distant Drosophila species and in other insects.) IFT122 is encoded in D. melanogaster by the oseg1 locus [28]. When REMPA-YFP was crossed into an oseg1) mutant background, no YFP signal was detected in oseg1 mutant cell bodies or cilia of this genotype at any stage (Figure 5). IFT122 is therefore required for stable expression of IFT140, implying that the IFT-A subcomplex must be at least partly assembled for stability and proper localization, possibly before entering the cilium.
Retrograde IFT is powered by a processive dynein, related to the cytoplasmic dynein that drives cellular minus-end directed transport on microtubules. The Drosophila IFT dynein heavy chain is encoded by the beethoven (btv) locus [35]; btv mutants have extremely reduced antennal sound-evoked potentials [20]. Cilia in btv chordotonal organs are variably disrupted: some retain their connections to the dendritic caps, but their ciliary dilations are always disorganized [20]. In chordotonal cilia of btv mutant larvae and pupal legs, REMPA-YFP is delocalized and redistributed toward the distal tip of the scolopidium. In antennal chordotonal organs, btv mutants show ectopic deposits of REMPA-YFP along the dendritic caps, (Figure 5F, G) suggesting either a failure to retract ciliary material during differentiation, or leakage from disrupted cilia.
A ciliary TRPV ion channel requires IFT140 for expression and IFT dynein for localization
The TRPV channel subunits encoded by the nanchung (nan) and inactive (iav) loci are expressed specifically in chordotonal neurons and localized in their cilia, proximal to the ciliary dilations [21, 22]. Mutations in either nan or iav eliminate antennal sound-evoked potentials, and each subunit requires the other for ciliary expression, suggesting that they form a heteromeric channel required to depolarize the chordotonal neurons enough to fire action potentials [22]. The channel is also needed to regulate active motility of the distal antennal segments, which vibrate with higher amplitude in iav or nan mutants [24].
To determine if the restricted TRPV ion channel distribution in Drosophila requires IFT-A or IFT dynein function, we introduced a functional IAV-GFP fusion transgene [22], under the control of its native promoter, into rempA and btv mutant flies (Figure 6). Surprisingly, instead of accumulating in chordotonal cilia like NOMPB-GFP, IAV-GFP was completely undetectable in rempA mutants. This appears to be a posttranscriptional depletion, as quantitative RT-PCR showed no significant difference in iav or nan transcript levels between rempA mutants and controls (supplemental table 1). Thus, IFT140 may be essential for stability of the expressed TRPV channel. However, REMPA-YFP is normally expressed and localized in chordotonal organs of iav mutants (supplemental figure S5), indicating that the ciliary dilation does not depend on TRPV channel expression or activity, nor is it affected by the increased antennal vibration in iav mutants [24].
Figure 6. The channel subunit IAV is not expressed in rempA mutants, and is delocalized in btv mutants.
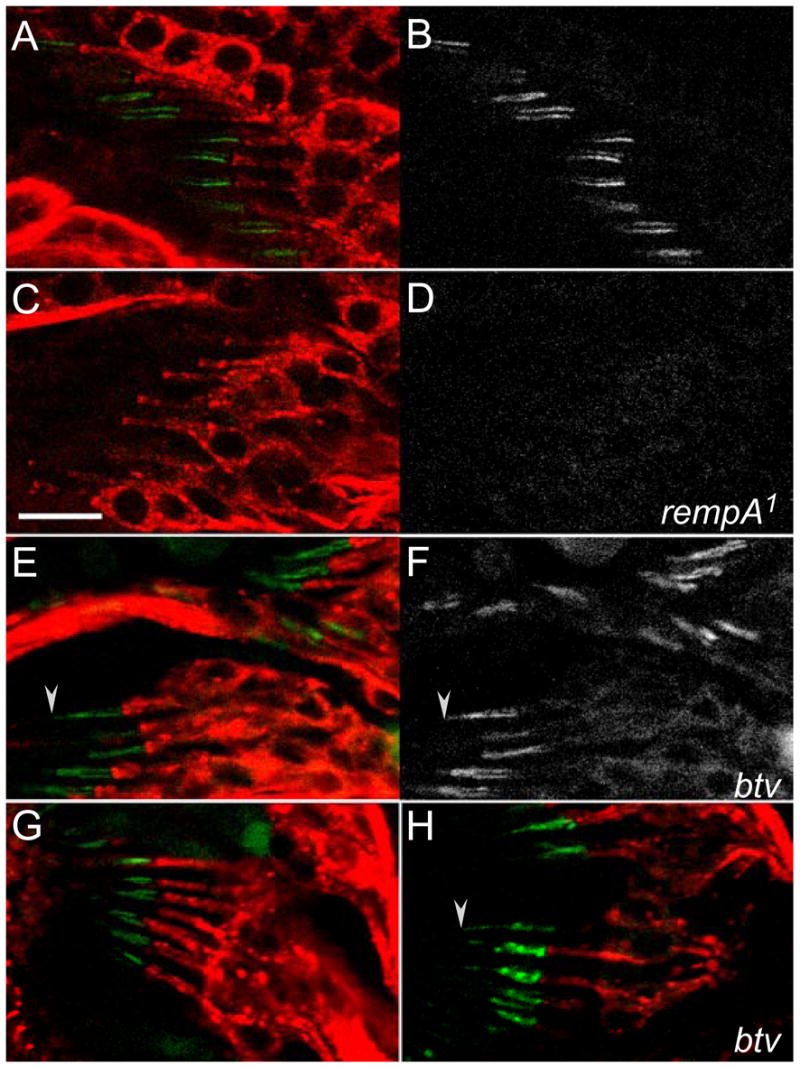
Scolopidia in femoral (A–F) and antennal (G, H) chordotonal organs, from pupae expressing the GFP-tagged IAV subunit (B, D, F), and counterstained with MAb 22C10 (A, C, E, G, H). A, B, G; wild type; C, D; rempA1 homozygotes; E–H, btv5P1 homozygotes. The IAV channel is normally restricted to the proximal part of chordotonal cilia, but is undetectable in the cilia of rempA mutants. In btv mutants, the channel is expressed and appears to extend further along the cilia, spreading into the zone distal to the ciliary dilation (arrowheads). Scalebar = 10μm; all panels at same scale.
In contrast to its absence from rempA mutants, IAV-GFP is still expressed in the chordotonal cilia of btv mutants, consistent with the small antennal potentials that can still be evoked in btv flies by high-amplitude sound stimuli [20]. However, in btv mutant cilia, some IAV-GFP signal penetrates beyond the ciliary dilation, into the distal zone from which it is normally excluded (Figure 6). This implies either that BTV dynein activity normally retrieves the TRPV channel from the distal zone, or that the disrupted ciliary dilations in the btv mutant cilia are insufficient to exclude it.
Discussion
rempA encodes the Drosophila homolog of IFT140, a conserved WD-TPR protein first identified in the IFT-A subcomplex. IFT-A proteins in other species are required for dynein-driven, retrograde transport within cilia and flagella, and the rempA mutant phenotypes are consistent with defective retrograde transport in developing chordotonal cilia. But the data also indicate unexpected functions for the Drosophila IFT-A proteins, as required elements of a sensory-specific structure, the ciliary dilation, and for stable expression of a TRPV ion channel. They show that IFT proteins, generally regarded as elements of the conserved IFT mechanism, can be reconfigured in some cells for specialized sensory functions, an important new perspective for studies both of IFT and of mechanosensory transduction.
IFT140 and IFT dynein function in differentiating ch and es organs
During the differentiation of chordotonal neurons, when REMPA/IFT140 is distributed along the growing cilia, it may function in retrograde transport. In contrast to IFT-B or kinesin-2 null mutants, which do not extend cilia beyond the basal body [25, 26], chordotonal cilia in rempA mutants are substantially longer and accumulate the IFT-B protein IFT88, indicating that anterograde IFT can still operate. These results are consistent with a specific defect in retrograde IFT, as in nematode and Tetrahymena IFT-A mutants [12–14, 36].
However, if the Drosophila IFT-A proteins are required only for retrograde IFT, then mutants lacking the IFT-associated dynein should show the same phenotype as rempA. Indeed, nematode and Chlamydomonas IFT dynein mutants have a similar phenotype, with shortened, distended cilia and flagella that accumulate IFT proteins and other material [8, 16, 37]. But the Drosophila IFT dynein, BTV, has a less severe mutant phenotype in chordotonal organs than rempA: btv mutant cilia retain their distal segments, and most are still connected to the dendritic caps. Moreover, BTV appears not to be required for the operation of other ciliary mechanosensors: unlike rempA mutants, btv mutants have normal bristle receptor potentials [20], and their mildly uncoordinated behavior, similar to that of the TRPV channel mutants iav and nan, is consistent with a chordotonal-specific loss of mechanotransduction. This suggests that the IFT-A proteins have additional functions, not wholly dependent on dynein-driven transport, in chordotonal and other cilia.
The ciliary dilation delimits structurally and molecularly distinct ciliary segments
As chordotonal cilia complete differentiation, REMPA-YFP concentrates to a focus at the same time and site as the CD forms. A distal shift of the REMPA-YFP signal in kinesin-2 hypomorphs parallels the displacement of the CD previously seen in these mutants [26]. The CD is missing in rempA mutants, and the REMPA-YFP focus is missing in mutants lacking IFT122, the other IFT-A protein in Drosophila. Thus IFT-A proteins are localized at the CD, and are probably both required for its formation. In addition, OSEG4, the homolog of the retrograde IFT-associated protein IFTA-1 [13], also localizes to the CD [34].
As a characteristic feature of chordotonal cilia, the CD has been proposed to have a role in mechanotransduction [17], but its precise function is unknown. Chordotonal cilia are unusual among sensory cilia in that they are potentially motile [38, 39]: their axonemal microtubules bear extensions similar to dynein arms (supplementary figure 2). They are a likely source of the mechanical energy that increases antennal compliance and sensitivity to low-intensity vibrations, and causes the antennae to oscillate even in the absence of any stimulus [40]. Mutants with cilia that are structurally defective (btv, tilB) or disconnected from the distal antennal segments (nompA) lack active antennal mechanics, implying that the cilia themselves are indeed the motor elements [41]. The active mechanics are also absent and the sensitivity reduced in nompC mutants, but the TRPV mutants iav or nan show much larger antennal oscillations than wild type [24]. These different mutant phenotypes imply opposing functions for the TRPN and TRPV ion channels in regulating ciliary motility: the NOMPC/TRPN channel may be required to trigger motility whereas the TRPV channel normally reduces it.
The opposite effects of the TRPN and TRPV channels on motility could result from different locations relative to the ciliary motors. The TRPV channel is normally restricted proximal to the ciliary dilation [22]. The apparent axonemal dynein arms are also located only in the proximal zone [17, 42] (supplementary figure 2). Thus potential drivers and negative regulators of ciliary motility are both restricted to the proximal part of the cilium. Conversely, new immunostaining data (Dr. Y.D. Chung, personal communication) show the TRPN/NOMPC channel to be located specifically in the distal zone of chordotonal cilia, and bounded proximally by the CD. The CD is therefore located between ciliary segments with different channel populations and axonemal structures. In btv mutants, in which the CD is disorganized, the partitioning is compromised, and some of the TRPV channel is located in the distal zone, suggesting that an intact CD and/or localized dynein activity is required to maintain the localization, and possibly to sort specific channels into each segment.
A role for IFT-A in preciliary transport?
In differentiating wild-type neurons, REMPA-YFP was observed in the cell body and inner dendrite as well as in the nascent cilia, but it is undetectable at any of these sites, at any stage, in oseg1 mutants. This suggests that IFT140 requires IFT122 for stable expression, even before arrival at the basal body and entry into the cilium. A similar interdependence was observed in Chlamydomonas, in which levels of multiple IFT-A proteins are reduced in each of three retrograde IFT mutants [11, 43].
Similarly, the lack of expression of the IAV channel subunit in rempA mutants indicates that TRPV protein expression or stability also requires the IFT-A proteins. (There is, however, no reciprocal requirement: IFT140 is normally localized at the ciliary dilation in TRPV mutants.) We speculate that the IFT-A proteins may be required for the extraciliary, vesicular transport of specific membrane proteins to the basal body region, before insertion into the ciliary membrane. Both OSEG1/IFT22 and REMPA/IFT140 share a common WD/TPR domain architecture with coat proteins (COP) of the coated vesicle transport pathways [28], from which IFT may be derived [44]. Prior evidence for preciliary, vesicular transport of ciliary membrane proteins includes the AP1-dependent transport of ODR-10 [45] and PKD-2 [46] in C. elegans, the presence of IFT20 at the Golgi apparatus [47], and the Rab8-dependent transport of rhodopsin [48] and BBS module proteins [49] to the cilium. Preciliary transport could be driven by a minus end-directed microtubule motor such as dynein, as the microtubules in the inner dendritic segment have their minus ends oriented distally [50]. It cannot, however, be the IFT dynein, as the IFT-A proteins accumulate in the cilia, not in the cell bodies or inner segments, of a btv mutant.
Conclusion
In conclusion, the involvement of REMPA/IFT140 in forming the ciliary dilation and localizing ciliary ion channels demonstrates an unexpected versatility for the IFT-A proteins. It will be interesting to see if reconfigurations and adaptations of the IFT proteins also underlie the many diverse forms of other sensory cilia.
Experimental Procedures
Genetic alleles, stocks, and transgene constructs
rempA1 [27] and btv5P1 [20] were isolated as described. The rempA allele P{lacW}exk12913 and the Oseg1 allele P[32]Oseg1EP3616 were obtained from the Bloomington Drosophila stock center. The rempA allele l(2)21Ci1 and other lethal mutations in the 21C region were obtained from Dr. P Heitzler. Transgenic stocks expressing GFP fused to NOMPB/IFT88 [25] and to the TRPV channel subunit Inactive [22] were described previously. Construction of the rescuing fusion transgene P{rempA+-YFP} is described in supplemental figure 1B.
Sensory electrophysiology
Antennal sound-evoked potentials [20], and bristle transepithelial potentials and mechanoreceptor potentials [27], were recorded from adult flies as previously described.
Electron Microscopy
Fly heads, with proboscis removed to facilitate infiltration, were fixed by immersion overnight at 4°C in a fixative containing 2.5% glutaraldehyde, 2.0% paraformaldehyde in 0.1 M phosphate buffer (PB) at pH 7.4. Heads were washed in PB, postfixed with OsO4, dehydrated in an ethanol series, and embedded in Polybed 812. Ultrathin sections (75 nm) were stained with aqueous uranyl acetate and lead citrate and were examined with a Hitachi 7000 electron microscope.
Fluorescence microscopy and immunostaining
Fluorescently tagged or immunostained proteins were visualized by scanning confocal microscopy in embryos, larvae, and late pupae or pharate adults. Embryos were collected 13–15 hours after egglaying and dechorionated in dilute bleach solution. Heterozygous sibs were distinguished from mutants by GFP-expressing balancer chromosomes. Pupal antennae and legs were dissected 24–48 hours after pupariation in PBT (0.2% Triton-X in PBS) and fixed in 4% formaldehyde in PBT for 20 min, then washed three times in PBT for 10 minutes. For immmunostaining, samples were first blocked for 1hr in 5% normal goat serum in PBT before incubating with primary antibodies overnight at 4°C in the blocking solution, then washed in PBT as before and incubated with secondary antibody for 2 hours. After washing with PBT for 3 times 20 minutes, samples were mounted in Vectashield (Vectorlabs, CA) and imaged with a laser scanning confocal microscope (Leica TCS SP2).
Monoclonal antisera 21A6 (used at a dilution of 1:250) and 22C10 (1:100) were obtained from the Developmental Studies Hybridoma Bank (DSHB, http://dshb.biology.uiowa.edu/). Phalloidin-Alexa568 (1:1000) and the secondary antibodies Alexa647 conjugated goat anti-rabbit (1:1000), Alexa647 conjugated goat anti-mouse (1:1000), Alexa568 conjugated goat anti-mouse (1:500), and Alexa546 goat anti-mouse (1:1000) were obtained from Molecular Probes/Invitrogen (http://probes.invitrogen.com/) and used at the dilutions indicated.
Supplementary Material
Figure S1. Positional cloning and transgene construction of rempA. A. Cytogenetic mapping & molecular identification. rempA1 was mapped by recombination to distal chromosome arm 2L, and by complementation testing to the overlap (shaded) of the deletions Df(2L)L124 and Df(2L)BSC16, in cytogenetic interval 21C-D; it is excluded from the regions deleted in Df(2L)BSC4 and Df(2L)al. Endpoints of the Df(2L)BSC4 and Df(2L)BSC16 in this region are defined on the molecular map below by their progenitor P element insertions P{PZ}a-adaptin06694 and P{lacW}exk12913.
Predicted genes (gene spans shown as shaded boxes) in the 21C-D interval includes predicted genes for two known ciliary proteins: CG13691/BBS8, the Drosophila homolog of a human Bardet-Biedl syndrome protein, and CG11838/oseg3, which encodes IFT140. A conservative missense mutation (A373V) was found in the BBS8 gene of rempA1 mutants, but no mutations of this gene were found in l(2)21Ci1 genomic DNA, and homozygotes for a deletion of the BBS8 coding region show no mechanosensory defects (E. Lee & M. Kernan, unpublished data). Thus, BBS8 is not rempA. The P{lacW}exk12913 chromosome is recessive lethal and fails to complement rempA1 - but another expanded allele, ex1, complemented the viability and behavior defects of both l(2)k12913 and rempA1, suggesting that a mutation other than the P insertion causes lethality. The P{lacW}exk12913 chromosome was subsequently found to have a 3kbp deletion within rempA/oseg3, causing a frameshift after codon 475 that truncated the predicted open reading frame after 34 more codons.
B, rempA-YFP transgene construction and transformation. To make the rescuing fusion construct P{rempA+-YFP}, an 8.34 kbp EcoR1 genomic DNA fragment, including the entire rempA coding region plus 0.66 kbp upstream and 2.86 kbp downstream, was subcloned from the BAC clone BACR48E08 (Drosophila Genome Resource Center, Bloomington, IN) into pBluescript KSII+ (Promega) to give pBS-rempA. To add the C-terminal YFP tag, a 3kb XmaI-NotI fragment including part of exon 6 and all downstream sequences was first removed from pBS-rempA. The deleted part of exon 6 was replaced as a 90 bp amplified XmaI-(NotI) fragment. The Venus-YFP coding sequence, preceded by the codon AAG and followed by a stop codon, was amplified from pPWV (Clontech, Mountain View, CA) and inserted as a NotI-SacII fragment. The tagged gene was transferred into pCaSpeR4 as an EcoRI-SacII fragment, and a 1.8kb 3′ UTR fragment amplified from pBS-rempA was inserted as a SacII-(XbaI) fragment. Transgene constructs were coinjected with the transposase-expressing helper plasmid pTurbo into y w embryos, and w+ offspring were selected.
Figure S2. Ciliary morphology and zonal differentiation in wild type chordotonal organs. A. Schematic of antennal scolopidia, which are offset so that a transverse section cuts adjacent scolopidia at progressively different levels. At level 1 scolopidia are cut though the dendritic cap (dc), distal to the ciliary axoneme; at level 2, through the dendritic cap where it encloses one or both cilia; at level 3, through or close to the ciliary dilation (cd); at level 4, through the cilia proximal to the ciliary dilation. B, Composite electron micrograph of cross-sectioned scolopidia in a wild type Johnston’s organ. Scolopidia are cut progressively more basally from top right to bottom left of the image; individual profiles are numbered according to the schematic in A. C, D: Enlarged sections of wild type chordotonal cilia with (C) and without (D) protrusions (arrowhead) similar to axonemal dynein arms. C is from a different section but similar to the level 4 sections at lower left in B; D is an enlargement of the bracketed ciliary profile at lower right in B. Scale bars: B, 0.5μm; C, 0.125 μm.
Figure S3. REMPA/IFT140 localizes to the connecting cilium in es organs. A, B, Abdominal bristles in pharate adults expressing REMPA-YFP from the rescue construct in Figure 1. The fluorescence image (B) includes both a REMPA-YFP signal, seen as a dot (arrowhead) at the posterior rim of each socket, and cuticular autofluorescence emitted in the same wavelength range. C–E, REMPA-YFP expressing bristle (C) counterstained with anti-NOMPA (D); merged image in (E). The NOMPA-containing dendritic sheath, which encloses the outer segment, is distal to the REMPA-YFP focus. F, bristle base from a non-transgenic wild type fly, also stained with anti-NOMPA: the YFP dot is absent. G, REMPA-YFP expressing bristle counterstained with 21A6 in the merged image (H) the YFP and 21A6 signals are coincident.
Figure S4. REMPA/IFT140 expression in kinesin-2 null and hypomorphic mutants. A–D: Scolopidia in femoral chordotonal organs in Klp64Dn123/CyO heterozygotes (A) and Klp64Dk1/n123 null trans-heterozygotes (B) expressing REMPA-YFP. Arrows point toward the apical ends of the scolopidia. The null mutants, which lack extended cilia, have a weak YFP signal near the base of the scolopale, at the site of the basal bodies. E–J: Merged DIC/fluorescence (E, H) and fluorescence (F, I) images of scolopidia in femoral chordotonal organs, focused on the arrowhead-shaped dendritic caps (bracketed). In wild type heterozygotes (E, F), the REMPA foci are located just proximal to the caps. In hypomorphic Klp64Dk5/l4 trans-heterozygotes (H, I), the foci are slightly weaker and are shifted distally to lie within the caps. B, D, G, J: schematic interpretations. Scale bar = 10μm; all panels at same scale.
Figure S5. Normal REMPA-YFP expression and location in iav mutants. Antennal scolopidia in an iav mutant expressing REMPA-YFP, counterstained with phalloidin (A) and 21A6 (B). Normal paired YFP foci are evident; the distribution of the 21A6 antigen is also unchanged.
Acknowledgments
We are very grateful to Dr. Pascal Heitzler for mutant stocks including the l(2)21Ci1 allele and for sharing unpublished mapping data, and to Dr. Joan Hooper and Dr. Markus Noll for other mutant stocks. Dr. Emiko Shishido and Simon Wong carried out preliminary work on positional cloning. We thank Dr. Yun Doo Chung for allowing us to refer to unpublished data and for informative discussions. This project was funded by grants from the NIH-NIDCD to M. K. (DC002780), and to D.E. (DC004848).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3:813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- 2.Scholey JM. Intraflagellar transport. Annu Rev Cell Dev Biol. 2003;19:423–443. doi: 10.1146/annurev.cellbio.19.111401.091318. [DOI] [PubMed] [Google Scholar]
- 3.Cole DG. The intraflagellar transport machinery of Chlamydomonas reinhardtii. Traffic. 2003;4:435–442. doi: 10.1034/j.1600-0854.2003.t01-1-00103.x. [DOI] [PubMed] [Google Scholar]
- 4.Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc Natl Acad Sci U S A. 1993;90:5519–5523. doi: 10.1073/pnas.90.12.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pazour GJ, Wilkerson CG, Witman GB. A dynein light chain is essential for the retrograde particle movement of intraflagellar transport (IFT) J Cell Biol. 1998;141:979–992. doi: 10.1083/jcb.141.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pazour GJ, Dickert BL, Witman GB. The DHC1b (DHC2) isoform of cytoplasmic dynein is required for flagellar assembly. J Cell Biol. 1999;144:473–481. doi: 10.1083/jcb.144.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porter ME, Bower R, Knott JA, Byrd P, Dentler W. Cytoplasmic dynein heavy chain 1b is required for flagellar assembly in Chlamydomonas. Mol Biol Cell. 1999;10:693–712. doi: 10.1091/mbc.10.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Signor D, Wedaman KP, Orozco JT, Dwyer ND, Bargmann CI, Rose LS, Scholey JM. Role of a class DHC1b dynein in retrograde transport of IFT motors and IFT raft particles along cilia, but not dendrites, in chemosensory neurons of living Caenorhabditis elegans. J Cell Biol. 1999;147:519–530. doi: 10.1083/jcb.147.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piperno G, Mead K. Transport of a novel complex in the cytoplasmic matrix of Chlamydomonas flagella. Proc Natl Acad Sci U S A. 1997;94:4457–4462. doi: 10.1073/pnas.94.9.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole DG, Diener DR, Himelblau AL, Beech PL, Fuster JC, Rosenbaum JL. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J Cell Biol. 1998;141:993–1008. doi: 10.1083/jcb.141.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piperno G, Siuda E, Henderson S, Segil M, Vaananen H, Sassaroli M. Distinct mutants of retrograde intraflagellar transport (IFT) share similar morphological and molecular defects. J Cell Biol. 1998;143:1591–1601. doi: 10.1083/jcb.143.6.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perkins LA, Hedgecock EM, Thomson JN, Culotti JG. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev Biol. 1986;117:456–487. doi: 10.1016/0012-1606(86)90314-3. [DOI] [PubMed] [Google Scholar]
- 13.Blacque OE, Li C, Inglis PN, Esmail MA, Ou G, Mah AK, Baillie DL, Scholey JM, Leroux MR. The WD repeat-containing protein IFTA-1 is required for retrograde intraflagellar transport. Mol Biol Cell. 2006;17:5053–5062. doi: 10.1091/mbc.E06-06-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsao CC, Gorovsky MA. Tetrahymena IFT122A is not essential for cilia assembly but plays a role in returning IFT proteins from the ciliary tip to the cell body. J Cell Sci. 2008;121:428–436. doi: 10.1242/jcs.015826. [DOI] [PubMed] [Google Scholar]
- 15.Absalon S, Blisnick T, Kohl L, Toutirais G, Dore G, Julkowska D, Tavenet A, Bastin P. Intraflagellar Transport and Functional Analysis of Genes Required for Flagellum Formation in Trypanosomes. Mol Biol Cell. 2008;19:929–944. doi: 10.1091/mbc.E07-08-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schafer JC, Haycraft CJ, Thomas JH, Yoder BK, Swoboda PK. #14} (2003). XBX-1 encodes a dynein light intermediate chain required for retrograde intraflagellar transport and cilia assembly in Caenorhabditis elegans. Mol Biol Cell. 2005;14:2057–2070. doi: 10.1091/mbc.E02-10-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Field L, Matheson T. Chordotonal organs of insects. Advances in Insect Physiology. 1998;27:1–228. [Google Scholar]
- 18.Yack JE. The structure and function of auditory chordotonal organs in insects. Microsc Res Tech. 2004;63:315–337. doi: 10.1002/jemt.20051. [DOI] [PubMed] [Google Scholar]
- 19.Kernan MJ. Mechanotransduction and auditory transduction in Drosophila. Pflugers Arch. 2007;454:703–720. doi: 10.1007/s00424-007-0263-x. [DOI] [PubMed] [Google Scholar]
- 20.Eberl DF, Hardy RW, Kernan MJ. Genetically similar transduction mechanisms for touch and hearing in Drosophila. J Neurosci. 2000;20:5981–5988. doi: 10.1523/JNEUROSCI.20-16-05981.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J, Chung YD, Park DY, Choi S, Shin DW, Soh H, Lee HW, Son W, Yim J, Park CS, et al. A TRPV family ion channel required for hearing in Drosophila. Nature. 2003;424:81–84. doi: 10.1038/nature01733. [DOI] [PubMed] [Google Scholar]
- 22.Gong Z, Son W, Chung YD, Kim J, Shin DW, McClung CA, Lee Y, Lee HW, Chang DJ, Kaang BK, et al. Two interdependent TRPV channel subunits, inactive and Nanchung, mediate hearing in Drosophila. J Neurosci. 2004;24:9059–9066. doi: 10.1523/JNEUROSCI.1645-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker RG, Willingham AT, Zuker CS. A Drosophila mechanosensory transduction channel. Science. 2000;287:2229–2234. doi: 10.1126/science.287.5461.2229. [DOI] [PubMed] [Google Scholar]
- 24.Gopfert MC, Albert JT, Nadrowski B, Kamikouchi A. Specification of auditory sensitivity by Drosophila TRP channels. Nat Neurosci. 2006;9:999–1000. doi: 10.1038/nn1735. [DOI] [PubMed] [Google Scholar]
- 25.Han YG, Kwok BH, Kernan MJ. Intraflagellar transport is required in Drosophila to differentiate sensory cilia but not sperm. Curr Biol. 2003;13:1679–1686. doi: 10.1016/j.cub.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 26.Sarpal R, Todi SV, Sivan-Loukianova E, Shirolikar S, Subramanian N, Raff EC, Erickson JW, Ray K, Eberl DF. Drosophila KAP interacts with the kinesin II motor subunit KLP64D to assemble chordotonal sensory cilia, but not sperm tails. Curr Biol. 2003;13:1687–1696. doi: 10.1016/j.cub.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 27.Kernan M, Cowan D, Zuker C. Genetic dissection of mechanosensory transduction: mechanoreception-defective mutations of Drosophila. Neuron. 1994;12:1195–1206. doi: 10.1016/0896-6273(94)90437-5. [DOI] [PubMed] [Google Scholar]
- 28.Avidor-Reiss T, Maer AM, Koundakjian E, Polyanovsky A, Keil T, Subramaniam S, Zuker CS. Decoding cilia function: defining specialized genes required for compartmentalized cilia biogenesis. Cell. 2004;117:527–539. doi: 10.1016/s0092-8674(04)00412-x. [DOI] [PubMed] [Google Scholar]
- 29.Chung YD, Zhu J, Han Y, Kernan MJ. nompA encodes a PNS-specific, ZP domain protein required to connect mechanosensory dendrites to sensory structures. Neuron. 2001;29:415–428. doi: 10.1016/s0896-6273(01)00215-x. [DOI] [PubMed] [Google Scholar]
- 30.Qin H, Rosenbaum JL, Barr MM. An autosomal recessive polycystic kidney disease gene homolog is involved in intraflagellar transport in C. elegans ciliated sensory neurons. Curr Biol. 2001;11:457–461. doi: 10.1016/s0960-9822(01)00122-1. [DOI] [PubMed] [Google Scholar]
- 31.Carlson SD, Hilgers SL, Juang JL. First developmental signs of the scolopale (glial) cell and neuron comprising the chordotonal organ in the Drosophila embryo. Glia. 1997;19:269–274. [PubMed] [Google Scholar]
- 32.Husain N, Pellikka M, Hong H, Klimentova T, Choe KM, Clandinin TR, Tepass U. The agrin/perlecan-related protein eyes shut is essential for epithelial lumen formation in the Drosophila retina. Dev Cell. 2006;11:483–493. doi: 10.1016/j.devcel.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 33.Zelhof AC, Hardy RW, Becker A, Zuker CS. Transforming the architecture of compound eyes. Nature. 2006;443:696–699. doi: 10.1038/nature05128. [DOI] [PubMed] [Google Scholar]
- 34.Cook B, Hardy RW, McConnaughey WB, Zuker CS. Preserving cell shape under environmental stress. Nature. 2008;452:361–364. doi: 10.1038/nature06603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma Y. Biology. PhD. Iowa City: University of Iowa; 2004. The Drosophila deafness gene beethoven encodes the dynein heavy chain 1b isoform required for intraflagellar transport. [Google Scholar]
- 36.Albert PS, Brown SJ, Riddle DL. Sensory control of dauer larva formation in Caenorhabditis elegans. J Comp Neurol. 1981;198:435–451. doi: 10.1002/cne.901980305. [DOI] [PubMed] [Google Scholar]
- 37.Wicks SR, de Vries CJ, van Luenen HG, Plasterk RH. CHE-3, a cytosolic dynein heavy chain, is required for sensory cilia structure and function in Caenorhabditis elegans. Dev Biol. 2000;221:295–307. doi: 10.1006/dbio.2000.9686. [DOI] [PubMed] [Google Scholar]
- 38.Moran DT, Varela FJ, Rowley JC., 3rd Evidence for active role of cilia in sensory transduction. Proc Natl Acad Sci U S A. 1977;74:793–797. doi: 10.1073/pnas.74.2.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gopfert MC, Robert D. Motion generation by Drosophila mechanosensory neurons. Proc Natl Acad Sci U S A. 2003;100:5514–5519. doi: 10.1073/pnas.0737564100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gopfert MC, Humphris AD, Albert JT, Robert D, Hendrich O. Power gain exhibited by motile mechanosensory neurons in Drosophila ears. Proc Natl Acad Sci U S A. 2005;102:325–330. doi: 10.1073/pnas.0405741102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gopfert MC, Robert D. The mechanical basis of Drosophila audition. J Exp Biol. 2002;205:1199–1208. doi: 10.1242/jeb.205.9.1199. [DOI] [PubMed] [Google Scholar]
- 42.Young D. Fine structure of the sensory cilium of an insect auditory receptor. J Neurocytol. 1973;2:47–58. doi: 10.1007/BF01099207. [DOI] [PubMed] [Google Scholar]
- 43.Iomini C, Babaev-Khaimov V, Sassaroli M, Piperno G. Protein particles in Chlamydomonas flagella undergo a transport cycle consisting of four phases. J Cell Biol. 2001;153:13–24. doi: 10.1083/jcb.153.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jekely G, Arendt D. Evolution of intraflagellar transport from coated vesicles and autogenous origin of the eukaryotic cilium. Bioessays. 2006;28:191–198. doi: 10.1002/bies.20369. [DOI] [PubMed] [Google Scholar]
- 45.Dwyer ND, Adler CE, Crump JG, L’Etoile ND, Bargmann CI. Polarized dendritic transport and the AP-1 mu1 clathrin adaptor UNC-101 localize odorant receptors to olfactory cilia. Neuron. 2001;31:277–287. doi: 10.1016/s0896-6273(01)00361-0. [DOI] [PubMed] [Google Scholar]
- 46.Bae YK, Qin H, Knobel KM, Hu J, Rosenbaum JL, Barr MM. General and cell-type specific mechanisms target TRPP2/PKD-2 to cilia. Development. 2006;133:3859–3870. doi: 10.1242/dev.02555. [DOI] [PubMed] [Google Scholar]
- 47.Follit JA, Tuft RA, Fogarty KE, Pazour GJ. The Intraflagellar Transport Protein IFT20 Is Associated with the Golgi Complex and Is Required for Cilia Assembly. Mol Biol Cell. 2006 doi: 10.1091/mbc.E06-02-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moritz OL, Tam BM, Hurd LL, Peranen J, Deretic D, Papermaster DS. Mutant rab8 Impairs docking and fusion of rhodopsin-bearing post-Golgi membranes and causes cell death of transgenic Xenopus rods. Mol Biol Cell. 2001;12:2341–2351. doi: 10.1091/mbc.12.8.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, et al. A Core Complex of BBS Proteins Cooperates with the GTPase Rab8 to Promote Ciliary Membrane Biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 50.Clark IE, Jan LY, Jan YN. Reciprocal localization of Nod and kinesin fusion proteins indicates microtubule polarity in the Drosophila oocyte, epithelium, neuron and muscle. Development. 1997;124:461–470. doi: 10.1242/dev.124.2.461. [DOI] [PubMed] [Google Scholar]
- 51.Laurencon A, Dubruille R, Efimenko E, Grenier G, Bissett R, Cortier E, Rolland V, Swoboda P, Durand B. Identification of novel regulatory factor X (RFX) target genes by comparative genomics in Drosophila species. Genome Biol. 2007;8:R195. doi: 10.1186/gb-2007-8-9-r195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andrade MA, Ponting CP, Gibson TJ, Bork P. Homology-based method for identification of protein repeats using statistical significance estimates. J Mol Biol. 2000;298:521–537. doi: 10.1006/jmbi.2000.3684. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Positional cloning and transgene construction of rempA. A. Cytogenetic mapping & molecular identification. rempA1 was mapped by recombination to distal chromosome arm 2L, and by complementation testing to the overlap (shaded) of the deletions Df(2L)L124 and Df(2L)BSC16, in cytogenetic interval 21C-D; it is excluded from the regions deleted in Df(2L)BSC4 and Df(2L)al. Endpoints of the Df(2L)BSC4 and Df(2L)BSC16 in this region are defined on the molecular map below by their progenitor P element insertions P{PZ}a-adaptin06694 and P{lacW}exk12913.
Predicted genes (gene spans shown as shaded boxes) in the 21C-D interval includes predicted genes for two known ciliary proteins: CG13691/BBS8, the Drosophila homolog of a human Bardet-Biedl syndrome protein, and CG11838/oseg3, which encodes IFT140. A conservative missense mutation (A373V) was found in the BBS8 gene of rempA1 mutants, but no mutations of this gene were found in l(2)21Ci1 genomic DNA, and homozygotes for a deletion of the BBS8 coding region show no mechanosensory defects (E. Lee & M. Kernan, unpublished data). Thus, BBS8 is not rempA. The P{lacW}exk12913 chromosome is recessive lethal and fails to complement rempA1 - but another expanded allele, ex1, complemented the viability and behavior defects of both l(2)k12913 and rempA1, suggesting that a mutation other than the P insertion causes lethality. The P{lacW}exk12913 chromosome was subsequently found to have a 3kbp deletion within rempA/oseg3, causing a frameshift after codon 475 that truncated the predicted open reading frame after 34 more codons.
B, rempA-YFP transgene construction and transformation. To make the rescuing fusion construct P{rempA+-YFP}, an 8.34 kbp EcoR1 genomic DNA fragment, including the entire rempA coding region plus 0.66 kbp upstream and 2.86 kbp downstream, was subcloned from the BAC clone BACR48E08 (Drosophila Genome Resource Center, Bloomington, IN) into pBluescript KSII+ (Promega) to give pBS-rempA. To add the C-terminal YFP tag, a 3kb XmaI-NotI fragment including part of exon 6 and all downstream sequences was first removed from pBS-rempA. The deleted part of exon 6 was replaced as a 90 bp amplified XmaI-(NotI) fragment. The Venus-YFP coding sequence, preceded by the codon AAG and followed by a stop codon, was amplified from pPWV (Clontech, Mountain View, CA) and inserted as a NotI-SacII fragment. The tagged gene was transferred into pCaSpeR4 as an EcoRI-SacII fragment, and a 1.8kb 3′ UTR fragment amplified from pBS-rempA was inserted as a SacII-(XbaI) fragment. Transgene constructs were coinjected with the transposase-expressing helper plasmid pTurbo into y w embryos, and w+ offspring were selected.
Figure S2. Ciliary morphology and zonal differentiation in wild type chordotonal organs. A. Schematic of antennal scolopidia, which are offset so that a transverse section cuts adjacent scolopidia at progressively different levels. At level 1 scolopidia are cut though the dendritic cap (dc), distal to the ciliary axoneme; at level 2, through the dendritic cap where it encloses one or both cilia; at level 3, through or close to the ciliary dilation (cd); at level 4, through the cilia proximal to the ciliary dilation. B, Composite electron micrograph of cross-sectioned scolopidia in a wild type Johnston’s organ. Scolopidia are cut progressively more basally from top right to bottom left of the image; individual profiles are numbered according to the schematic in A. C, D: Enlarged sections of wild type chordotonal cilia with (C) and without (D) protrusions (arrowhead) similar to axonemal dynein arms. C is from a different section but similar to the level 4 sections at lower left in B; D is an enlargement of the bracketed ciliary profile at lower right in B. Scale bars: B, 0.5μm; C, 0.125 μm.
Figure S3. REMPA/IFT140 localizes to the connecting cilium in es organs. A, B, Abdominal bristles in pharate adults expressing REMPA-YFP from the rescue construct in Figure 1. The fluorescence image (B) includes both a REMPA-YFP signal, seen as a dot (arrowhead) at the posterior rim of each socket, and cuticular autofluorescence emitted in the same wavelength range. C–E, REMPA-YFP expressing bristle (C) counterstained with anti-NOMPA (D); merged image in (E). The NOMPA-containing dendritic sheath, which encloses the outer segment, is distal to the REMPA-YFP focus. F, bristle base from a non-transgenic wild type fly, also stained with anti-NOMPA: the YFP dot is absent. G, REMPA-YFP expressing bristle counterstained with 21A6 in the merged image (H) the YFP and 21A6 signals are coincident.
Figure S4. REMPA/IFT140 expression in kinesin-2 null and hypomorphic mutants. A–D: Scolopidia in femoral chordotonal organs in Klp64Dn123/CyO heterozygotes (A) and Klp64Dk1/n123 null trans-heterozygotes (B) expressing REMPA-YFP. Arrows point toward the apical ends of the scolopidia. The null mutants, which lack extended cilia, have a weak YFP signal near the base of the scolopale, at the site of the basal bodies. E–J: Merged DIC/fluorescence (E, H) and fluorescence (F, I) images of scolopidia in femoral chordotonal organs, focused on the arrowhead-shaped dendritic caps (bracketed). In wild type heterozygotes (E, F), the REMPA foci are located just proximal to the caps. In hypomorphic Klp64Dk5/l4 trans-heterozygotes (H, I), the foci are slightly weaker and are shifted distally to lie within the caps. B, D, G, J: schematic interpretations. Scale bar = 10μm; all panels at same scale.
Figure S5. Normal REMPA-YFP expression and location in iav mutants. Antennal scolopidia in an iav mutant expressing REMPA-YFP, counterstained with phalloidin (A) and 21A6 (B). Normal paired YFP foci are evident; the distribution of the 21A6 antigen is also unchanged.