Abstract
Luteolin, 3′,4′,5,7-tetrahydroxyflavone, is a common flavonoid that exists in many types of plants including fruits, vegetables, and medicinal herbs. Plants rich in luteolin have been used in Chinese traditional medicine for treating various diseases such as hypertension, inflammatory disorders, and cancer. Having multiple biological effects such as anti-inflammation, anti-allergy and anticancer, luteolin functions as either an antioxidant or a pro-oxidant biochemically. The biological effects of luteolin could be functionally related to each other. For instance, the anti-inflammatory activity may be linked to its anticancer property. Luteolin's anticancer property is associated with the induction of apoptosis, and inhibition of cell proliferation, metastasis and angiogenesis. Furthermore, luteolin sensitizes cancer cells to therapeutic-induced cytotoxicity through suppressing cell survival pathways such as phosphatidylinositol 3′-kinase (PI3K)/Akt, nuclear factor kappa B (NF-κB), and X-linked inhibitor of apoptosis protein (XIAP), and stimulating apoptosis pathways including those that induce the tumor suppressor p53. These observations suggest that luteolin could be an anticancer agent for various cancers. Furthermore, recent epidemiological studies have attributed a cancer prevention property to luteolin. In this review, we summarize the progress of recent research on luteolin, with a particular focus on its anticancer role and molecular mechanisms underlying this property of luteolin.
Keywords: luteolin, cancer, therapy, prevention, ROS, apoptosis, carcinogenesis, flavonoid
INTRODUCTION
Luteolin, 3′,4′,5,7-tetrahydroxyflavone, belongs to a group of naturally occurring compounds called flavonoids that are found widely in the plant kingdom. Flavonoids are polyphenols that play an important role in defending plant cells against microorganisms, insects, and UV irradiation [1]. Evidence from cell culture, animal, and human population studies have suggested that flavonoids are also beneficial to human and animal health. Because of their abundance in foods, e.g., vegetables, fruits, and medicinal herbs, flavonoids are common nutrients that are antioxidants, estrogenic regulators, and antimicrobial agents [2]. It has been noticed that flavonoids may be a cancer preventive [3,4]. Flavonoids may block several points in the progression of carcinogenesis, including cell transformation, invasion, metastasis, and angiogenesis, through inhibiting kinases, reducing transcription factors, regulating cell cycle, and inducing apoptotic cell death [2].
Belonging to the flavone group of flavonoids, luteolin has a C6-C3-C6 structure and possesses two benzene rings (A, B), a third, oxygen-containing (C) ring, and a 2−3 carbon double bond. Luteolin also possesses hydroxyl groups at carbons 5, 7, 3’, and 4’ positions (Fig. 1) [5]. The hydroxyl moieties and 2−3 double bond are important structure features in luteolin that are associated with its biochemical and biological activities [6]. As in other flavonoids, luteolin is often glycosylated in plants, and the glycoside is hydrolyzed to free luteolin during absorption [7]. Some portion of luteolin is converted to glucuronides when passing through the intestinal mucosa [8]. Luteolin is heat stable and losses due to cooking are relatively low [9].
Fig. 1.
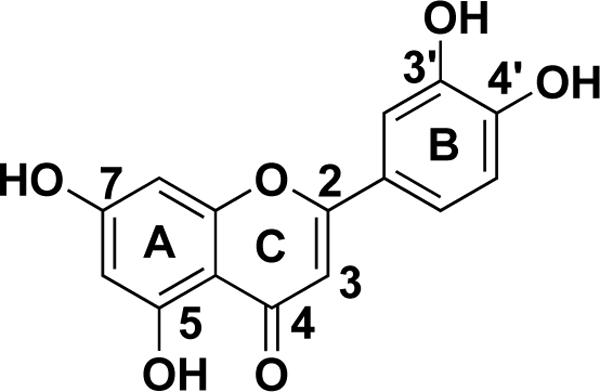
Structure of luteolin.
Vegetables and fruits such as celery, parsley, broccoli, onion leaves, carrots, peppers, cabbages, apple skins, and chrysanthemum flowers are luteolin rich [4,10-13]. Plants rich in luteolin have been used as Chinese traditional medicine for hypertension, inflammatory diseases, and cancer [1]. The pharmacological activities of luteolin could be functionally related to each other. For instance, the anti-inflammatory effect of luteolin also may be linked to its anticancer function. The anticancer property of luteolin is associated with inducing apoptosis, which involves redox regulation, DNA damage, and protein kinases in inhibiting proliferation of cancer cells and suppressing metastasis and angiogenesis. Furthermore, luteolin sensitizes a variety of cancer cells to therapeutically induced cytotoxicity through suppressing cell survival pathways and stimulating apoptosis pathways. Notably, luteolin is blood-brain barrier permeable, rendering it applicable to the therapy of central nerve system diseases, including brain cancer [14]. Furthermore, recent studies have attributed a cancer prevention potential to luteolin. In this review, we summarize recent progress in luteolin researches. Particularly, we focus on the roles and molecular mechanisms underlying luteolin's anticancer property.
REDOX MODULATION ACTIVITY
Antioxidant Activity
Most flavonoids, including luteolin, are regarded as antioxidants. Reactive oxygen species (ROS) refers to a diverse group of reactive, short-lived, oxygen-containing species, such as superoxide (O2•−), hydrogen peroxide (H2O2), hydroxyl radical (•OH), singlet oxygen (1O2), and lipid peroxyl radical (LOO•). ROS serve as second messengers for cellular signaling [15]. However, excessive production of ROS results in oxidative stress and damage to DNA, lipids, and protein that is involved in cancer as well as cardiovascular and neurodegenerative diseases. Luteolin was found to inhibit ROS-induced damage of lipids, DNA, and protein [16,17].
Multiple mechanisms may underlie luteolin's antioxidant effect. First, luteolin functions as a ROS scavenger through its own oxidation [18]. Luteolin possesses the structures essential to flavonoid's antioxidant activity: 3′, 4′ hydroxylation, the presence of a double bond between carbons 2 and 3, and a carbonyl group on carbon 4 [18]. The hydrogen atom from an aromatic hydroxyl group can be donated to free radicals. As an aromatic compound, luteolin can support unpaired electrons around the M-electron system [17,18]. Direct evidence showing luteolin as a ROS scavenger was obtained in cell-free systems [19]. Second, luteolin inhibits ROS-generating oxidases. For example, luteolin suppresses O2•− formation by inhibiting xanthine oxidase activity [20]. However, it is unclear in mammalian cells whether luteolin affects ROS generation in the mitochondria, the main ROS generation site, although it interferes with the mitochondrial electron transportation chain in parasite (leishmanial) cells [21]. Third, luteolin may exert its antioxidant effect by protecting or enhancing endogenous antioxidants such as glutathione-S-transferase (GST), glutathione reductase (GR), superoxide dismutase (SOD) and catalase (CAT) [5,22,23]. Fourth, luteolin may directly inhibit the enzymes that catalyze oxidation of the cellular components. For example, luteolin suppresses lipoxygenase, cyclooxygenase, and ascorbic acid-stimulated malonaldehyde formation in liver lipids [16]. Lastly, luteolin may chelate transition metal ions responsible for the generation of ROS and therefore inhibit lipooxygenase reaction, or suppress nontransition metal-dependent oxidation [5,17]. It should be noted that concordant antioxidant mechanisms of luteolin may occur in vivo. For example, inhibition of LPS-induced •OH production in macrophages by luteolin may be through scavenging O2•−, inhibiting xanthine oxidase activity, or a combination of both [24].
Pro-oxidant Activity
Although the ability of flavonoids to protect cells from oxidative stress has been well-documented, there is increasing evidence for their pro-oxidant property [25,26]. The pro-oxidant activity of flavonoids may be related to their ability to undergo autoxidation catalyzed by transition metals to produce superoxide anions [27]. In other reports, however, it was observed that the phenol rings of flavonoids are metabolized by peroxidase to form pro-oxidant phenoxyl radicals, which are sufficiently reactive to co-oxidize glutathione (GSH) or nicotinamide-adenine hydrogen (NADH) accompanied by extensive oxygen uptake and ROS formation [28]. The structure-activity relationship study on pro-oxidant cytotoxicity of flavonoids shows that flavonoids with a phenol ring are generally more bioactive than the catechol ring-containing ones [28]. Cytotoxicity induced by flavonoids is correlated with their electrochemical oxidation susceptibility and lipophilicity [29]. Luteolin has been shown to induce ROS in untransformed and cancer cells [30,31]. In lung cancer cells, luteolin induced accumulation of O2•− while it reduced H2O2 concentration. Although a suppression of manganese superoxide dismutase (MnSOD) activity, which converts O2•− to H2O2, was observed, it remains to be determined whether other mechanisms underlie luteolin-induced pro-oxidation [31].
How the anti- or pro-oxidant effects of luteolin ensue has not been well determined. It is believed that flavonoids could behave as antioxidants or pro-oxidants, depending on the concentration and the source of the free radicals [32]. Also, the context and microenvironment of the cell may be important determinants of the outcome of luteolin-induced effects on cellular redox status. For example, the antioxidant activity of luteolin is dependent on Cu, V, and Cd ions in the cells. Changes in the Fe ion concentrations dramatically impact the effect of luteolin's redox-regulating activities. With low Fe ion concentrations (< 50 μM), luteolin behaves as an antioxidant while high Fe concentrations (>100 μM) induce luteolin's pro-oxidative effect [33].
Understanding whether and how luteolin's redox regulation activity is involved in its cellular effects is key to evaluating its potential as an anticancer agent, a cardioprotectant, or an inhibitor of neurodegeneration [34]. Because oxidative stresses are closely related to mutagenesis and carcinogenesis, luteolin, as an antioxidant, may act as a chemopreventive agent to protect cells from various forms of oxidant stresses and thus prevent cancer development. On the other hand, the pro-oxidant properties of luteolin may be involved in its ability to induce tumor cell apoptosis, which is achieved partly through direct oxidative damage of DNA, RNA, and/or protein in the cells [30,35]. Interference of cellular signaling by ROS may also contribute to luteolin-induced apoptosis in cancer cells. We found that luteolin-induced oxidative stress causes suppression of the NF-κB pathway while it triggers JNK activation, which potentiates TNF-induced cytotoxicity in lung cancer cells [31]. It was suggested that the antioxidant activity of luteolin is associated with apoptosis in lung cancer cell line CH27. However, the induction of SOD-1 and −2 proteins by luteolin is moderate, and no causative relationship between the induction of SOD proteins and suppression of ROS or apoptosis was established [22]. Thus, the anti-and pro-oxidant roles of luteolin in cytotoxicity need to be further investigated.
ESTROGENIC AND ANTI-ESTROGENIC ACTIVITY
Estrogens are hormones involved in the proliferation and differentiation of their target cells. In response to estrogens, the estrogen receptor (ER) is activated to stimulate DNA synthesis and cell proliferation [36]. Flavonoids are naturally occurring phytoestrogens because they can bind to ERs and activate their signaling pathways [37-39]. Because luteolin possesses potent estrogenic activity at low concentrations, it could be a useful agent for hormone replacement therapy [40].
However, there are also reports showing anti-estrogenic effects of luteolin [38]. The mechanism behind this apparently contradictory effect may be attributed to its relative low estrogenic activity when it binds to ERs. Flavonoids bind and activate ERs when estrogen is deficient. However, due to their relative weak estrogenic activity, which is 103- to 105-fold lower than 17-β-estradialluteolin, they may function as anti-estrogenic agents through competition with estrogens for binding to ERs [40,41]. Another mechanism of luteolin's anti-estrogenic activity is that it inhibits aromatase whose function is to aromatize androgens and produce estrogens [42]. Additionally, luteolin reduces the ER expression level through inhibiting transcription of the ER gene or potentiating degradation of the ER protein [43,44]. Finally, some alternative signaling mechanisms unrelated to ERs could also be involved [37]. Although the interaction of estrogen agonists and antagonists with the ER is a primary event in estrogen action, mammalian cells contain a second binding site (type II site) for estrogen to control cell growth, which resides in endogenous proteins such as histone [45]. Luteolin was found to bind to nuclear type II sites irreversibly and to compete for estradiol binding to these sites [46].
The etiology of breast, prostate, ovarian, and endometrial cancers is associated with estrogen activity. Thus, consumption of luteolin in diet may reduce risk of these cancers through regulation of estrogen-induced cellular effects. Indeed, luteolin, as well as other flavonoids, is able to inhibit DNA synthesis and proliferation in mammary epithelial cells and breast cancer cells induced by estrogens, both in vitro and in vivo [38,47]. Suppressing estrogen-induced cancer cell proliferation may contribute to luteolin's therapeutic and preventive activities against estrogen-associated cancer.
ANTI-INFLAMMATION
Inflammation is one of the body's defense mechanisms that guard against infection and help heal injury. However, chronic inflammation may result in harmful diseases such as arthritis, chronic obstructive pulmonary disease, and cancer [48-50]. During inflammation macrophages are activated by various molecules, including cytokines from the host and toxins from the pathogens. Lipopolysaccharide (LPS), an outer membrane component of Gram-negative bacteria, is a common endotoxin and inflammation trigger. The activated macrophages vigorously produce inflammatory molecules such as tumor necrosis factor α (TNFα), interleukins (ILs), and free radicals (ROS and reactive nitrogen species, RNS), leading to recruitment of inflammatory cells, such as neutrophils and lymphocytes, to the infection site and clearance of the pathogens [48,50]. Persistent production of these molecules during chronic inflammation can result in diseases such as cancer. Luteolin exerts its anti-inflammatory effect through suppressing the production of these cytokines and their signal transduction pathways [51-53]. Experiments with animals show that luteolin suppresses LPS or bacteria-induced inflammation in vivo [54,55]. LPS-induced-high mortality was effectively alleviated by luteolin, which is associated with reduction of LPS-stimulated TNFα release in serum and intercellular adhesion molecule-1 (ICAM-1) expression in the liver [54]. Luteolin was found to suppress inflammation in lung tissue that was caused by Chlamydia pneumoniae [55].
In vitro experiments provided more direct evidence of luteolin's anti-inflammatory effect. Pretreating murine macrophages (RAW 264.7) with luteolin inhibited LPS-stimulated TNFα and IL-6 release, which was associated with blockage of LPS-induced activation of nuclear factor kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) family members ERK, p38, and JNK [51,52,56,57]. NF-κB and MAPK are two major pathways that are involved in macrophage activation and in responses of tissue epithelial and stromal cells to inflammation mediators such as TNFα and ILs [58]. Suppression of these pathways by luteolin underlies the main mechanism of its inhibitory effect on both acute and chronic inflammation. The suppression of inflammatory cytokine-induced signaling is at least partly on the level of the receptor, because accumulation of lipid rafts, which is the critical step for receptor signaling, was blocked by luteolin [53].
NF-κB can be activated by both the primary (LPS) and secondary (TNFα and IL-1) inflammatory stimulators. As a heterodimer typically consisting of RelA (p65)/p50, NF-κB is retained in the cytoplasm as an inactive form by association with IκB proteins. Through binding to Toll-like receptor 4 (TLR-4), LPS activates the IκB kinase (IKK), which in turn phosphorylates IκB to trigger its rapid degradation. This allows NF-κB to migrate into the nucleus and activate its targets, including a number of genes with anti-apoptotic properties and cytokines such as TNFα and IL-1 [59]. A positive feedback loop for NF-κB activation is established by these cytokines through binding to their cognate receptors. The NF-κB pathways activated by LPS and the inflammatory cytokines converge at IKK activation [59]. Luteolin can effectively block the NF-κB pathway and interfere with the functions of the primary (LPS) and secondary (TNFα and IL-1) inflammatory stimulators through inhibiting IKK activation and IκB degradation [51,56,60]. However, it remains to be determined whether luteolin directly inhibits IKK activity or blocks the upstream steps in the IKK activation pathway such as the formation of the receptor signaling complex. On the other hand, the mechanism by which luteolin suppresses MAPK, which is awaiting the dissection of the MAPKKK-MAPKK-MAPK cascade for each MAPK activation, is less well understood. It is unlikely that luteolin suppresses the binding TNFα and IL-1 to their respective receptors because luteolin selectively suppresses each MAPK in macrophages [57].
Based on the observations that some flavonoids with strong antioxidant activities are completely ineffective in suppressing LPS-stimulated TNFα production, it is assumed that the inhibitory action of flavonoids on proinflammatory cytokine production is not directly associated with their antioxidant properties [61]. However, because luteolin is able to scavenge ROS directly and to suppress the LPS-activated nitric oxide production in activated macrophages, the antioxidant activity of luteolin at least in part contributes to luteolin's anti-inflammatory effect [62,63]. Because inflammation and its involved signaling pathways are strongly associated with carcinogenesis [64,65], luteolin's anti-inflammation role may contribute to cancer prevention.
ANTI-CANCER ACTIVITIES
Carcinogenesis is a long-lasting and multi-stage process that results from clonal expansion of mutated cells. A typical carcinogenic process can be divided into three stages: initiation, promotion, and progression. During initiation, a potential carcinogen (pro-mutagen) is converted to a mutagen by enzymes such as cytochrome P450. The mutagen then reacts with DNA to induce irreversible genetic alteration including mutations, transversions, transitions, and/or small deletions in DNA. During the promotion stage, alterations in genome expression occur to favor cell growth and proliferation. During the progression stage tumorigenicity is established and becomes irreversible; it is characterized by karyotypic instability and malignant growth in an uncontrolled manner [66]. The transformed cells acquire a number of characteristic alterations, including the capacity to proliferate in an exogenous growth-promoting signal-independent manner, to invade surrounding tissues and metastasize to distant sites. In addition, cancer cells elicit an angiogenic response, evade mechanisms that limit cell proliferation (such as apoptosis and senescence), and elude immune surveillance [67]. These properties of cancer cells are reflected by alterations in the cellular signaling pathways that control cell proliferation, motility, and survival in normal cells [67]. Luteolin is able to interfere with almost all of the characteristics of cancer cells, mainly through the following mechanisms [68]. The main potential molecular targets for luteolin's anticancer activity are summarized in Table 1.
Table 1.
Potential molecular targets for luteolin's anticancer activity*
| Luteolin's effects | Processes involved in carcinogenesis | |||||
|---|---|---|---|---|---|---|
| Carcinogen Activation | Cell proliferation | Cell survival signaling | Apoptosis | Angiogenesis | Metastasis | |
| p27/kip1 | DR5 | |||||
| p21/waf1 | Caspases | |||||
| Activation | p53 | Fas | ||||
| Bax | ||||||
| p53 | ||||||
| |
|
|
|
JNK |
|
|
| CYP1A1 | NFκB | IGF-IR | DNA topoisomerases | VEGF | TNFα | |
| CYP1A2 | MAPKs | PDGF | XIAP | VEGFR | IL-6 | |
| CYP1B1 | PI3K/Akt | PI3k/Akt | Bcl-XL | MMP-9 | MMP-1 | |
| CDK2 | EGFR | Fatty acid synthase | NF-κB | FAK | ||
| Inhibition | ER | HIF-1α | Twist | |||
| PKC | Hyaluronidase | NF-κB | ||||
| NF-κB | PI3k/Akt | PI3k/Akt | ||||
| MAPKs | ERK | |||||
Some factors are involved in multiple processes. For details please refer the papers cited in the text.
Preventing Carcinogen Metabolic Activation
In earlier reports, luteolin was found to inhibit the metabolism of carcinogens that generates active mutagens in liver microsomes [69,70]. Recently, it was determined that luteolin potently inhibits human cytochrome P450 (CYP) 1 family enzymes such as CYP1A1, CYP1A2, and CYP1B1, thereby suppressing the mutagenic activation of carcinogens [71]. Suppressing these enzymes reduces the generation of active mutagens such as benzo[a]pyrene diol epoxide, a metabolite of the tabacco-specific carcinogen benzo[a]pyrene carcinogenesis [72].
Inhibiting Cancer Cell Proliferation
Unrestricted proliferation, which is often due to lose of cell cycle control, allows cancer cells to outgrow and form tumors. Like many other flavonoids, luteolin is able to inhibit the proliferation of cancer cells derived from nearly all types of cancers, mainly through regulating the cell cycle [38,73-75].
In eukaryotic cells, proliferation proceeds through DNA replication followed by division of the nucleus and separation of the cytoplasm to yield daughter cells. The sequential process, called cell cycle, consists of four distinct phases, G1, S, G2, and M [76]. Cell cycle progression is timely regulated by cyclin-dependent kinases (CDKs) and their cyclin subunits at the two checkpoints, G1/S and G2/M [76]. The G1/S checkpoint is regulated by CDK4-cyclin D, CDK6-cyclin D, and CDK2-cyclin E. When associated with cyclin A, CDK2 controls the S-phase, while the G2/M transition is regulated by CDK1 in combination with cyclins A and B [76]. CDK activity is negatively controlled by two groups of CDK inhibitors (CKI), INK4 and CIP/KIP families. The INK4 family members inhibit CDK4 and CDK6; while the CIP/KIP family, consisting of p21cip1/waf1, p27kip1, and p57kip2, inhibits a broad range of CDKs [76].
Inhibiting cell cycle progression
Flavonoids have been found to inhibit the proliferation of many cancer cells by arresting cell cycle progression either at the G1/S or G2/M checkpoint [77,78]. Luteolin is able to arrest the cell cycle during the G1 phase in human gastric and prostate cancer, and in melanoma cells [79-81]. The G1 cell cycle arrest induced by luteolin is associated with inhibition of the CDK2 activity in melanoma OCM-1 and colorectal cancer HT-29 cells. This arrest is achieved by up-regulation of the CDK inhibitors p27/kip1 and p21/waf1, or direct inhibition on the CDK2 activity [81,82]. Luteolin arrests mouse cancer cell tsFT210 at the G2/M checkpoint [83]. DNA damage-activated tumor suppressor protein p53 is involved in both the G1/S and G2/M transition regulation [84,85]. Luteolin can bind and suppress DNA topoisomerases I and II, enzymes essential for repairing damaged DNA, and intercalates directly with the substrate DNA to cause DNA double-strand breaks [85-87]. This action of luteolin induces cell cycle arrest though p53-mediated expression of p21/waf1[81].
Suppressing growth factor receptor-mediated cell proliferation signaling
Growth factors promote DNA synthesis and cell cycle progression through binding to their respective receptors. Common growth factors include epidermal growth factor (EGF), platelet-derived growth factor (PDGF), insulin-like growth factor (IGF), and fibroblast growth factor (FGF). TNFα can also stimulate cancer cell proliferation through NF-κB. The inhibitory effect of luteolin on cancer cell proliferation is partly achieved through blocking the proliferation signaling pathways induced by these factors.
EGF receptor (EGFR) is a typical receptor protein tyrosine kinase (PTK) that mediates cell growth and proliferation. When activated by its ligands, EGFR is phosphorylated to mediate activation of downstream signaling pathways, including MAPK and PI3K/Akt [88]. Luteolin was found to inhibit the proliferation of pancreatic and prostate cancer and human epidermoid carcinoma cells, which is closely associated with the inhibition of the PTK activity and autophosphorylation of EGFR, transphosphorylation of EGFR downstream effector protein enolase, and activation of MAPK/ERK [89].
Luteolin is able to inhibit IGF-1-induced activation of IGF-1R and Akt, and phosphorylation of the Akt targets p70S6K1, GSK-3β, and FKHR/FKHRL1. This inhibition is associated with suppressed expression of cyclin D1, and increased expression of p21/waf1 and proliferation in prostate cancer cells in vitro [90]. Luteolin also suppressed prostate tumor growth in vivo through suppressing IGF-1R/Akt signaling [90]. Similarly, luteolin inhibits PDGF-induced proliferation by inhibiting PDGF receptor phosphorylation in vascular smooth muscle cells [91]. As a consequence, luteolin significantly inhibits PDGF-induced ERK, PI3K/Akt and phospholipase C (PLC)-γ1 activation, and c-fos gene expression. These results suggest that the inhibitory effect of luteolin on the PDGF-induced proliferation may be mediated by blocking phosphorylation of the PDGF receptor [91]. As PDGF stimulates cancer cell proliferation [92], it remains to be determined whether luteolin can block PDGF-induced signaling to suppress cancer cell proliferation.
As discussed above, ER induces proliferation in several types of cancer cells [5]. Luteolin suppresses proliferation of prostate and breast cancer cells in both an androgen-dependent and -independent manner, suggesting that luteolin's anti-estrogen activity may at least partly contribute to its anti-proliferation effect[38,44,75]. Similar observations were also made in thyroid carcinoma cell lines bearing the ER [93]. Further experiments suppressing the expression and function of the ER are needed to confirm the role of ER-mediated signaling in luteolin-induced anti-proliferation in ER-responsive cancer cells.
In addition to affecting the receptors, luteolin may directly target the downstream pathways that are involved in cell proliferation. For example, protein kinase C, a family of serine-threonine protein kinases that regulates growth factor response and cell proliferation, differentiation and apoptosis [94,95], can be inhibited in a concentration-dependent manner by luteolin in both cell-free systems and in intact cells [96].
Taken together, the above reports suggest that luteolin suppresses cell proliferation signaling on distinct components of the growth factor receptor signaling pathways. In addition, carcinogens activate cell survival pathways such as NF-κB and MAPK during the course of carcinogenesis; these pathways could be additional targets for flavonoids, including luteolin, in anti-carcinogenesis [97,98].
Eliminating Transformed Cells by Induction of Apoptosis
Accumulating evidence shows that uncontrolled proliferation of mutated cells due to lack of programmed cell death or apoptosis is closely associated with tumorigenesis [99]. Cancer cells’ resistance to apoptosis is acquired through a variety of biochemical changes that also contribute to cells’ reduced responsiveness to anticancer therapy. Apoptosis is a tightly regulated cell death process that is critical for maintaining tissue homeostasis as well as preventing cancer development. Two apoptosis pathways, the death receptor pathway (extrinsic) and the mitochondrial (intrinsic) pathway, are established during evolution. The intrinsic pathway involves functional incapacitation of mitochondria by pro-apoptotic Bcl2 family members, including Bax, Bak, and Bik, that cause mitochondria potential loss and release cytochrome c to activate caspase 9, which in turn activates executor caspases (−3, −7) and destroys cellular proteins [100]. The extrinsic pathway is initiated by the binding of TNF family cytokines (TNFα, Fas and TNF-related apoptosis-inducing ligand, TRAIL) to their cognate death receptors, to activate caspase 8, which in turn activates downstream executor caspases [101].
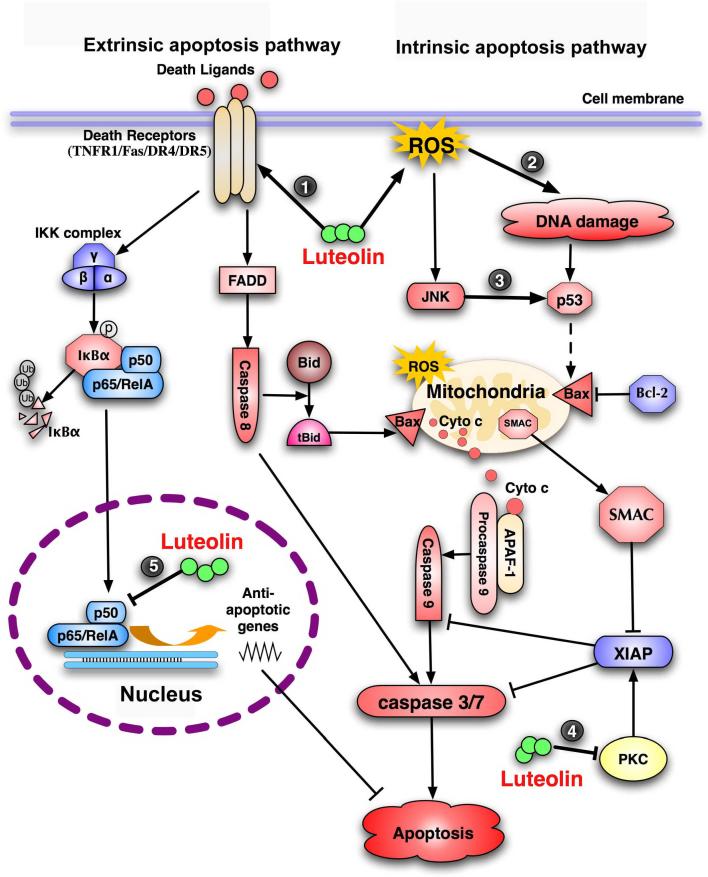
Luteolin kills cancer cells by inducing apoptotic cell death in many types of cancer cells, including epidermoid carcinoma, leukemia, pancreatic tumor, and hepatoma [89,102-104]. Although the mechanisms underlying luteolin-induced apoptosis are complex, they can be generalized as breaking the cell survival and death balance by either enhancing apoptosis or decreasing the survival signaling in cancer cells, which is summarized in Fig. 2.
Fig. 2. Apoptosis pathways and the points targeted by luteolin.
The extrinsic apoptosis pathway is mediated by death receptors, resulting in sequential activation of initiator caspase 8 and executor caspases 3 and 7. The intrinsic apoptosis pathway is initiated by lose of mitochondrial potential, which leads to release of cytochrome c. Cytochrome c binds to APAF-1 and procaspase 9, resulting in activation of initiator caspase 9 and downstream executor caspases. Cleavage of Bid by caspase 8 establishes a crosstalk between the extrinsic and intrinsic apoptosis pathways. Luteolin triggers apoptotic cell death through potentiation of both apoptosis pathways and suppression of cell survival pathways. The points targeted by luteolin in the apoptosis pathways are highlighted with numbers in filled circles.
Activating the apoptosis pathway
Luteolin is potent to activate both the extrinsic and intrinsic apoptosis pathways. Direct increase in expression of the death receptor 5 (DR5), the functional receptor for TRAIL, has been demonstrated in cervical and prostate cancer cells, which is accompanied by activation of caspase-8, −10, −9 and −3, and cleavage of Bcl-2-interacting domain (BID). The increase of DR5 expression is likely through activated transcription of the dr5 gene [105]. Interestingly, DR5 was not induced and no cytotoxicity was observed in luteolin-treated normal human peripheral blood mononuclear cells [105]. Luteolin was also found to enhance expression of Fas to induce apoptosis in human hepatoma cells through triggering the degradation of STAT3, a known negative regulator of fas transcription [106].
Luteolin also activates the intrinsic apoptosis pathway through inducing DNA damage and activating p53 [107,108]. This is achieved by inhibiting DNA topoisomerases [85,87]. Additionally, luteolin triggers sustained JNK activation that can promote the apoptosis pathway, presumably through modulation of BAD or p53 [31,108-110]. The JNK-mediated p53 activation results in transcriptional expression of Bax that facilitates apoptosis [108,110]. JNK activation causes the mitochondria translocation of Bax and Bak to initiate the intrinsic apoptosis pathway [103,104].
Suppressing cell survival signaling
On the other hand, luteolin suppresses cell survival pathways to decrease the threshold of apoptosis. As discussed above, luteolin inhibits survival pathways, such as PI3K/Akt, NF-κB, and MAPKs in cancer cells, which may mimic deprivation of growth factors that blocks the growth factor-triggered signaling pathways. Suppressing the death receptors-mediated cell survival pathway NF-κB augments apoptosis induced by their cognate ligands TNFα or TRAIL. TNFα plays a critical role in inflammation-associated carcinogenesis through NF-κB-mediated cell survival and proliferation [97,111]. Blockage of NF-κB by luteolin shifts the cell survival and death balance to the side of death[31, 109], converting TNFα from a tumor promoter to a tumor suppressor. TRAIL can promote proliferation and metastasis in TRAIL-resistant cancer cells via a mechanism involving NF-κB [112]; thus, suppressing NF-κB with luteolin can sensitize cancer cells to TRAIL-induced apoptosis and prevent the detrimental effect of TRAIL.
Luteolin also suppresses cell survival by inhibiting apoptosis inhibitors and anti-apoptotic Bcl2 family members. It was found that luteolin inhibits PKC activity, which results in a decrease in the protein level of XIAP by ubiquitination and proteasomal degradation of this anti-apoptotic protein. Reducing XIAP sensitizes cancer cells to TRAIL-induced apoptosis [113]. In addition to increasing Bax protein, luteolin decreases the Bcl-XL level in hepatocellular carcinoma cells, which elevates the Bax/Bcl-XL ratio and lowers the threshold for apoptosis [114]. Additionally, luteolin-induced apoptosis in prostate and breast cancer cells is associated with its ability to inhibit fatty acid synthase (FAS), a key lipogenic enzyme overexpressed in many human cancers [115]. Although presently the mechanism is unclear, inhibiting FAS causes apoptosis in cancer cells [116].
Anti-angiogenesis
Due to lack of sufficient nutrition and oxygen, avascular tumors cannot grow beyond a diameter of 1−2 mm [117]. Angiogenesis, a process to generate new blood vessels, is critical for solid tumor growth and metastasis. Growing in a hypoxic microenvironment, tumor cells secret angiogenic factors such as vascular endothelial growth factor (VEGF) and matrix metalloproteases (MMP) to trigger angiogenesis [118]. Luteolin was found to be a potent angiogenesis inhibitor [119]. In a murine xenograft tumor model, luteolin inhibited tumor growth and angiogenesis in xenografted tumors [120].
Suppression of VEGF secretion and signaling induced by VEGF appears to be the main mechanism of luteolin-induced anti-angiogenesis. Transcription of the VEGF gene is enhanced by hypoxia-inducible factor-1α (HIF-1α) [121]. Luteolin may suppress VEGF expression by inhibiting HIF-1α through p53-mediated proteasomal degradation of this transcription factor [122]. Additionally, luteolin can suppress VEGF-induced signaling in endothelial cells [73,120]. Luteolin effectively blocked activation of the VEGF receptor and its downstream PI3K/Akt and PI3K/p70S6 kinase pathways, which may directly contribute to luteolin-induced anti-angiogenesis, resulting in suppression of proliferation and survival of human umbilical vein endothelial cells [120].
Luteolin may also suppress angiogenesis by stabilizing hyaluronic acid, a neovascularization barrier. Hyaluronic acid is one of the most abundant constituents of the extracellular matrix that block neovacuole formation and extension [123]. Hyaluronidase catalyzes hyaluronic acid to break the barrier and to promote angiogenesis through the processed product. Oligosaccharides generated from hyaluronic acid bind to the CD44 receptor on the membranes of endothelial cells to trigger their proliferation, migration, and eventually angiogenesis. Luteolin has been found to be a potent inhibitor of hyaluronidase and maintain the neovascularization barrier [124].
Furthermore, tumor angiogenesis is dependent on the activity of MMPs, especially that of MMP-9, which renders MMP inhibitors a potential choice for blocking tumor angiogenesis [125]. Thus, luteolin's additional anti-angiogenesis mechanism may be via its suppression of MMPs. Indeed, luteolin is a potent MMP inhibitor that suppresses MMP expression through suppressing NF-κB or directly inhibiting MMP activity [126].
Anti-metastasis
In addition to rapid and continuous division and proliferation, another important and unique feature of cancer cells is their ability to invade surrounding tissues and to migrate from their primary site to distal sites. This process, namely metastasis, contributes to over 90% of human cancer mortality [127]. The metastasis cascade is thought to consist of multiple steps: local invasion; intravasation into the systemic circulation; survival during transport, extravasation, and establishment of micrometastases in distant organs; and colonization of macroscopic metastases [128].
Although direct evidence showing luteolin suppresses cancer metastasis is not seen in literature, available results strongly suggest that luteolin has this function. First, luteolin suppresses production and secretion of cytokines such as TNFα and IL-6 that can stimulate cancer cell migration and metastasis [51,56,129,130]. TNFα stimulates expression of molecules involved in cancer cell migration and metastasis such as intercellular adhesion molecule-1, which can be blocked by luteolin [52]. IL-6 is known to induce MMP-1 expression. Luteolin potently inhibits the production of IL-6 and IL-6-induced expression of MMP-1 [131]. Second, luteolin blocks critical signal transduction pathways for migration and metastasis in cancer cells. For example, activation of the EGFR is involved cell migration. By blocking the EGFR-signaling pathway, luteolin reduces cell invasion and metastasis [102,132]. Luteolin blocks NF-κB[31,109], which is critical for the expression of Twist and MMP expression. Twist is a transcription factor that is important for epithelial-mesenchymal transition to facilitate metastasis [128]. MMPs are involved in several stages of metastasis, including the escape of individual tumor cells from the primary tumor, their intravasation, extravasation, and establishment of tumor foci at secondary sites [125]. Focal adhesion kinase (FAK) activity in human carcinoma cells is associated with increased invasive potential; luteolin's inhibitory effect on FAK phosphorylation may contribute to suppressing FAK's cell invasion ability [133]. Finally, luteolin directly inhibits MMP or hyaluronidase enzyme activity to maintain the neovascularization barrier [124,126], which may also contribute to suppressing cancer cell metastasis. In vitro studies have shown that luteolin potently inhibits migration and invasion of cancer cells through blocking the MAPK/ERKs and PI3K-Akt pathways [134,135]. Experiments with cancer metastasis animal models are needed to verify luteolin's anti-metastasis activity.
LUTEOLIN AS AN ANTICANCER OR CHEMOPREVENTION AGENT
As discussed above, luteolin induces apoptotic cell death in a variety of cancers [103,104,136,137], inhibits cancer cell proliferation [82,90,138], and suppresses tumor angiogenesis [120]. Thus, luteolin is expected to be a putative anticancer therapeutic. Supporting the in vitro results, in vivo experiments in nude mice with xenografted tumors showed that luteolin suppressed growth of tumors formed from human skin carcinoma, hepatoma, and human ovarian cancer cells [106,120,137] or mouse Lewis lung carcinoma [139] in a dosage-dependent manner. Interestingly, in a 7,12-dimethylbenz(a)anthracene (DMBA)-induced mammary carcinogenesis in Wistar rat model, luteolin inhibited the incidence rate of tumors and decreased tumor volume significantly without changing the total body weight of the animals. Long-term treatment did not show any apparent toxicity in rats (30mg/kg, p.o. for 20 days)[140]. Consistently, luteolin induces marginal cytotoxicity in normal cells[105,141]. These results imply that luteolin is relatively safe when used as an anticancer agent.
Combination therapy with distinct anticancer drugs can improve the therapeutic value of the combined agents by allowing the use of lower, subtoxic doses to achieve more effective cancer cell killing. Luteolin has been tested with other anticancer drugs for its anticancer cell properties, and has sensitized different drug-induced cytotoxicity in a variety of cancer cells. The drugs tested include cisplatin [108], TRAIL [105,113], TNFα [31,109], and the mTOR inhibitor rapamycin [137]. Although the mechanism of this sensitization vary in different cancer cells or with different drugs, it is generally thought to be through suppressing cell survival signals in cancer cells or activating apoptosis pathways. Cancer cells often have constitutively activated cell survival pathways such as NF-κB and Akt. Cancer therapeutics also activate these pathways, dampening their cancer cell-killing activities [142,143]. Thus, luteolin's suppression of the constitutive or drug-induced cell survival pathways contributes to the sensitized anticancer activity. Additionally, luteolin is also capable of promoting apoptotic pathways. For instance, luteolin-induced upregulation of the TRAIL receptor DR5 contributes to sensitizing not only TRAIL-induced, but also other chemotherapeutic-induced cytotoxicity [144].
Thus, data from previous studies suggest luteolin is a promising agent for anticancer therapy. More preclinical work is needed for establishing the efficacy and safety of luteolin alone or in combination with other therapeutics before conducting clinical trials. Because extracts from fruits such as black raspberries, apples and grapes exerts anticancer activities that are associated with suppressing of cell survival and potentiation of apoptosis pathways, it is interesting to determine if luteolin or other flavonoids contributes to the anticancer activity of these fruits [145-149].
Based on the observations that luteolin is able to interfere with almost all the aspects of carcinogenesis, and it is relatively safe for animals and humans, it is assumed to be a potential chemopreventive agent against cancer through blocking cell transformation, suppressing tumor growth, and killing tumor cells. Using luteolin to suppress chronic inflammation can potentially prevent inflammation-associated carcinogenesis.
In a 20-methycholanyrene-induced fibrosarcoma model using Swiss albino mice, luteolin administered in diet significantly suppressed tumor incidences, which are associated with reduction in lipid peroxides and cytochrome P450, increased activity of GST, and suppressed DNA synthesis [150]. In a murine two-stage skin carcinogenesis model, topical application of luteolin prior to 12-tetradecanoylphorbol 13-acetate (TPA) treatment in DMBA-initiated mouse skin resulted in a significant reduction in tumor incidence and multiplicity, which is associated with inhibiting the inflammatory response and scavenging reactive oxygen radicals [151,152]. In a colon carcinogenesis model induced by 1, 2-dimethyl hydrazine (DMH), luteolin (0.1, 0.2, or 0.3 mg/kg body weight/daily p.o.) significantly reduced colon cancer incidence when it was administered at either the initiation or post-initiation stages [153]. The results demonstrate that luteolin exerts chemopreventive and anticarcinogenic effects, in association with its antiperoxidative and antioxidant effects, against colon cancer [153].
Epidemiological studies suggest that dietary intake of flavonoids is inversely associated with risk of lung, prostate, stomach, and breast cancer in humans [4,154,155]. However, there are few epidemiological reports designed to study the role of luteolin in cancer prevention. A recent population study on the association between intake of dietary flavonoids and incidence of epithelial ovarian cancer among 66,940 women showed a significant (34%) decrease in cancer incidence for the highest versus lowest luteolin intake (RR = 0.66, 95% CI = 0.49−0.91; p-trend = 0.01) [11]. The data suggest that dietary intake of luteolin may reduce ovarian cancer risk, although additional prospective studies are needed [11]. Dietary intake of flavonols and flavones was found to be inversely associated with the risk of lung cancer [3,156]. However, because of many confounding factors, luetolin's preventive potential for lung cancer still remains unclear [156,157]. It should be noted that mixed bioactive compounds, such as different flavonoids that exist in foods, may impact each others’ biological effects. Lifestyle differences of the subjects in a study may interfere with the results. Furthermore, variations in epidemiological studies, including differences in questionnaire design, databases for flavonoid content in foods, and methods for data analysis, may substantially vary the outcomes of different studies. Thus, caution should be exercised when interpreting epidemiological study results [4]. Nevertheless, further prospective animal and human studies are warranted to verify luteolin's cancer prevention properties.
CONCLUSIONS AND PERSPECTIVES
Documented results suggest that luteolin has a variety of beneficial properties, including those as an anti-inflammatory and anticancer agent. The mechanisms underlying these properties have not been fully understood but are attributed partly to luteolin's redox- and estrogen-regulating properties. It is interesting and important to determine the mechanism for luteolin's selective cytotoxicity in cancerous but not normal cells. It is apparent that distinct mechanisms for modulating cellular signaling pathways exist in normal cells and in malignant cancer cells. For example, luteolin suppresses JNK in macrophages while it activates this kinase in cancer cells [31,57,109]. Also, luteolin suppresses NF-κB through inhibiting IKK activation during inflammation in epithelial cells and macrophages [51,56,158]. However, in cancer cells suppression of NF-κB by luteolin is apparently a nuclear event [31,109]. It remains to be determined whether the distinct mechanisms are due to differences in cell contexts. Because luteolin inhibits NF-κB in lung cancer cells and is associated with its pro-oxidant effect [31], it will be interesting to determine if the distinct mechanisms in NF-κB suppression are dependent on the redox status of the cell or the redox-regulating function of luteolin. Understanding the mechanisms will undoubtedly facilitate the use of luteolin in cancer prevention and therapy. Finally, although it is relatively safe, luteolin (2% in chow diet) was found to worsen chemically induced colitis in mice [159]. Further studies are needed to address the safety issues of luteolin with doses effective for cancer prevention and therapy in humans.
ACKNOWLEDGEMENTS
The authors thank Dr. Guodong Lu for helping figure preparation and Vicki Fisher for editing the manuscript. This study is partly supported by grants from the National Cancer Institute (R03CA125796 to Y.L.), National Medical Research Council (NMRC) and Biomedical Research Council (BMRC), Singapore (to H-M.S) and National Natural Science Foundation of China (30772539, to X.W.).
ABBREVIATIONS
- •OH
hydroxyl radical (•OH)
- 1O2
singlet oxygen
- 1SO2
singlet oxygen
- BID
Bcl-2-interacting domain
- CAT
catalase
- CDK
cyclin-dependent kinase
- CKI
CDK inhibitors
- CYP
cytochrome P450
- DMBA
7,12-dimethylbenz[a]anthracene
- DMH
1, 2-dimethyl hydrazine
- DR5
death receptor 5
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- ER
estrogen receptor
- FAK
focal adhesion kinase
- FGF
fibroblast growth factor
- GR
glutathione reductase
- GSH
glutathione
- GST
glutathione-S-transferase
- H2O2
hydrogen peroxide
- HIF-1α
hypoxia-inducible factor-1α
- IGF
insulin-like growth factor
- ICAM-1
intercellular adhesion molecule-1
- IKK
IκB kinase
- IL
interleukin
- LPS
lipopolysaccharide
- LOO•
lipid peroxyl radical
- MAPK
mitogen-activated protein kinase
- MMP
matrix metalloproteases
- MnSOD
manganese superoxide dismutase
- NADH
nicotinamide-adenine hydrogen
- NF-κB
nuclear factor kappa B
- O2•−
superoxide
- PDGF
platelet-derived growth factor
- PI3K
phosphatidylinositol 3′-kinase
- PKC
protein kinase C
- PTK
protein tyrosine kinase
- ROS
Reactive oxygen species
- RNS
reactive nitrogen species
- SOD
superoxide dismutase
- TLR-4
Toll-like receptor-4
- TNFα
tumor necrosis factor alpha
- TRAIL
TNF-related apoptosis-inducing ligand
- TPA
12-tetradecanoylphorbol 13-acetate
- VEGF
vascular endothelial growth factor
- XIAP
X-linked inhibitor of apoptosis protein
REFERENCES
- 1.Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry. 2000;55:481–504. doi: 10.1016/s0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- 2.Birt DF, Hendrich S, Wang W. Dietary agents in cancer prevention: flavonoids and isoflavonoids. Pharmacol. Ther. 2001;90:157–177. doi: 10.1016/s0163-7258(01)00137-1. [DOI] [PubMed] [Google Scholar]
- 3.Knekt P, Jarvinen R, Seppanen R, Hellovaara M, Teppo L, Pukkala E, Aromaa A. Dietary flavonoids and the risk of lung cancer and other malignant neoplasms. Am. J. Epidemiol. 1997;146:223–230. doi: 10.1093/oxfordjournals.aje.a009257. [DOI] [PubMed] [Google Scholar]
- 4.Neuhouser ML. Dietary flavonoids and cancer risk: evidence from human population studies. Nutr. Cancer. 2004;50:1–7. doi: 10.1207/s15327914nc5001_1. [DOI] [PubMed] [Google Scholar]
- 5.Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- 6.Chan TS, Galati G, Pannala AS, Rice-Evans C, O'Brien PJ. Simultaneous detection of the antioxidant and pro-oxidant activity of dietary polyphenolics in a peroxidase system. Free Radic. Res. 2003;37:787–794. doi: 10.1080/1071576031000094899. [DOI] [PubMed] [Google Scholar]
- 7.Hempel J, Pforte H, Raab B, Engst W, Bohm H, Jacobasch G. Flavonols and flavones of parsley cell suspension culture change the antioxidative capacity of plasma in rats. Nahrung. 1999;43:201–204. doi: 10.1002/(SICI)1521-3803(19990601)43:3<201::AID-FOOD201>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 8.Shimoi K, Okada H, Furugori M, Goda T, Takase S, Suzuki M, Hara Y, Yamamoto H, Kinae N. Intestinal absorption of luteolin and luteolin 7-O-beta-glucoside in rats and humans. FEBS Lett. 1998;438:220–224. doi: 10.1016/s0014-5793(98)01304-0. [DOI] [PubMed] [Google Scholar]
- 9.Le Marchand L. Cancer preventive effects of flavonoids--a review. Biomed. Pharmacother. 2002;56:296–301. doi: 10.1016/s0753-3322(02)00186-5. [DOI] [PubMed] [Google Scholar]
- 10.Miean KH, Mohamed S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J. Agric. Food Chem. 49:3106–3112. doi: 10.1021/jf000892m. 200. [DOI] [PubMed] [Google Scholar]
- 11.Gates MA, Tworoger SS, Hecht JL, De Vivo I, Rosner B, Hankinson SE. A prospective study of dietary flavonoid intake and incidence of epithelial ovarian cancer. Int. J. Cancer. 2007;121:2225–2232. doi: 10.1002/ijc.22790. [DOI] [PubMed] [Google Scholar]
- 12.Sun T, Xu Z, Wu CT, Janes M, Prinyawiwatkul W, No HK. Antioxidant activities of different colored sweet bell peppers (Capsicum annuum L.). J. Food Sci. 2007;72:S98–102. doi: 10.1111/j.1750-3841.2006.00245.x. [DOI] [PubMed] [Google Scholar]
- 13.Mencherini T, Picerno P, Scesa C, Aquino R. Triterpene, Antioxidant, and Antimicrobial Compounds from Melissa officinalis. J. Nat. Prod. 2007;70:1889–1894. doi: 10.1021/np070351s. [DOI] [PubMed] [Google Scholar]
- 14.Wruck CJ, Claussen M, Fuhrmann G, Romer L, Schulz A, Pufe T, Waetzig V, Peipp M, Herdegen T, Gotz ME. Luteolin protects rat PC12 and C6 cells against MPP+ induced toxicity via an ERK dependent Keap1-Nrf2-ARE pathway. J. Neural Transm. Suppl. 2007;72:57–67. doi: 10.1007/978-3-211-73574-9_9. [DOI] [PubMed] [Google Scholar]
- 15.Nimnual AS, Taylor LJ, Bar-Sagi D. Redox-dependent downregulation of Rho by Rac. Nat. Cell. Biol. 2003;5:236–241. doi: 10.1038/ncb938. [DOI] [PubMed] [Google Scholar]
- 16.Robak J, Shridi F, Wolbis M, Krolikowska M. Screening of the influence of flavonoids on lipoxygenase and cyclooxygenase activity, as well as on nonenzymic lipid oxidation. Pol. J. Pharmacol. Pharm. 1988;40:451–458. [PubMed] [Google Scholar]
- 17.Brown JE, Rice-Evans CA. Luteolin-rich artichoke extract protects low density lipoprotein from oxidation in vitro. Free Radic. Res. 1998;29:247–255. doi: 10.1080/10715769800300281. [DOI] [PubMed] [Google Scholar]
- 18.Lien EJ, Ren S, Bui HH, Wang R. Quantitative structure-activity relationship analysis of phenolic antioxidants. Free Radic. Biol. Med. 1999;26:285–294. doi: 10.1016/s0891-5849(98)00190-7. [DOI] [PubMed] [Google Scholar]
- 19.Shimoi K, Masuda S, Furugori M, Esaki S, Kinae N. Radioprotective effect of antioxidative flavonoids in gamma-ray irradiated mice. Carcinogenesis. 1994;15:2669–2672. doi: 10.1093/carcin/15.11.2669. [DOI] [PubMed] [Google Scholar]
- 20.Nagao A, Seki M, Kobayashi H. Inhibition of xanthine oxidase by flavonoids. Biosci. Biotechnol. Biochem. 1999;63:1787–1790. doi: 10.1271/bbb.63.1787. [DOI] [PubMed] [Google Scholar]
- 21.Sen N, Das BB, Ganguly A, Banerjee B, Sen T, Majumder HK. Leishmania donovani: intracellular ATP level regulates apoptosis-like death in luteolin induced dyskinetoplastid cells. Exp. Parasitol. 2006;114:204–214. doi: 10.1016/j.exppara.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 22.Leung HW, Kuo CL, Yang WH, Lin CH, Lee HZ. Antioxidant enzymes activity involvement in luteolin-induced human lung squamous carcinoma CH27 cell apoptosis. Eur. J. Pharmacol. 2006;534:12–18. doi: 10.1016/j.ejphar.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 23.Manju V, Nalini N. Chemopreventive potential of luteolin during colon carcinogenesis induced by 1,2-dimethylhydrazine. Ital. J. Biochem. 2005;54:268–275. [PubMed] [Google Scholar]
- 24.Harris GK, Qian Y, Leonard SS, Sbarra DC, Shi X. Luteolin and chrysin differentially inhibit cyclooxygenase-2 expression and scavenge reactive oxygen species but similarly inhibit prostaglandin-E2 formation in RAW 264.7 cells. J. Nutr. 2006;136:1517–1521. doi: 10.1093/jn/136.6.1517. [DOI] [PubMed] [Google Scholar]
- 25.Lapidot T, Walker MD, Kanner J. Antioxidant and Prooxidant Effects of Phenolics on Pancreatic Cells in Vitro. J. Agric. Food Chem. 2002;50:7220–7225. doi: 10.1021/jf020615a. [DOI] [PubMed] [Google Scholar]
- 26.Galati G, O'Brien PJ. Potential toxicity of flavonoids and other dietary phenolics: significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 2004;37:287–303. doi: 10.1016/j.freeradbiomed.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 27.Hanasaki Y, Ogawa S, Fukui S. The correlation between active oxygens scavenging and antioxidative effects of flavonoids Free Radic. Biol. Med. 1994;16:845–850. doi: 10.1016/0891-5849(94)90202-x. [DOI] [PubMed] [Google Scholar]
- 28.Galati G, Sabzevari O, Wilson JX, O'Brien PJ. Prooxidant activity and cellular effects of the phenoxyl radicals of dietary flavonoids and other polyphenolics. Toxicology. 2002;177:91–104. doi: 10.1016/s0300-483x(02)00198-1. [DOI] [PubMed] [Google Scholar]
- 29.Sergediene E, Jonsson K, Szymusiak H, Tyrakowska B, Rietjens IMCM, Cenas N. Prooxidant toxicity of polyphenolic antioxidants to HL-60 cells: description of quantitative structure-activity relationships. FEBS Lett. 1999;462:392–396. doi: 10.1016/s0014-5793(99)01561-6. [DOI] [PubMed] [Google Scholar]
- 30.Matsuo M, Sasaki N, Saga K, Kaneko T. Cytotoxicity of flavonoids toward cultured normal human cells. Biol. Pharm. Bull. 2005;28:253–259. doi: 10.1248/bpb.28.253. [DOI] [PubMed] [Google Scholar]
- 31.Ju W, Wang X, Shi H, Chen W, Belinsky SA, Lin Y. A critical role of luteolin-induced reactive oxygen species in blockage of tumor necrosis factor-activated nuclear factor-kappaB pathway and sensitization of apoptosis in lung cancer cells. Mol. Pharmacol. 2007;71:1381–1388. doi: 10.1124/mol.106.032185. [DOI] [PubMed] [Google Scholar]
- 32.Cao G, Sofic E, Prior RL. Antioxidant and Prooxidant Behavior of Flavonoids: Structure-Activity Relationships. Free Radic. Biol. Med. 1997;22:749–760. doi: 10.1016/s0891-5849(96)00351-6. [DOI] [PubMed] [Google Scholar]
- 33.Sugihara N, Arakawa T, Ohnishi M, Furuno K. Anti- and pro-oxidative effects of flavonoids on metal-induced lipid hydroperoxide-dependent lipid peroxidation in cultured hepatocytes loaded with alpha-linolenic acid. Free Radic. Biol. Med. 1999;27:1313–1323. doi: 10.1016/s0891-5849(99)00167-7. [DOI] [PubMed] [Google Scholar]
- 34.Williams RJ, Spencer JP, Rice-Evans C. Flavonoids: antioxidants or signalling molecules? Free Radic. Biol. Med. 2004;36:838–849. doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Shen S-C, Ko CH, Tseng S-W, Tsai S-H, Chen Y-C. Structurally related antitumor effects of flavanones in vitro and in vivo: involvement of caspase 3 activation, p21 gene expression, and reactive oxygen species production. Toxicol. Appl. Pharmacol. 2004;197:84–95. doi: 10.1016/j.taap.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Colditz GA. Estrogen, Estrogen Plus Progestin Therapy, and Risk of Breast Cancer. Clin. Cancer Res. 2005;11:909–917. [PubMed] [Google Scholar]
- 37.Collins-Burow BM, Burow ME, Duong BN, McLachlan JA. Estrogenic and antiestrogenic activities of flavonoid phytochemicals through estrogen receptor binding-dependent and -independent mechanisms. Nutr. Cancer. 2000;38:229–244. doi: 10.1207/S15327914NC382_13. [DOI] [PubMed] [Google Scholar]
- 38.Han DH, Denison MS, Tachibana H, Yamada K. Relationship between estrogen receptor-binding and estrogenic activities of environmental estrogens and suppression by flavonoids. Biosci. Biotechnol. Biochem. 2002;66:1479–1487. doi: 10.1271/bbb.66.1479. [DOI] [PubMed] [Google Scholar]
- 39.Ise R, Han D, Takahashi Y, Terasaka S, Inoue A, Tanji M, Kiyama R. Expression profiling of the estrogen responsive genes in response to phytoestrogens using a customized DNA microarray. FEBS Lett. 2005;579:1732–1740. doi: 10.1016/j.febslet.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 40.Zand RS, Jenkins DJ, Diamandis EP. Steroid hormone activity of flavonoids and related compounds. Breast Cancer Res. Treat. 2000;62:35–49. doi: 10.1023/a:1006422302173. [DOI] [PubMed] [Google Scholar]
- 41.Murkies AL, Wilcox G, Davis SR. Phytoestrogens. J. Clin. Endocrinol. Metab. 1998;83:297–303. doi: 10.1210/jcem.83.2.4577. [DOI] [PubMed] [Google Scholar]
- 42.Wang C, Makela T, Hase T, Adlercreutz H, Kurzer MS. Lignans and flavonoids inhibit aromatase enzyme in human preadipocytes. J. Steroid. Biochem. Mol. Biol. 1994;50:205–212. doi: 10.1016/0960-0760(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 43.Wang TT, Milner MJ, Milner JA, Kim YS. Estrogen receptor alpha as a target for indole-3-carbinol. J. Nutr. Biochem. 2006;17:659–664. doi: 10.1016/j.jnutbio.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 44.Chiu FL, Lin JK. Downregulation of androgen receptor expression by luteolin causes inhibition of cell proliferation and induction of apoptosis in human prostate cancer cells and xenografts. Prostate. 2008;68:61–71. doi: 10.1002/pros.20690. [DOI] [PubMed] [Google Scholar]
- 45.Shoulars K, Rodriguez MA, Crowley J, Turk J, Thompson T, Markaverich BM. Reconstitution of the type II [3H]estradiol binding site with recombinant histone H4. J. Steroid. Biochem. Mol. Biol. 2006;99:1–8. doi: 10.1016/j.jsbmb.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 46.Markaverich BM, Roberts RR, Alejandro MA, Johnson GA, Middleditch BS, Clark JH. Bioflavonoid interaction with rat uterine type II binding sites and cell growth inhibition. J. Steroid. Biochem. 1988;30:71–78. doi: 10.1016/0022-4731(88)90078-7. [DOI] [PubMed] [Google Scholar]
- 47.Holland MB, Roy D. Estrone-induced cell proliferation and differentiation in the mammary gland of the female Noble rat. Carcinogenesis. 1995;16:1955–1961. doi: 10.1093/carcin/16.8.1955. [DOI] [PubMed] [Google Scholar]
- 48.Brody JS, Spira A. State of the art. Chronic obstructive pulmonary disease, inflammation, and lung cancer. Proc. Am. Thorac. Soc. 2006;3:535–537. doi: 10.1513/pats.200603-089MS. [DOI] [PubMed] [Google Scholar]
- 49.Perwez Hussain S, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int. J. Cancer. 2007;121:2373–2380. doi: 10.1002/ijc.23173. [DOI] [PubMed] [Google Scholar]
- 50.Karin M, Lawrence T, Nizet V. Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell. 2006;124:823–835. doi: 10.1016/j.cell.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 51.Xagorari A, Papapetropoulos A, Mauromatis A, Economou M, Fotsis T, Roussos C. Luteolin inhibits an endotoxin-stimulated phosphorylation cascade and proinflammatory cytokine production in macrophages. J. Pharmacol. Exp. Ther. 2001;296:181–187. [PubMed] [Google Scholar]
- 52.Chen C-C, Chow M-P, Huang W-C, Lin Y-C, Chang Y-J. Flavonoids Inhibit Tumor Necrosis Factor-{alpha}-Induced Up-Regulation of Intercellular Adhesion Molecule-1 (ICAM-1) in Respiratory Epithelial Cells through Activator Protein-1 and Nuclear Factor-{kappa}B: Structure-Activity Relationships. Mol. Pharmacol. 2004;66:683–693. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- 53.Kumazawa Y, Kawaguchi K, Takimoto H. Immunomodulating effects of flavonoids on acute and chronic inflammatory responses caused by tumor necrosis factor alpha. Curr. Pharm. Des. 2006;12:4271–4279. doi: 10.2174/138161206778743565. [DOI] [PubMed] [Google Scholar]
- 54.Kotanidou A, Xagorari A, Bagli E, Kitsanta P, Fotsis T, Papapetropoulos A, Roussos C. Luteolin Reduces Lipopolysaccharide-induced Lethal Toxicity and Expression of Proinflammatory Molecules in Mice. Am. J. Respir. Crit. Care Med. 2002;165:818–823. doi: 10.1164/ajrccm.165.6.2101049. [DOI] [PubMed] [Google Scholar]
- 55.Tormakangas L, Vuorela P, Saario E, Leinonen M, Saikku P, Vuorela H. In vivo treatment of acute Chlamydia pneumoniae infection with the flavonoids quercetin and luteolin and an alkyl gallate, octyl gallate, in a mouse model. Biochem. Pharmacol. 2005;70:1222–1230. doi: 10.1016/j.bcp.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 56.Chen CY, Peng WH, Tsai KD, Hsu SL. Luteolin suppresses inflammation-associated gene expression by blocking NF-kappaB and AP-1 activation pathway in mouse alveolar macrophages. Life Sci. 2007;81:1602–1614. doi: 10.1016/j.lfs.2007.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xagorari A, Roussos C, Papapetropoulos A. Inhibition of LPS-stimulated pathways in macrophages by the flavonoid luteolin. Br. J. Pharmacol. 2002;136:1058–1064. doi: 10.1038/sj.bjp.0704803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karin M. Mitogen activated protein kinases as targets for development of novel anti-inflammatory drugs. Ann. Rheum. Dis. 2004;63(Suppl 2):ii62–ii64. doi: 10.1136/ard.2004.028274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 60.Chen CC, Chow MP, Huang WC, Lin YC, Chang YJ. Flavonoids inhibit tumor necrosis factor-alpha-induced up-regulation of intercellular adhesion molecule-1 (ICAM-1) in respiratory epithelial cells through activator protein-1 and nuclear factor-kappaB: structure-activity relationships. Mol. Pharmacol. 2004;66:683–693. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- 61.Devasagayam TP, Subramanian M, Singh BB, Ramanathan R, Das NP. Protection of plasmid pBR322 DNA by flavonoids against single-stranded breaks induced by singlet molecular oxygen. J. Photochem. Photobiol. B. 1995;30:97–103. doi: 10.1016/1011-1344(95)07159-y. [DOI] [PubMed] [Google Scholar]
- 62.Kim HK, Cheon BS, Kim YH, Kim SY, Kim HP. Effects of naturally occurring flavonoids on nitric oxide production in the macrophage cell line RAW 264.7 and their structure-activity relationships. Biochem. Pharmacol. 1999;58:759–765. doi: 10.1016/s0006-2952(99)00160-4. [DOI] [PubMed] [Google Scholar]
- 63.Hu C, Kitts DD. Luteolin and luteolin-7-O-glucoside from dandelion flower suppress iNOS and COX-2 in RAW264.7 cells. Mol. Cell. Biochem. 2004;265:107–113. doi: 10.1023/b:mcbi.0000044364.73144.fe. [DOI] [PubMed] [Google Scholar]
- 64.Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 65.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 66.Pitot HC. Multistage carcinogenesis--genetic and epigenetic mechanisms in relation to cancer prevention. Cancer Detect. Prev. 1993;17:567–573. [PubMed] [Google Scholar]
- 67.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 68.Galati G, Teng S, Moridani MY, Chan TS, O'Brien PJ. Cancer chemoprevention and apoptosis mechanisms induced by dietary polyphenolics. Drug. Metabol. Drug. Interact. 2000;17:311–349. doi: 10.1515/dmdi.2000.17.1-4.311. [DOI] [PubMed] [Google Scholar]
- 69.Buening MK, Chang RL, Huang MT, Fortner JG, Wood AW, Conney AH. Activation and inhibition of benzo(a)pyrene and aflatoxin B1 metabolism in human liver microsomes by naturally occurring flavonoids. Cancer Res. 1981;41:67–72. [PubMed] [Google Scholar]
- 70.Huang MT, Wood AW, Newmark HL, Sayer JM, Yagi H, Jerina DM, Conney AH. Inhibition of the mutagenicity of bay-region diolepoxides of polycyclic aromatic hydrocarbons by phenolic plant flavonoids. Carcinogenesis. 1983;4:1631–1637. doi: 10.1093/carcin/4.12.1631. [DOI] [PubMed] [Google Scholar]
- 71.Kim HJ, Lee SB, Park SK, Kim HM, Park YI, Dong MS. Effects of hydroxyl group numbers on the B-ring of 5,7-dihydroxyflavones on the differential inhibition of human CYP 1A and CYP1B1 enzymes. Arch. Pharm. Res. 2005;28:1114–1121. doi: 10.1007/BF02972971. [DOI] [PubMed] [Google Scholar]
- 72.Miller KP, Ramos KS. Impact of cellular metabolism on the biological effects of benzo[a]pyrene and related hydrocarbons. Drug Metab. Rev. 2001;33:1–35. doi: 10.1081/dmr-100000138. [DOI] [PubMed] [Google Scholar]
- 73.Fotsis T, Pepper MS, Aktas E, Breit S, Rasku S, Adlercreutz H, Wahala K, Montesano R, Schweigerer L. Flavonoids, dietary-derived inhibitors of cell proliferation and in vitro angiogenesis. Cancer Res. 1997;57:2916–2921. [PubMed] [Google Scholar]
- 74.Ko WG, Kang TH, Lee SJ, Kim YC, Lee BH. Effects of luteolin on the inhibition of proliferation and induction of apoptosis in human myeloid leukaemia cells. Phytother Res. 2002;16:295–298. doi: 10.1002/ptr.871. [DOI] [PubMed] [Google Scholar]
- 75.Knowles LM, Zigrossi DA, Tauber RA, Hightower C, Milner JA. Flavonoids suppress androgen-independent human prostate tumor proliferation. Nutr. Cancer. 2000;38:116–120. doi: 10.1207/S15327914NC381_16. [DOI] [PubMed] [Google Scholar]
- 76.Massague J. G1 cell-cycle control and cancer. Nature. 2004;432:298. doi: 10.1038/nature03094. [DOI] [PubMed] [Google Scholar]
- 77.Zi X, Feyes DK, Agarwal R. Anticarcinogenic effect of a flavonoid antioxidant, silymarin, in human breast cancer cells MDA-MB 468: induction of G1 arrest through an increase in Cip1/p21 concomitant with a decrease in kinase activity of cyclin-dependent kinases and associated cyclins. Clin. Cancer Res. 1998;4:1055–1064. [PubMed] [Google Scholar]
- 78.Lindenmeyer F, Li H, Menashi S, Soria C, Lu H. Apigenin acts on the tumor cell invasion process and regulates protease production. Nutr. Cancer. 2001;39:139–147. doi: 10.1207/S15327914nc391_19. [DOI] [PubMed] [Google Scholar]
- 79.Matsukawa Y, Marui N, Sakai T, Satomi Y, Yoshida M, Matsumoto K, Nishino H, Aoike A. Genistein arrests cell cycle progression at G2-M. Cancer Res. 1993;53:1328–1331. [PubMed] [Google Scholar]
- 80.Kobayashi T, Nakata T, Kuzumaki T. Effect of flavonoids on cell cycle progression in prostate cancer cells. Cancer Lett. 2002;176:17–23. doi: 10.1016/s0304-3835(01)00738-8. [DOI] [PubMed] [Google Scholar]
- 81.Casagrande F, Darbon JM. Effects of structurally related flavonoids on cell cycle progression of human melanoma cells: regulation of cyclin-dependent kinases CDK2 and CDK1. Biochem. Pharmacol. 2001;61:1205–1215. doi: 10.1016/s0006-2952(01)00583-4. [DOI] [PubMed] [Google Scholar]
- 82.Lim do Y, Jeong Y, Tyner AL, Park JH. Induction of cell cycle arrest and apoptosis in HT-29 human colon cancer cells by the dietary compound luteolin. Am J Physiol Gastrointest Liver Physiol. 2007;292:G66–75. doi: 10.1152/ajpgi.00248.2006. [DOI] [PubMed] [Google Scholar]
- 83.Li WX, Cui CB, Cai B, Wang HY, Yao XS. Flavonoids from Vitex trifolia L. inhibit cell cycle progression at G2/M phase and induce apoptosis in mammalian cancer cells. J. Asian Nat. Prod. Res. 2005;7:615–626. doi: 10.1080/10286020310001625085. [DOI] [PubMed] [Google Scholar]
- 84.Caino MC, Oliva JL, Jiang H, Penning TM, Kazanietz MG. Benzo[a]pyrene-7,8-dihydrodiol promotes checkpoint activation and G2/M arrest in human bronchoalveolar carcinoma H358 cells. Mol. Pharmacol. 2007;71:744–750. doi: 10.1124/mol.106.032078. [DOI] [PubMed] [Google Scholar]
- 85.Helton ES, Chen X. p53 modulation of the DNA damage response. J. Cell. Biochem. 2007;100:883–896. doi: 10.1002/jcb.21091. [DOI] [PubMed] [Google Scholar]
- 86.Yamashita N, Kawanishi S. Distinct mechanisms of DNA damage in apoptosis induced by quercetin and luteolin. Free Radic. Res. 2000;33:623–633. doi: 10.1080/10715760000301141. [DOI] [PubMed] [Google Scholar]
- 87.Chowdhury AR, Sharma S, Mandal S, Goswami A, Mukhopadhyay S, Majumder HK. Luteolin, an emerging anti-cancer flavonoid, poisons eukaryotic DNA topoisomerase I. Biochem. J. 2002;366:653–661. doi: 10.1042/BJ20020098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang L, Lau YK, Xi L, Hong RL, Kim DS, Chen CF, Hortobagyi GN, Chang C, Hung MC. Tyrosine kinase inhibitors, emodin and its derivative repress HER-2/neu-induced cellular transformation and metastasis-associated properties. Oncogene. 1998;16:2855–2863. doi: 10.1038/sj.onc.1201813. [DOI] [PubMed] [Google Scholar]
- 89.Lee LT, Huang YT, Hwang JJ, Lee PP, Ke FC, Nair MP, Kanadaswam C, Lee MT. Blockade of the epidermal growth factor receptor tyrosine kinase activity by quercetin and luteolin leads to growth inhibition and apoptosis of pancreatic tumor cells. Anticancer Res. 2002;22:1615–1627. [PubMed] [Google Scholar]
- 90.Fang J, Zhou Q, Shi XL, Jiang BH. Luteolin inhibits insulin-like growth factor 1 receptor signaling in prostate cancer cells. Carcinogenesis. 2007;28:713–723. doi: 10.1093/carcin/bgl189. [DOI] [PubMed] [Google Scholar]
- 91.Kim JH, Jin YR, Park BS, Kim TJ, Kim SY, Lim Y, Hong JT, Yoo HS, Yun YP. Luteolin prevents PDGF-BB-induced proliferation of vascular smooth muscle cells by inhibition of PDGF beta-receptor phosphorylation. Biochem. Pharmacol. 2005;69:1715–1721. doi: 10.1016/j.bcp.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 92.Matei D, Emerson RE, Lai YC, Baldridge LA, Rao J, Yiannoutsos C, Donner DD. Autocrine activation of PDGFRalpha promotes the progression of ovarian cancer. Oncogene. 2006;25:2060–2069. doi: 10.1038/sj.onc.1209232. [DOI] [PubMed] [Google Scholar]
- 93.Yin F, Giuliano AE, Van Herle AJ. Growth inhibitory effects of flavonoids in human thyroid cancer cell lines. Thyroid. 1999;9:369–376. doi: 10.1089/thy.1999.9.369. [DOI] [PubMed] [Google Scholar]
- 94.Lucas M, Sanchez-Margalet V. Protein kinase C involvement in apoptosis. Gen. Pharmacol. 1995;26:881–887. doi: 10.1016/0306-3623(94)00295-x. [DOI] [PubMed] [Google Scholar]
- 95.Weinstein IB, Kahn SM, O'Driscoll K, Borner C, Bang D, Jiang W, Blackwood A, Nomoto K. The role of protein kinase C in signal transduction, growth control and lipid metabolism. Adv. Exp. Med. Biol. 1997;400A:313–321. doi: 10.1007/978-1-4615-5325-0_44. [DOI] [PubMed] [Google Scholar]
- 96.Ferriola PC, Cody V, Middleton E., Jr. Protein kinase C inhibition by plant flavonoids. Kinetic mechanisms and structure-activity relationships. Biochem. Pharmacol. 1989;38:1617. doi: 10.1016/0006-2952(89)90309-2. [DOI] [PubMed] [Google Scholar]
- 97.Stathopoulos GT, Sherrill TP, Cheng DS, Scoggins RM, Han W, Polosukhin VV, Connelly L, Yull FE, Fingleton B, Blackwell TS. Epithelial NF-kappaB activation promotes urethane-induced lung carcinogenesis. Proc. Natl. Acad Sci. U.S.A. 2007;104:18514–18519. doi: 10.1073/pnas.0705316104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee SH, Lee SJ, Kim JH, Park BJ. Chemical carcinogen, N-methyl-N′-nitro-N-nitrosoguanidine, is a specific activator of oncogenic Ras. Cell Cycle. 2007;6:1257–1264. doi: 10.4161/cc.6.10.4243. [DOI] [PubMed] [Google Scholar]
- 99.Hanahan D, Weinberg RA. The Hallmarks of Cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 100.Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–2933. [PubMed] [Google Scholar]
- 101.Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death. Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- 102.Huang YT, Hwang JJ, Lee PP, Ke FC, Huang JH, Huang CJ, Kandaswami C, Middleton E, Jr., Lee MT. Effects of luteolin and quercetin, inhibitors of tyrosine kinase, on cell growth and metastasis-associated properties in A431 cells overexpressing epidermal growth factor receptor. Br. J. Pharmacol. 1999;128:999–1010. doi: 10.1038/sj.bjp.0702879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cheng A-C, Huang T-C, Lai C-S, Pan M-H. Induction of apoptosis by luteolin through cleavage of Bcl-2 family in human leukemia HL-60 cells. Eur. J. Pharmacol. 2005;509:1–10. doi: 10.1016/j.ejphar.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 104.Lee HJ, Wang CJ, Kuo HC, Chou FP, Jean LF, Tseng TH. Induction apoptosis of luteolin in human hepatoma HepG2 cells involving mitochondria translocation of Bax/Bak and activation of JNK. Toxicol. Appl. Pharmacol. 2005;203:124–131. doi: 10.1016/j.taap.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 105.Horinaka M, Yoshida T, Shiraishi T, Nakata S, Wakada M, Nakanishi R, Nishino H, Matsui H, Sakai T. Luteolin induces apoptosis via death receptor 5 upregulation in human malignant tumor cells. Oncogene. 2005;24:7180–7189. doi: 10.1038/sj.onc.1208874. [DOI] [PubMed] [Google Scholar]
- 106.Selvendiran K, Koga H, Ueno T, Yoshida T, Maeyama M, Torimura T, Yano H, Kojiro M, Sata M. Luteolin promotes degradation in signal transducer and activator of transcription 3 in human hepatoma cells: an implication for the antitumor potential of flavonoids. Cancer Res. 2006;66:4826–4834. doi: 10.1158/0008-5472.CAN-05-4062. [DOI] [PubMed] [Google Scholar]
- 107.Plaumann B, Fritsche M, Rimpler H, Brandner G, Hess RD. Flavonoids activate wild-type p53. Oncogene. 1996;13:1605–1614. [PubMed] [Google Scholar]
- 108.Shi R, Huang Q, Zhu X, Ong YB, Zhao B, Lu J, Ong CN, Shen HM. Luteolin sensitizes the anticancer effect of cisplatin via c-Jun NH2-terminal kinase-mediated p53 phosphorylation and stabilization. Mol. Cancer Ther. 2007;6:1338–1347. doi: 10.1158/1535-7163.MCT-06-0638. [DOI] [PubMed] [Google Scholar]
- 109.Shi RX, Ong CN, Shen HM. Luteolin sensitizes tumor necrosis factor-alpha-induced apoptosis in human tumor cells. Oncogene. 2004;23:7712–7721. doi: 10.1038/sj.onc.1208046. [DOI] [PubMed] [Google Scholar]
- 110.Yu C, Minemoto Y, Zhang J, Liu J, Tang F, Bui TN, Xiang J, Lin A. JNK suppresses apoptosis via phosphorylation of the proapoptotic Bcl-2 family protein BAD. Mol. Cell. 2004;13:329–340. doi: 10.1016/s1097-2765(04)00028-0. [DOI] [PubMed] [Google Scholar]
- 111.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 112.Malhi H, Gores GJ. TRAIL resistance results in cancer progression: a TRAIL to perdition? Oncogene. 2006;25:7333–7335. doi: 10.1038/sj.onc.1209765. [DOI] [PubMed] [Google Scholar]
- 113.Shi R-X, Ong C-N, Shen H-M. Protein Kinase C Inhibition and X-Linked Inhibitor of Apoptosis Protein Degradation Contribute to the Sensitization Effect of Luteolin on Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand-Induced Apoptosis in Cancer Cells. Cancer Res. 2005;65:7815–7823. doi: 10.1158/0008-5472.CAN-04-3875. [DOI] [PubMed] [Google Scholar]
- 114.Chang J, Hsu Y, Kuo P, Kuo Y, Chiang L, Lin C. Increase of Bax/Bcl-XL ratio and arrest of cell cycle by luteolin in immortalized human hepatoma cell line. Life Sci. 2005;76:1883–1893. doi: 10.1016/j.lfs.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 115.Brusselmans K, Vrolix R, Verhoeven G, Swinnen JV. Induction of cancer cell apoptosis by flavonoids is associated with their ability to inhibit fatty acid synthase activity. J. Biol. Chem. 2005;280:5636–5645. doi: 10.1074/jbc.M408177200. [DOI] [PubMed] [Google Scholar]
- 116.Lupu R, Menendez JA. Pharmacological inhibitors of Fatty Acid Synthase (FASN)--catalyzed endogenous fatty acid biogenesis: a new family of anticancer agents? Curr. Pharm. Biotechnol. 2006;7:483–493. doi: 10.2174/138920106779116928. [DOI] [PubMed] [Google Scholar]
- 117.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 118.Benassayag C, Perrot-Applanat M, Ferre F. Phytoestrogens as modulators of steroid action in target cells. J. Chromatogr. B. 2002;777:233–248. doi: 10.1016/s1570-0232(02)00340-9. [DOI] [PubMed] [Google Scholar]
- 119.Joussen AM, Rohrschneider K, Reichling J, Kirchhof B, Kruse FE. Treatment of Corneal Neovascularization with Dietary Isoflavonoids and Flavonoids. Exp. Eye Res. 2000;71:483–487. doi: 10.1006/exer.2000.0900. [DOI] [PubMed] [Google Scholar]
- 120.Bagli E, Stefaniotou M, Morbidelli L, Ziche M, Psillas K, Murphy C, Fotsis T. Luteolin inhibits vascular endothelial growth factor-induced angiogenesis; inhibition of endothelial cell survival and proliferation by targeting phosphatidylinositol 3′-kinase activity. Cancer Res. 2004;64:7936–7946. doi: 10.1158/0008-5472.CAN-03-3104. [DOI] [PubMed] [Google Scholar]
- 121.Semenza GL. HIF-1 and mechanisms of hypoxia sensing. Curr. Opin. Cell Biol. 2001;13:167–171. doi: 10.1016/s0955-0674(00)00194-0. [DOI] [PubMed] [Google Scholar]
- 122.Hasebe Y, Egawa K, Yamazaki Y, Kunimoto S, Hirai Y, Ida Y, Nose K. Specific inhibition of hypoxia-inducible factor (HIF)-1 alpha activation and of vascular endothelial growth factor (VEGF) production by flavonoids. Biol. Pharm. Bull. 2003;26:1379–1383. doi: 10.1248/bpb.26.1379. [DOI] [PubMed] [Google Scholar]
- 123.Trochon V, Mabilat-Pragnon C, Bertrand P, Legrand Y, Soria J, Soria C, Delpech B, Lu H. Hyaluronectin blocks the stimulatory effect of hyaluronan-derived fragments on endothelial cells during angiogenesis in vitro. FEBS Lett. 1997;418:6–10. doi: 10.1016/s0014-5793(97)01337-9. [DOI] [PubMed] [Google Scholar]
- 124.Kuppusamy UR, Khoo HE, Das NP. Structure-activity studies of flavonoids as inhibitors of hyaluronidase. Biochem. Pharmacol. 1990;40:397–401. doi: 10.1016/0006-2952(90)90709-t. [DOI] [PubMed] [Google Scholar]
- 125.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 126.Ende C, Gebhardt R. Inhibition of matrix metalloproteinase-2 and -9 activities by selected flavonoids. Planta Med. 2004;70:1006–1008. doi: 10.1055/s-2004-832630. [DOI] [PubMed] [Google Scholar]
- 127.Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat. Rev. Mol. Cell Biol. 2002;3:207–214. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- 128.Yang J, Mani SA, Weinberg RA. Exploring a new twist on tumor metastasis. Cancer Res. 2006;66:4549–4552. doi: 10.1158/0008-5472.CAN-05-3850. [DOI] [PubMed] [Google Scholar]
- 129.Beuvink I, Boulay A, Fumagalli S, Zilbermann F, Ruetz S, O'Reilly T, Natt F, Hall J, Lane HA, Thomas G. The mTOR Inhibitor RAD001 Sensitizes Tumor Cells to DNA-Damaged Induced Apoptosis through Inhibition of p21 Translation. Cell. 2005;120:747–759. doi: 10.1016/j.cell.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 130.Szlosarek P, Charles KA, Balkwill FR. Tumour necrosis factor-alpha as a tumour promoter. Eur. J. Cancer. 2006;42:745–750. doi: 10.1016/j.ejca.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 131.Kim JH, Cho YH, Park SM, Lee KE, Lee JJ, Lee BC, Pyo HB, Song KS, Park HD, Yun YP. Antioxidants and inhibitor of matrix metalloproteinase-1 expression from leaves of Zostera marina L. Arch. Pharm. Res. 2004;27:177–183. doi: 10.1007/BF02980103. [DOI] [PubMed] [Google Scholar]
- 132.Lee L-T, Huang Y-T, Hwang J-J, Lee AYL, Ke F-C, Huang C-J, Kandaswami C, Lee P-PH, Lee M-T. Transinactivation of the epidermal growth factor receptor tyrosine kinase and focal adhesion kinase phosphorylation by dietary flavonoids: effect on invasive potential of human carcinoma cells. Biochem. Pharmacol. 2004;67:2103–2114. doi: 10.1016/j.bcp.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 133.Huang YT, Lee LT, Lee PP, Lin YS, Lee MT. Targeting of focal adhesion kinase by flavonoids and small-interfering RNAs reduces tumor cell migration ability. Anticancer Res. 2005;25:2017–2025. [PubMed] [Google Scholar]
- 134.Lansky EP, Harrison G, Froom P, Jiang WG. Pomegranate (Punica granatum) pure chemicals show possible synergistic inhibition of human PC-3 prostate cancer cell invasion across Matrigel. Invest. New Drugs. 2005;23:121–122. doi: 10.1007/s10637-005-5856-7. [DOI] [PubMed] [Google Scholar]
- 135.Lee WJ, Wu LF, Chen WK, Wang CJ, Tseng TH. Inhibitory effect of luteolin on hepatocyte growth factor/scatter factor-induced HepG2 cell invasion involving both MAPK/ERKs and PI3K-Akt pathways. Chem. Biol. Interact. 2006;160:123–133. doi: 10.1016/j.cbi.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 136.Monasterio A, Urdaci MC, Pinchuk IV, Lopez-Moratalla N, Martinez-Irujo JJ. Flavonoids induce apoptosis in human leukemia U937 cells through caspase- and caspase-calpain-dependent pathways. Nutr. Cancer. 2004;50:90–100. doi: 10.1207/s15327914nc5001_12. [DOI] [PubMed] [Google Scholar]
- 137.Chiang CT, Way TD, Lin JK. Sensitizing HER2-overexpressing cancer cells to luteolin-induced apoptosis through suppressing p21(WAF1/CIP1) expression with rapamycin. Mol. Cancer Ther. 2007;6:2127–2138. doi: 10.1158/1535-7163.MCT-07-0107. [DOI] [PubMed] [Google Scholar]
- 138.Kandaswami C, Lee LT, Lee PP, Hwang JJ, Ke FC, Huang YT, Lee MT. The antitumor activities of flavonoids. In Vivo. 2005;19:895–909. [PubMed] [Google Scholar]
- 139.Kim JH, Lee EO, Lee HJ, Ku JS, Lee MH, Yang DC, Kim SH. Caspase activation and extracellular signal-regulated kinase/Akt inhibition were involved in luteolin-induced apoptosis in Lewis lung carcinoma cells. Ann. N. Y. Acad. Sci. 2007;1095:598–611. doi: 10.1196/annals.1397.102_2. [DOI] [PubMed] [Google Scholar]
- 140.Samy RP, Gopalakrishnakone P, Ignacimuthu S. Anti-tumor promoting potential of luteolin against 7,12-dimethylbenz(a)anthracene-induced mammary tumors in rats. Chem. Biol. Interact. 2006;164:1–14. doi: 10.1016/j.cbi.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 141.Chen D, Chen MS, Cui QC, Yang H, Dou QP. Structure-proteasome-inhibitory activity relationships of dietary flavonoids in human cancer cells. Front. Biosci. 2007;12:1935–1945. doi: 10.2741/2199. [DOI] [PubMed] [Google Scholar]
- 142.Belyanskaya LL, Hopkins-Donaldson S, Kurtz S, Simoes-Wust AP, Yousefi S, Simon HU, Stahel R, Zangemeister-Wittke U. Cisplatin activates Akt in small cell lung cancer cells and attenuates apoptosis by survivin upregulation. Int. J. Cancer. 2005;117:755–763. doi: 10.1002/ijc.21242. [DOI] [PubMed] [Google Scholar]
- 143.Lin Y, Devin A, Cook A, Keane MM, Kelliher M, Lipkowitz S, Liu ZG. The death domain kinase RIP is essential for TRAIL (Apo2L)-induced activation of IkappaB kinase and c-Jun N-terminal kinase. Mol. Cell. Biol. 2000;20:6638–6645. doi: 10.1128/mcb.20.18.6638-6645.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Friesen C, Fulda S, Debatin KM. Cytotoxic drugs and the CD95 pathway. Leukemia. 1999;13:1854–1858. doi: 10.1038/sj.leu.2401333. [DOI] [PubMed] [Google Scholar]
- 145.Huang C, Huang Y, Li J, Hu W, Aziz R, Tang MS, Sun N, Cassady J, Stoner GD. Inhibition of benzo(a)pyrene diol-epoxide-induced transactivation of activated protein 1 and nuclear factor kappaB by black raspberry extracts. Cancer Res. 2002;62:6857–6863. [PubMed] [Google Scholar]
- 146.Lu H, Li J, Zhang D, Stoner GD, Huang C. Molecular mechanisms involved in chemoprevention of black raspberry extracts: from transcription factors to their target genes. Nutr. Cancer. 2006;54:69–78. doi: 10.1207/s15327914nc5401_8. [DOI] [PubMed] [Google Scholar]
- 147.Li J, Zhang D, Stoner GD, Huang C. Differential effects of black raspberry and strawberry extracts on BaPDE-induced activation of transcription factors and their target genes. Mol. Carcinog. 2008;47:286–294. doi: 10.1002/mc.20377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Raina K, Singh RP, Agarwal R, Agarwal C. Oral grape seed extract inhibits prostate tumor growth and progression in TRAMP mice. Cancer Res. 2007;67:5976–5982. doi: 10.1158/0008-5472.CAN-07-0295. [DOI] [PubMed] [Google Scholar]
- 149.Veeriah S, Kautenburger T, Habermann N, Sauer J, Dietrich H, Will F, Pool-Zobel BL. Apple flavonoids inhibit growth of HT29 human colon cancer cells and modulate expression of genes involved in the biotransformation of xenobiotics. Mol. Carcinog. 2006;45:164–174. doi: 10.1002/mc.20158. [DOI] [PubMed] [Google Scholar]
- 150.Elangovan V, Sekar N, Govindasamy S. Chemopreventive potential of dietary bioflavonoids against 20-methylcholanthrene-induced tumorigenesis. Cancer Lett. 1994;87:107–113. doi: 10.1016/0304-3835(94)90416-2. [DOI] [PubMed] [Google Scholar]
- 151.Osakabe N, Yasuda A, Natsume M, Yoshikawa T. Rosmarinic acid inhibits epidermal inflammatory responses: anticarcinogenic effect of Perilla frutescens extract in the murine two-stage skin model. Carcinogenesis. 2004;25:549–557. doi: 10.1093/carcin/bgh034. [DOI] [PubMed] [Google Scholar]
- 152.Ueda H, Yamazaki C, Yamazaki M. Inhibitory effect of Perilla leaf extract and luteolin on mouse skin tumor promotion. Biol. Pharm. Bull. 2003;26:560–563. doi: 10.1248/bpb.26.560. [DOI] [PubMed] [Google Scholar]
- 153.Manju V, Nalini N. Protective role of luteolin in 1,2-dimethylhydrazine induced experimental colon carcinogenesis. Cell Biochem. Funct. 2007;25:189–194. doi: 10.1002/cbf.1305. [DOI] [PubMed] [Google Scholar]
- 154.Knekt P, Jarvinen R, Seppanen R, Hellovaara M, Teppo L, Pukkala E, Aromaa A. Dietary flavonoids and the risk of lung cancer and other malignant neoplasms. Am. J. Epidemiol. 1997;146:223–230. doi: 10.1093/oxfordjournals.aje.a009257. [DOI] [PubMed] [Google Scholar]
- 155.Wright ME, Mayne ST, Stolzenberg-Solomon RZ, Li Z, Pietinen P, Taylor PR, Virtamo J, Albanes D. Development of a comprehensive dietary antioxidant index and application to lung cancer risk in a cohort of male smokers. Am. J. Epidemiol. 2004;160:68–76. doi: 10.1093/aje/kwh173. [DOI] [PubMed] [Google Scholar]
- 156.Hirvonen T, Virtamo J, Korhonen P, Albanes D, Pietinen P. Flavonol and flavone intake and the risk of cancer in male smokers (Finland). Cancer Causes Control. 2001;12:789–796. doi: 10.1023/a:1012232008016. [DOI] [PubMed] [Google Scholar]
- 157.Garcia-Closas R, Agudo A, Gonzalez CA, Riboli E. Intake of specific carotenoids and flavonoids and the risk of lung cancer in women in Barcelona, Spain. Nutr. Cancer. 1998;32:154–158. doi: 10.1080/01635589809514734. [DOI] [PubMed] [Google Scholar]
- 158.Kim JS, Jobin C. The flavonoid luteolin prevents lipopolysaccharide-induced NF- B signalling and gene expression by blocking I B kinase activity in intestinal epithelial cells and bone-marrow derived dendritic cells. Immunology. 2005;115:375–387. doi: 10.1111/j.1365-2567.2005.02156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Karrasch T, Kim JS, Jang BI, Jobin C. The flavonoid luteolin worsens chemical-induced colitis in NF-kappaB(EGFP) transgenic mice through blockade of NF-kappaB-dependent protective molecules. PLoS ONE. 2007;2:e596. doi: 10.1371/journal.pone.0000596. [DOI] [PMC free article] [PubMed] [Google Scholar]