Abstract
Focus upon therapeutic strategies that intersect between pathways that govern cellular metabolism and cellular survival may offer the greatest impact for the treatment of a number of neurodegenerative and metabolic disorders, such as diabetes mellitus. In this regard, we investigated the role of a Drosophila nicotinamidase (DN) in mammalian SH-SY5Y neuronal cells during oxidative stress. We demonstrate that during free radical exposure to nitric oxide generators DN neuronal expression significantly increased cell survival and blocked cellular membrane injury. Furthermore, DN neuronal expression prevented both apoptotic late DNA degradation and early phosphatidylserine exposure that may serve to modulate inflammatory cell activation in vivo. Nicotinamidase activity that limited nicotinamide cellular concentrations appeared to be necessary for DN neuroprotection, since application of progressive nicotinamide concentrations could abrogate the benefits of DN expression during oxidative stress. Pathways that involved sirtuin activation and SIRT1 were suggested to be vital, at least in part, for DN to confer protection through a series of studies. First, application of resveratrol increased cell survival during oxidative stress either alone or in conjunction with the expression of DN to a similar degree, suggesting that DN may rely upon SIRT1 activation to foster neuronal protection. Second, the overexpression of either SIRT1 or DN in neurons prevented apoptotic injury specifically in neurons expressing these proteins during oxidative stress, advancing the premise that DN and SIRT1 may employ similar pathways for neuronal protection. Third, inhibition of sirtuin activity with sirtinol was detrimental to neuronal survival during oxidative stress and prevented neuronal protection during overexpression of DN or SIRT1, further supporting that SIRT1 activity may be necessary for DN neuroprotection during oxidative stress. Implementation of further work to elucidate the cellular mechanisms that govern nicotinamidase activity in mammalian cells may offer novel avenues for the treatment of disorders tied to oxidative stress and cellular metabolic dysfunction.
Keywords: Apoptosis, neuron, nicotinamidase, oxidative stress, phosphatidylserine, resveratrol, sirtinol, sirtuin, SIRT1
INTRODUCTION
Diabetes mellitus (DM) is a significant health concern for both young and mature adult populations (Won, et al., 2006). Approximately 16 million individuals in the United States and more than 165 million individuals worldwide suffer from DM. By the year 2030, it is predicted that more than 360 million individuals will be afflicted with DM (Wild, et al., 2004). Type 2 DM represents at least 80 percent of all diabetics and the presence of type 2 DM is increasing in incidence as a result of changes in human behavior and increased body mass index (Maiese, et al., 2007a, Maiese, et al., 2007c). Type 1 insulin-dependent DM is present in 5–10 percent of all diabetics with an increased incidence in adolescent minority groups (Dabelea, et al., 2007). Furthermore, the incidence of undiagnosed diabetes, impaired glucose tolerance, and fluctuations in serum glucose in the young raises additional concerns (Jacobson, et al., 2007).
The development of insulin resistance and the complications of DM in the vascular system can be the result of cellular oxidative stress (Maiese, et al., 2007a, Maiese, et al., 2007c). Hyperglycemia can lead to increased production of reactive oxygen species (ROS) in neuronal cells, endothelial cells, liver cells, and pancreatic β-cells (Maiese, 2008a, Maiese and Chong, 2003, Slomka, et al., 2008a). Recent clinical correlates support these experimental studies to show that elevated levels of ceruloplasmin are suggestive of increased ROS (Memisogullari and Bakan, 2004). Furthermore, acute glucose swings in addition to chronic hyperglycemia can trigger oxidative stress mechanisms, illustrating the importance for therapeutic interventions to maintain cellular metabolism within physiological constraints (Monnier, et al., 2006).
As a result, the preservation of cellular energy reserves may be a critical factor for the prevention of cellular injury during oxidative stress and DM (Maiese, 2008a, Maiese, et al., 2008d). One potential agent to consider for the maintenance of cellular metabolism in DM is nicotinamide (Chong, et al., 2002b, Lee, et al., 2008b, Li, et al., 2006a, Maiese and Chong, 2003), the amide form of niacin or vitamin B3. Treatment with nicotinamide may be effective during DM (Li, et al., 2006a, Li, et al., 2004, Maiese and Chong, 2003), since nicotinamide can lead to the remission of type 1 DM in mice with acetyl-l-carnitine (Cresto, et al., 2006), preserve neuronal and endothelial cell function during oxidative stress (Biedron, et al., 2008, Chong, et al., 2005d, Chong, et al., 2002b, Chong, et al., 2004b, Hoane, et al., 2008, Slomka, et al., 2008a, Slomka, et al., 2008b), and maintain normal fasting blood glucose in animals with streptozotocin-induced diabetes (Hu, et al., 1996, Reddy, et al., 1995a). In patients with DM, oral nicotinamide protects β-cell function, prevents clinical disease in islet-cell antibody-positive first-degree relatives of type-1 DM (Olmos, et al., 2006), and can reduce HbA1c levels (Crino, et al., 2005).
Yet, it must be realized that strategies, such as with nicotinamide, to modulate cellular metabolism may yield a double-edge sword for both beneficial and detrimental clinical results. Some studies report that extended exposure to nicotinamide results in impaired β-cell function and reduction in cell growth (Liu, et al., 2004, Reddy, et al., 1995b). In addition, nicotinamide offers cellular protection in millimole concentrations against oxidative stress, but in relation to cell longevity, lower concentrations of nicotinamide can function as an inhibitor of sirtuins (Bitterman, et al., 2002, Gallo, et al., 2004, Tang and Chua, 2008), NAD+ - dependent protein deacetylases that have been associated with the promotion of increased lifespan in yeast and metazoans (Li, et al., 2006a, Saunders and Verdin, 2007). Furthermore, SIRT1, a mammalian sirtuin that has the greatest sequence similarity to the yeast sirtuin silent information regulator factor 2 (Sir2) (Frye, 2000), has been tied to the regulation of cellular metabolism in adipose tissue (Picard, et al., 2004). Reduction in cellular nicotinamide concentrations that convert nicotinamide to nicotinic acid through the NAD salvage pathway, such as by the nicotinamidase in yeast encoded by the PNC1 gene, effectively can potentiate sirtuin activity (Anderson, et al., 2002, Gallo, et al., 2004).
Given the intimate and inverse relationship between nicotinamide and sirtuins in controlling cell longevity and survival, unique strategies that focus upon nicotinamide regulation in mammalian cells during oxidative stress may offer new insights for novel therapeutic strategies for metabolic as well as degenerative disorders. We demonstrate that expression of a Drosophila nicotinamidase (DN) in human neuronal SH-SY5Y cells significantly enhances cellular tolerance to oxidative stress and increases neuronal survival during free radical exposure. Furthermore, DN expression prevents late as well as early apoptotic signaling that can be associated with inflammatory cell activation. Cytoprotection through DN expression appears to rely, at least in part, upon SIRT1 activation, highlighting the potential of signal transduction pathways that integrate components of the NAD salvage pathway in mammals.
MATERIALS AND METHODS
Human Neuroblastoma SH-SY5Y Cell Culture and Differentiation
Per our prior studies, human adrenergic neuroblastoma SH-SY5Y cells were purchased from American Type Culture Collection and maintained in regular Eagle’s Minimum Essential alpha medium (MEM) (Invitrogen, Carlsbad, CA), supplemented with 10% heat-inactivated fetal bovine serum, 1 mM pyruvate, 1.5 g/L sodium bicarbonate, 100 IU/ml penicillin, 100 µg/ml streptomycin at 37°C in 95%/5% (v/v) mixture of humidified atmospheric air and CO2 (Chong, et al., 2007). Cell suspension was prepared at a density of 3–4 × 104 (24 well plate) or 1–1.5× 105 (35 mm2 Petri dish). When confluent at 50–60%, cells were differentiated by MEM growth medium containing 10 µM all-trans retinoic acid (RA) (Sigma, St. Louis, MO) for 48 hours. Experiments were initiated until cells grew to 60% – 70% confluence between passages 4–10 after differentiation.
Experimental Treatments
Nitric oxide (NO) administration was performed by replacing the culture media with media containing 6-(2-hydroxy-1-methyl-2-nitrosohydrazino)-N-methyl-1-hexanamine (NOC-9) (Calbiochem, San Diego, CA), or sodium nitroprusside (SNP) (Sigma, St. Louis, MO) per the experimental paradigm (Kang, et al., 2003a, Kang, et al., 2003b). More than one NO generator was used as a control to demonstrate that cells were responding to NO rather than to other by-products of these agents. In experiments using sirtinol and resveratrol, cells were treated with varying concentrations of sirtinol 1 hour prior to exposure of an NO donor.
Assessment of Cell Survival
SH-SY5Y injury was determined by bright field microscopy using a 0.4% trypan blue dye exclusion method 24 hours following treatment with a NO donor per our previous protocols (Chong, et al., 2002a). The mean survival was determined by counting eight randomly selected non-overlapping fields with each containing approximately 10–20 cells (viable + non-viable). Each experiment was replicated 6 times independently with different cultures. In experiments using GFP-tagged proteins, images were acquired with "blinded" assessment with a Leitz DMIRB microscope and a Fuji/Nikon Super CCD (6.1 megapixels) and cell death was analyzed in all cells or was analyzed separately for GFP positive and negative cells.
Assessment of DNA Fragmentation
Genomic DNA fragmentation was determined by the terminal deoxynucleotidyl transferase nick end labeling (TUNEL) assay (Chong and Maiese, 2007a, Kang, et al., 2003b). Briefly, SH-SY5Y cells were fixed in 4% paraformaldehyde/0.2% picric acid/0.05% glutaraldehyde and the 3’-hydroxy ends of cut DNA were labeled with biotinylated dUTP using the enzyme terminal deoxytransferase (Promega, Madison, WI) followed by streptavidin-peroxidase and visualized with 3,3’-diaminobenzidine (Vector Laboratories, Burlingame, CA). For fluorescence staining of TUNEL, the 3'-hydroxy ends of cut DNA were labeled with biotinylated dUTP using an enzyme terminal deoxytransferase followed by Texas-red Avidin (1:50) and imaging was performed at the wavelengths of 565 nm (red) and 490 nm (green).
Assessment of Membrane Phosphatidylserine (PS) Residue Externalization
Phosphatidylserine (PS) exposure was assessed through the established use of annexin V. Per our prior protocols (Chong, et al., 2004a, Kang, et al., 2003b), a 30 µg/ml stock solution of annexin V conjugated to phycoerythrin (PE) (R&D Systems, Minneapolis, MN) was diluted to 3 µg/ml in warmed calcium containing binding buffer (10 mmol/L Hepes, pH 7.5, 150 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L MgCl2, 1.8 mmol/L CaCl2). Plates were incubated with 500 µl of diluted annexin V for 10 minutes. Images were acquired with "blinded" assessment with a Leitz DMIRB microscope (Leica, McHenry, IL) and a Fuji/Nikon Super CCD (6.1 megapixels) using transmitted light and fluorescent single excitation light at 490 nm and detected emission at 585 nm. For simultaneous assessment of PS and Drosophila nicotinamidase (DN), SH-SY5Y cells were fixed with 4% polyparaformaldhyde, blocked with 1.5% normal horse serum, and subsequently incubated with mouse anti-v5 antibody (1:500, Invitrogen, Carlsbad, CA). This was followed by biotinylated anti-mouse IgG and Texas-red-Avidin with annexin V labeling as previously described.
Transfection of Drosophila Nicotinamidase (DN), GFP-DN, and GFP-SIRT1 Constructs into SH-SY5Y Cells
The cDNA construct for Drosophila nicotinamidase (DN-v5) (in addition to GFP-DN and GFP-SIRT1 expression vectors were generously provided by Dr. Guri Tzivion, Karmanos Cancer Institute, Wayne State University School of Medicine) with full-length cDNA (LD05707) was subcloned with a carboxy-terminal V5-His6-epitope tag into the Drosophila expression vector pAc 5.1 (actin promoter) for cell expression. V5 epitope antibodies were obtained from Invitrogene (Carlsbad, CA) (Balan et al., 2008). Cells were seeded into 35 mm dishes at a concentration of 1–1.5× 105 and transfected with 8 µg cDNA by using lipofection with Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) according to the instructions of the manufacturer. The transfection was assayed by detection of v5 expression by Western analysis or fluorescent expression of EGFP or GFP under a Leitz DMIRB microscope.
Analysis of v5 Expression
For western analysis, cells were homogenized and following protein determination, each sample (50 µg/lane) was then subjected to 7.5% SDS-polyacrylamide gel electrophoresis. After blocking 1 hour at room temperature with 5% skim milk, the membrane was incubated overnight at 4 °C with the primary mouse antibodies against v5 (1:5000, Invitrogen, Carlsbad, CA). After incubation of the membranes with horseradish peroxidase conjugated secondary antibodies (goat anti-mouse IgG, 1:2000) (Pierce, Rockford, IL), the antibody-reactive bands were revealed by enhanced chemiluminescence’s detection on Hyperfilm (Amersham Pharmacia Biotech, Piscataway, NJ).
For immunohistochemistry of DN labeled by the v5 antibody, cultures were incubated with mouse anti-v5 antibody (1:500, Invitrogen, Carlsbad, CA) at 4° over night. After incubation with biotinylated anti-mouse IgG (1:50) (Vector Laboratories, Burlingame, CA) at room temperature for 2 hours, v5 expression was revealed by conjugated to fluorescein avidin (1:50) (Vector Laboratories, Burlingame, CA).
Statistical Analysis
For each experiment, the mean and standard error were determined. Statistical differences between groups were assessed by means of analysis of variance (ANOVA) from 6 replicate experiments with the post-hoc Dunnett's test. Statistical significance was considered at p<0.05.
RESULTS
Exposure to NO Leads to Progressive Neuronal Cell Death
A NO donor (SNP or NOC-9) was applied to neuronal cultures in a series of concentration (10, 50, 100, 300, 500, and 1000 µM) and cell survival was assessed 24 hours later by trypan blue dye exclusion. As shown in Fig. (1A), cell survival was significantly reduced from 93 ± 3% in untreated control cells to 70 ± 4%, 55 ± 4% (P<0.01), 40 ± 5% (P<0.01), 33 ± 5% (P<0.01), and 22 ± 5% (P<0.01) during administration of SNP or NOC-9 in the serial concentrations of 50 µM, 100 µM, 300 µM, 500 µM and 1000 µM respectively. Since the NO concentrations of 100 µM and 300 µM provided robust cell injury models similar to clinically relevant levels during oxidative stress injuries (Thomas, et al., 2008) and resulted in a large decrease in neuronal survival ranging from approximately 40–60% (40–60% SH-SY5Y cell loss), these concentrations of the NO donors were employed for the subsequent experimental paradigms to allow observation of potential changes in cell survival that would be statistically significant. In subsequent experiments, data for the two NO donors was combined since no significant differences in cell injury were present among the agents.
Fig. (1). NO exposure leads to neuronal injury and DN is significantly expressed in SH-SY5Y neurons.
(A) A NO donor (SNP or NOC-9) was applied to the cultures of SH-SY5Y neurons at the concentrations of 10, 50, 100, 300, 500, and 1000 µM and cell survival was determined 24 hours later by using trypan blue dye exclusion method. Cell survival was progressively decreased in SH-SY5Y neurons following NO exposure (*p<0.01 vs. Control). The data from two NO donors was combined since each led to similar cell survival at the corresponding concentrations. Each data point represents the mean and SEM. The mean value was determined by counting 8 randomly selected non-overlapping fields with each containing approximately 10–30 cells. (B) DN cDNA-v5 construct was transfected into SH-SY5Y neurons by using lipofectamine 2000 reagents over 72 hours. The expression of DN was detected by Western blot analysis for v5 epitope antibody following transfection. (C) Immunohistochemical staining was performed 72 hours following transfection by using anti-v5 antibody with fluorescence labeling. Representative pictures with transmitted light (Tran) and Fluorescence (Fluo) for v5 staining demonstrated the expression of v5-DN shown by fluorescence green color in DN transfected but not in wildtype (non-transfected) neurons. (D) The quantitative analysis of immunostaining for DN transfection into SH-SY5Y neurons demonstrated that transfection efficiency reached over 95%.
DN Protein is Significantly Expressed in SH-SY5Y Cells Following Transfection
Western blot assay was performed for DN protein expression in SH-SY5Y neurons following transfection of the cDNA construct for DN and detected by v5 protein. As shown in Fig. (1B) western analysis, DN expression was highly expressed in the SH-SY5Y neurons. We subsequently examined the immunocytochemical expression of DN-v5 in individual SH-SY5Y cells. Using transmitted light (Tran), untreated control cells, cells transfected with vector only and no cDNA, or cells transfected with cDNA for DN-v5 demonstrate nor morphological differences and no cell loss. With fluorescent imaging (Fluo), untreated control cells and cells transfected with vector only are without fluorescent labeling. However, SH-SY5Y neurons transfected with DN-v5 reveal a transfection efficiency equal to approximately 95% on quantitative analysis.
DN Prevents Neuronal Cell Injury and Apoptotic Genomic DNA Degradation During Oxidative Stress
To examine the ability of DN to prevent cell injury during oxidative stress with NO exposure, SH-SY5Y cells were exposed to a NO donor (SNP, NOC-9, 50 µM, 100 µM, 300 µM) and cell survival was assessed 24 hours later by the trypan blue dye exclusion method (Figs. 2A, and 2B). In Fig. (2A), untreated wildtype control cells are without trypan blue staining, but exposure to NO leads to cellular membrane breakdown and trypan blue staining of cells. In wildtype and vector transfected cells exposed to NO, cell survival was significantly reduced following NO donor concentrations of 50 µM, 100 µM and 300 µM (P<0.01). In contrast, cells with DN exposed to NO had significantly reduced trypan blue staining and had increased survival, illustrating that DN was capable of preventing NO toxicity (Fig. 2B).
Fig. (2). Overexpression of DN prevents neuronal cell injury and apoptotic DNA fragmentation during NO exposure.
(A) SH-SY5Y wildtype neurons or neurons transfected with DN were exposed to a NO donor (NOC-9 or SNP) at the concentrations of 50, 100, and 300 µM and cell survival was determined 24 hours later by using trypan blue exclusion method. Representative images demonstrate staining for trypan blue in wildtype neurons following NO exposure, but significantly reduced trypan blue staining was seen in neurons with DN overexpression. (B) Quantitative results demonstrate that the neuronal survival in wildtype (non-transfected neurons) was progressively decreased 24 hours following exposure to NO. In contrast, neuronal survival in SH-SY5Y cells over-expressing DN was significantly increased following NO exposure (*p<0.01 vs. Control; †p<0.01 vs. wildtype/NO). The mean value was determined by counting 8 randomly selected non-overlapping fields with each containing approximately 10–30 cells. (C) Representative images illustrate DNA fragmentation with TUNEL assay in both wildtype and DN transfected SH-SY5Y (DN) cells 24 h following exposure to a NO donor (NOC-9, 300 µM). NO resulted in DNA fragmentation in wildtype and vector transfected neurons, but markedly reduced DNA fragmentation was present in DN transfected neurons. (D) Quantitative results demonstrate that DNA fragmentation in neurons with DN transfection was significantly decreased when compared to wildtype neurons after exposure to a NO donor (NOC-9 or SNP, 100 and 300 µM) (*p<0.01 vs. Control; †p<0. vs. Wildtype/NO). In B and D, to simplify the figures, the results for the two NO donors were combined. In all cases, each data point represents the mean and SEM and vector represents transfection absent of the cDNA DN construct.
In Figs. (2C) and (2D), SH-SY5Y cells were exposed to a NO donor (SNP, NOC-9, 100 µM, 300 µM) and 24 hours later cell apoptosis was assessed by DNA fragmentation using TUNEL. Untreated wildtype control cells are without DNA fragmentation. In Fig. (2C), wildtype cells are without evidence of nuclear DNA damage, but exposure to NO results in significant nuclear DNA damage with TUNEL labeling. In contrast, cells with DN overexpression exposed to NO are absent of significant DNA degradation when compared to wildtype cells and vector transfected cells (Figs. 2C and 2D). Data show a transfection efficiency equal to or greater than 95% with no significant differences in cell injury or DNA fragmentation present among the individual clones with DN overexpression.
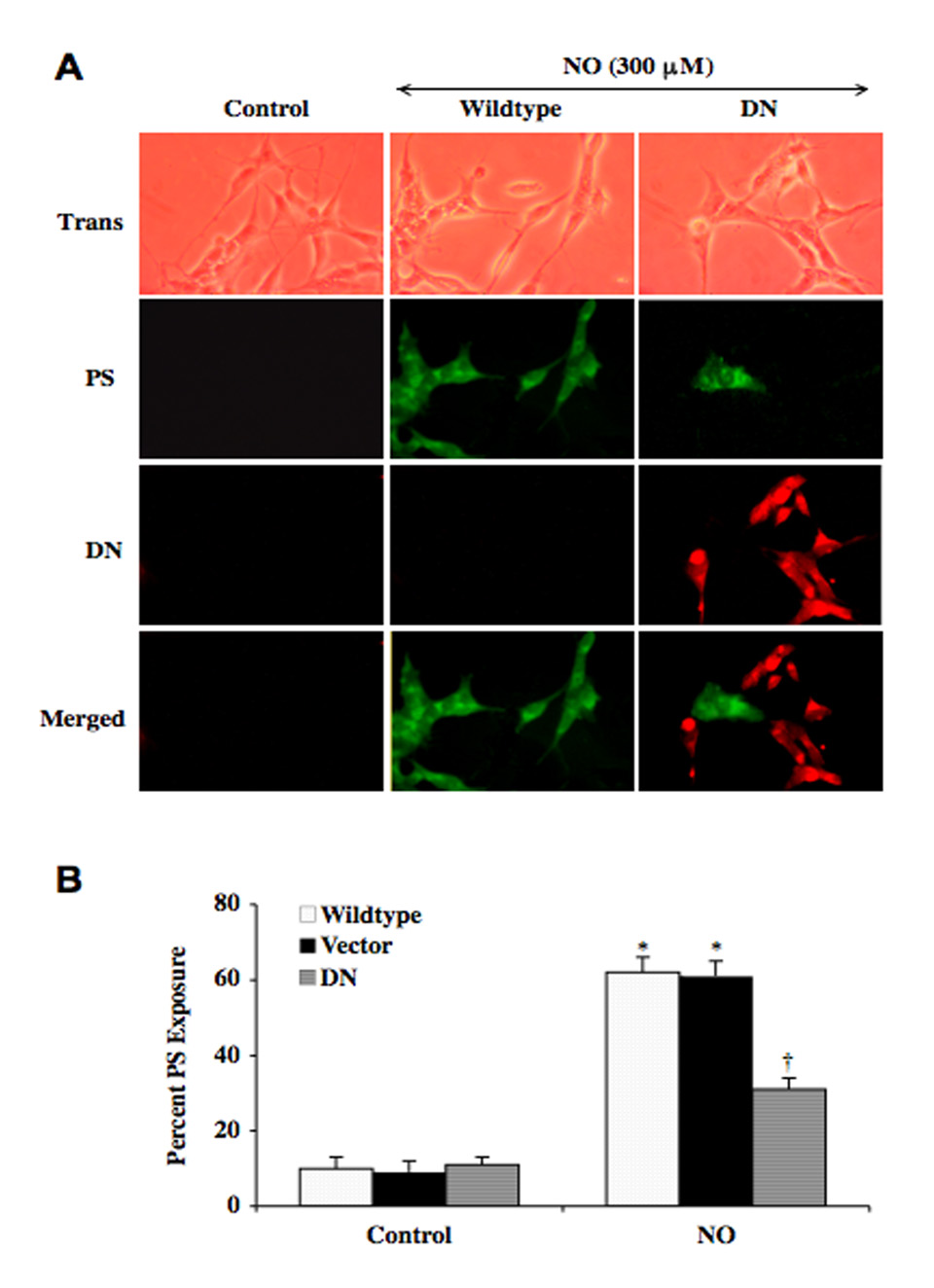
DN Blocks Apoptotic Membrane Phosphatidylserine (PS) Exposure in SH-SY5Y Cells
Apoptotic neuronal cell injury can be a dynamic process that involves genomic DNA degradation as well as early membrane PS exposure (Chong, et al., 2005c). Exposure of membrane PS residues in neurons undergoing apoptosis can compromise neuronal cell longevity since it can result in inflammatory cell activation and identify these cells for phagocytic disposal (Maiese, et al., 2008a, Mallat, et al., 2005, Ruifrok, et al., 2008). We therefore examined the ability of DN to modulate externalization of membrane PS exposure in SH-SY5Y neurons during exposure to the NO donors SNP and NOC-9. In Fig. (3A), SH-SY5Y neurons were exposed to the NOC-9 (300 µM) and 24 hours later cell membrane PS exposure was determined by annexin V. Untreated wildtype control neurons without exposure to NO were without PS externalization. In SH-SY5Y wildtype neurons and vector transfected neurons exposed to NO there was significant membrane PS exposure (labeled in green). In contrast, cells with DN overexpression (labeled in red) exposed to NO had minimal PS exposure upon examination of merged images (Fig. 3A).
Fig. (3). Overexpression of DN prevents membrane phosphatidylserine (PS) in SH-SY5Y neurons during NO exposure.
(A) Representative images of double staining for PS and DN illustrate PS exposure with annexin-V labeling in wildtype neurons or vector neurons (green) without DN transfection 24 hours following exposure to a NO donor (NOC-9, 300 µM). Minimal PS staining was seen in neurons with merged images during DN transfection (red) following NO exposure. (B) Quantitative results demonstrate that membrane PS exposure in neurons with transfection of DN were significantly decreased when compared to wildtype and vector transfected neurons after exposure to a NO donor (NOC-9 or SNP, 300 µM) (*p<0.01 vs. Control; †p<0. vs. Wildtype/NO). In B, to simplify the figures, the results for the two NO donors were combined. In all cases, each data point represents the mean and SEM and vector represents transfection absent of the cDNA DN construct.
Quantification of the data illustrated an increase in membrane PS exposure in SH-SY5Y wildtype neurons NO to 62 ± 4% and vector transfected neurons exposed to NO (Vector/NO) to 61 ± 5% at 24 hours after NO exposure when compared to untreated control cultures of 10 ± 3%. Neurons with DN overexpression demonstrated a significant reduction in membrane PS exposure to 31 ± 3% at 24 hours after exposure to NO (Fig. 3B).
Nicotinamide Eliminates Neuronal Protection During DN Expression in SH-SY5Y Neurons
To further examine the role of DN to offer protection in mammalian neuronal cells during oxidative stress through nicotinamidase activity, we administered increasing concentrations of the precursor for the coenzyme β-nicotinamide adenine dinucleotide (NAD+), namely nicotinamide that can potentially negate the nicotinamidase activity for DN. In the presence of a NO donor (SNP or NOC-9, 300 µM), nicotinamide was administered in several concentrations (0.25, 0.50, and 1.0 µM) and neuronal cell survival was assessed 24 hours later by trypan blue dye exclusion. In Fig. (4A), neurons exposed to NO (300 µM) alone as well as neurons receiving nicotinamide (NIC (1.00 µM)) and exposed to NO reveal significant trypan blue staining of cells. SH-SY5Y neuronal cells that overexpress DN are with minimal trypan blue staining, but cells that express DN and also are treated with nicotinamide (NIC (1.00 µM)/DN) have cellular membrane breakdown with trypan blue staining, negating the protective effects of DN. Quantification of this data in Fig. (4B) reveals that SH-SY5Y neurons exposed to NO with overexpression of DN have increased survival to 63 ± 5% when compared to wildtype cells without DN overexpression with a survival of 39 ± 4%, but SH-SY5Y neurons with DN overexpression lose the ability to protect in the presence of nicotinamide concentrations of 0.25, 0.50, and 1.00 µM. In all cases, control neurons were not exposed to NO.
Fig. (4). Nicotinamide eliminates the protection of DN in SH-SY5Y neurons during NO exposure.
Nicotinamide (NIC) at the concentrations indicated was applied at 1 hour prior to NO exposure (NOC-9 or SNP, 300 µM) to SH-SY5Y wildtype neurons or neurons transfected with DN for 3 days. Cell survival was determined 24 hours later using trypan blue exclusion method. (A) Representative images show that neurons without DN transfection or neurons with NIC alone (NIC (1.00 µM)) exposed to NO reveal significant trypan blue staining of cells. However, SH-SY5Y neurons that overexpress DN are with minimal trypan blue staining, but cells that express DN and also are treated with nicotinamide (NIC (1.00 µM)/DN) have cellular membrane breakdown with trypan blue staining, negating the protective effects of DN. (B) Quantitative analysis demonstrates that DN significantly increased neuronal survival during NO exposure, but that protection was diminished after administration of nicotinamide (0.25–1.00 µM) (*p<0.01 vs. DN/NO). In B, to simplify the figures, the results for the two NO donors were combined. In all cases, each data point represents the mean and SEM and control equals SH-SY5Y neurons not exposed to NO.
Resveratrol, a Sirtuin Activator, Prevents Neuronal SH-SY5Y Cell Injury to a Similar Degree as SH-SY5Y Cells Overexpressing DN
We examined the role of resveratrol (3,5,4' -trihydroxy-trans- stilbene) since it is known to activate the NAD+-dependent deacetylase SIRT1 (Tang and Chua, 2008) as well as offer anti-oxidant properties and cytoprotection against oxidative stress with ROS (Bastianetto, et al., 2000). In Fig. (5A), resveratrol (15 µM) significantly prevented SH-SY5Y neuronal cell injury and blocked the uptake of trypan blue staining in wildtype cells exposed to a NO donor (SNP or NOC-9, 300 µM). In addition, resveratrol (15 µM) provided a similar level of increased neuronal cell survival in SH-SY5Y cells overexpressing DN during NO exposure. In Fig. (5B), resveratrol in concentrations of 5 µM, 15 µM, and 30 µM increased neuronal cell survival during NO exposure to approximately 60% in neurons without DN overexpression (wildtype) or in neurons overexpressing DN, suggesting that neuronal protection with DN may require, at least in part, activation of SIRT1.
Fig. (5). Resveratrol increases survival in SH-SY5Y neurons during NO exposure.
Resveratrol at the concentrations of 5–30 µM was applied 1 hour prior to NO exposure (NOC-9 or SNP, 300 µM) to SH-SY5Y neurons without DN overexpression (wildtype) or to neurons transfected with DN for 3 days. Neuronal cell survival was determined 24 hours following NO exposure using trypan blue exclusion method. (A) Representative images indicate that NO exposure led to trypan blue staining in SH-SY5Y neurons. Resveratrol at the concentration of 15 µM significantly decreased trypan blue uptake in both wildtype and DN transfected neurons following NO exposure. (B) Quantitative analysis demonstrates that resveratrol (5–30 µM) alone or with DN overexpression significantly increased neuronal survival during NO (300 µM) exposure (*p<0.01 vs. Wildtype/NO; †p<0. vs. DN/NO). In B, to simplify the figures, the results for the two NO donors were combined. In all cases, each data point represents the mean and SEM and control neurons were not exposed to NO.
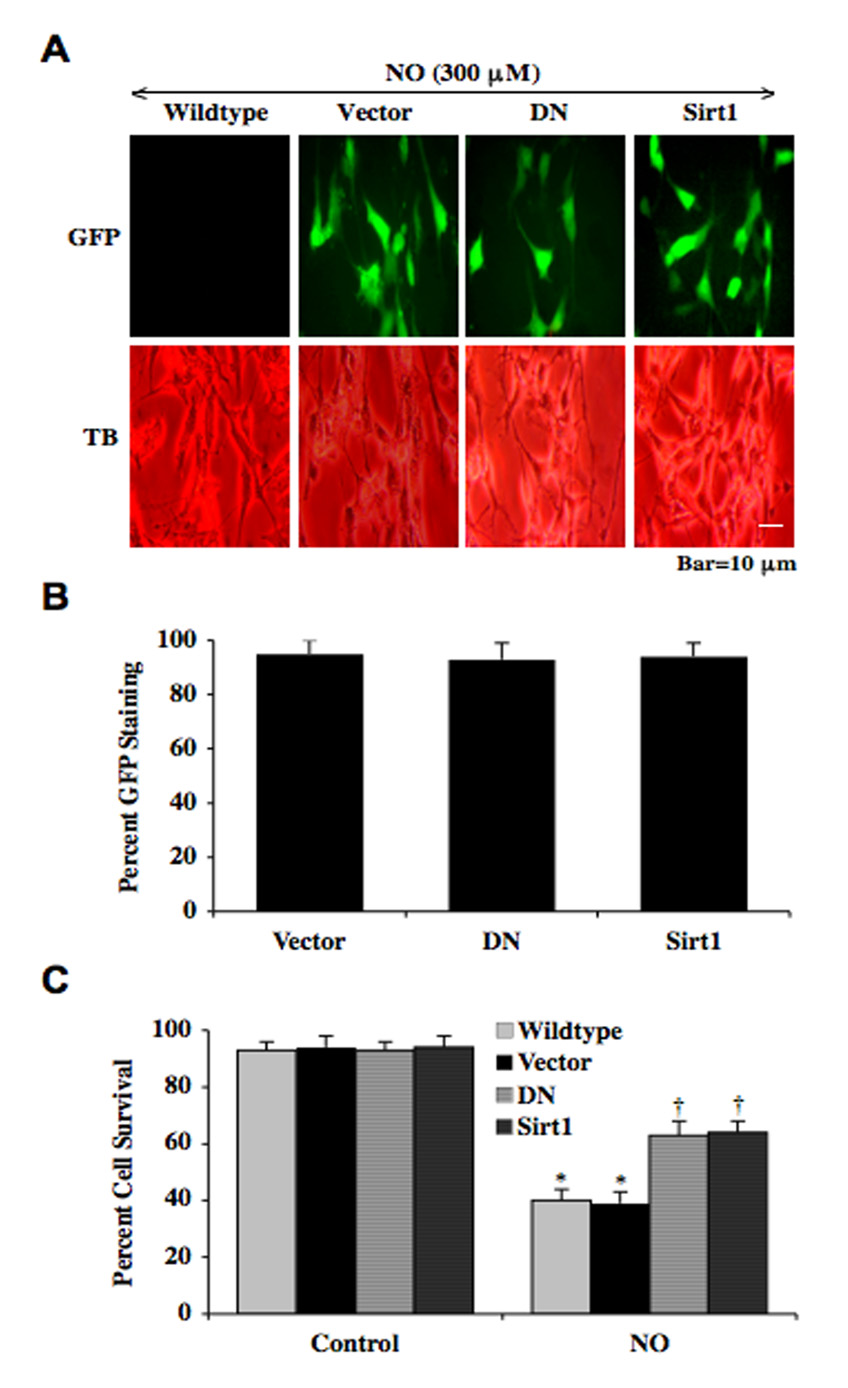
SIRT1 Overexpression Increases Neuronal SH-SY5Y Survival Similar to Protection with DN During Oxidative Stress
Given the results obtained with resveratrol that may be linked to SIRT1 activation, we next studied whether SIRT1 overexpression could alter cell survival in comparison with neurons overexpressing DN. In Fig. (6A), representative images show GFP labeling for neurons that are without transfection (wildtype), transfected with vector (without DN construct), transfected with DN, or transfected with SIRT1 (Sirt1). Exposure to a NO donor (SNP or NOC-9, 300 µM) leads to significant trypan blue uptake in neurons without transfection or transfected with vector only. In contrast, SH-SY5Y neurons with DN or SIRT1 overexpression during NO exposure had increased neuronal cell survival and minimal trypan blue uptake. Fig. (6B) illustrates that transfection efficiency was equal to or greater than 95% with vector, DN, and Sirt1. In Fig. (6C), quantitation of Fig. (6A) data reveals significant neuronal cell loss during NO exposure (SNP or NOC-9, 300 µM) in neurons without transfection (wildtype) or with vector transfection. Yet, neuronal cell survival is markedly increased to approximately 60% during NO exposure with either overexpression of DN or SIRT1, suggesting that DN and SIRT1 may employ similar pathways for neuronal protection during oxidative stress.
Fig. (6). Overexpression of SIRT1 increases survival in SH-SY5Y neurons during NO exposure similar to overexpression of DN.
(A) Representative images illustrate neuronal cell survival with a trypan blue dye exclusion in SH-SY5Y cells with no transfection (wildtype), transfection of GFP-vector (Vector),transfection of GFP-DN (DN), or transfection of GFP-SIRT1 (Sirt1) 24 hours following exposure to a NO donor (NOC-9, 300 µM). NO neuronal injury through trypan blue dye uptake is evident in wildtype and vector-transfected neurons, but neurons with overexpression DN or SIRT1 had markedly reduced trypan blue dye uptake. (B) Quantitation of transfection efficiency demonstrates by GFP labeling for vector, DN, and Sirt1 was greater than 95%. (C) Neuronal cell survival with overexpression of either DN or Sirt1 was significantly increased when compared to wildtype or vector neurons without either DN or Sirt1 overexpression after exposure to a NO donor (SNP or NOC-9, 300 µM) (*p<0.01 vs. Control; †p<0. vs. Wildtype/NO). In all cases, each data point represents the mean and SEM.
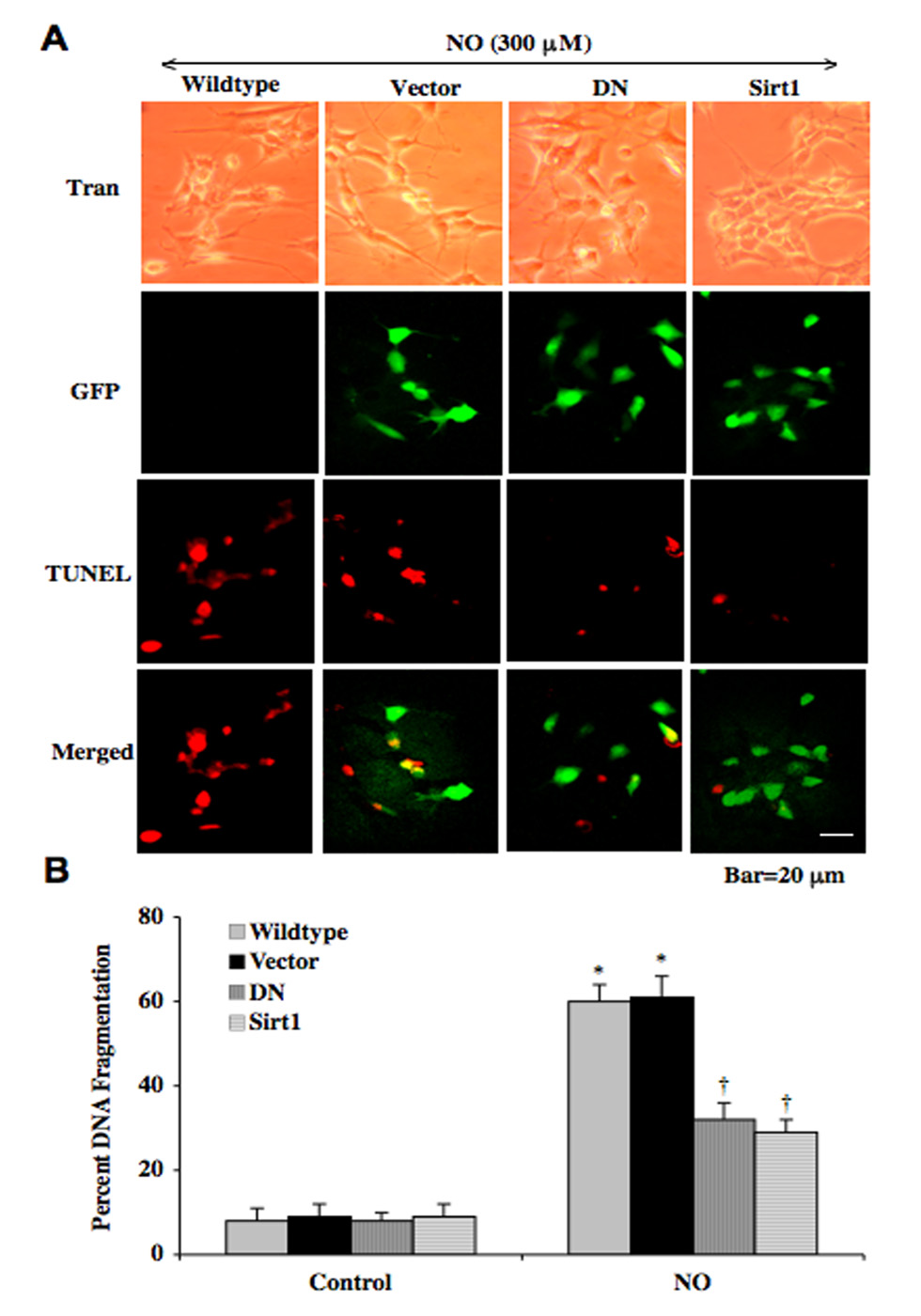
Overexpression of DN or SIRT1 in SH-SY5Y Neurons Correlates with Prevention of Nuclear DNA Degradation in the Same Cells During Oxidative Stress
To examine the individual neuronal cell relationship between expression of DN or SIRT1 and apoptotic neuronal cell injury during oxidative stress, we assessed co-localization of cells overexpressing either DN or SIRT1 and TUNEL with immunofluorescent double staining. In Fig. (7A), transmitted light (Tran) images show neuronal cell morphology during exposure to a NO donor (SNP or NOC-9, 300 µM) without transfection (wildtype) or with transfection of vector, DN, or SIRT1 (Sirt1). Flourescent images with GFP labeling (green) confirmed transfection of these cells with vector, DN, or SIRT1. During NO exposure, merged images reveal significant nuclear staining of TUNEL (red) in wildtype neurons and neurons transfected with vector only consistent with high apoptotic cell injury (Fig. 7A). In contrast, merged images of SH-SY5Y neuronal cells transfected with DN or SIRT1 during NO exposure illustrate minimal cells with nuclear staining for TUNEL (yellow), demonstrating that DN and SIRT1 expression protect neuronal cells from apoptotic cell death (Fig. 7A). Quantification of this data demonstrates a significant reduction in DNA fragmentation in cells with DN or SIRT1 overexpression when compared to wildtype neurons without transfection or neurons transfected with vector only during exposure to a NO donor (SNP or NOC-9, 300 µM) (Fig. 7B).
Fig. (7). Overexpression of DN and SIRT1 prevents DNA fragmentation in SH-SY5Y neurons during NO exposure.
(A) Representative images illustrate transmitted light (Tran) images that display neuronal cell morphology during exposure to a NO donor (NOC-9, 300 µM) without transfection (wildtype) or with transfection of vector, DN, or SIRT1 (Sirt1). Flourescent images with GFP labeling confirm transfection of these cells with vector, DN, or SIRT1. During NO exposure, merged images reveal significant nuclear staining of TUNEL (red) in wildtype neurons and neurons transfected with vector only consistent with high apoptotic cell injury, but neuronal cells transfected with DN or SIRT1 during NO exposure illustrate minimal cells with nuclear staining for TUNEL (yellow). (B) DNA fragmentation in neurons with transfection of DN or Sirt1 was significantly decreased when compared to wildtype cells after exposure to a NO donor (SNP or NOC-9 300 µM) (*p<0.01 vs. Control; †p<0. vs. Wildtype/NO). In B, to simplify the figures, the results for the two NO donors were combined. Each data point represents the mean ± SEM and wildtype neurons were not exposed to NO.
Loss of Sirtuin Eliminates the Ability of DN to Prevent Neuronal Cell Injury in SH-SY5Y Neurons During Oxidative Stress
Initially, the sirtuin inhibitor sirtinol (2-[(2-hydroxy-naphthalen-1-ylmethylene)-amino]-N-(1-phenyl-ethyl)-benzamide) (Grozinger, et al., 2001) was administered in concentrations of 25, 50, 75, and 100 µM 1 hour prior to the administration of a NO donor (SNP or NOC-9, 300 µM) and neuronal cell survival was assessed 24 hours later by trypan blue dye exclusion. SH-SY5Y cell survival was significantly reduced with increasing concentrations of sirtinol, suggesting that an endogenous level of sirtuin activity such as with SIRT1 may be required for neuronal protection during oxidative stress (data not shown). Next, sirtuin activity was blocked with sirtinol (50 µM) applied 1 hour prior to a NO donor (SNP or NOC-9, 300 µM) that significantly reduced neuronal survival in neurons without transfection (wildtype) and in transfected neurons with vector only. Furthermore, sirtinol eliminated neuronal protection by DN or SIRT1 in neurons overexpressing these proteins, illustrating that DN may require pathways tied to SIRT1 activation to foster neuronal protection during oxidative stress (Fig. 8).
Fig. (8). Sirtinol decreases survival in SH-SY5Y neurons following NO exposure.
(A) Sirtinol at the concentration of 50 µM was applied to the cultures of SH-SY5Y neuronal cells 1 hour prior to the exposure to a NO donor (SNP or NOC-9, 300 µM) in neurons without transfection (wildtype), in vector transfected neurons, and in neurons transfected with DN or SIRT1 (Sirt1). Neuronal cell survival was determined 24 hours following NO exposure with trypan blue dye exclusion method. Sirtinol administration significantly reduced neuronal survival in neurons without transfection (wildtype), in transfected neurons with vector only, and in neurons overexpressing DN or SIRT1 (Sirt1) (*p<0.01 vs. NO). To simplify the figures, the results for the two NO donors were combined and each data point represents the mean ± SEM.
DISCUSSION
In a number of disorders, cell survival and lifespan is closely tied to the presence of oxidative stress and the subsequent induction of apoptotic cell injury. Although several different mechanisms can account for the degeneration of neuronal cells in the body, the generation of cellular oxidative stress represents a significant component of the pathological complications that injure cells of the nervous system (Maiese, et al., 2008b). Oxidative stress is a result of the release of ROS that include superoxide free radicals, hydrogen peroxide, singlet oxygen, NO, and peroxynitrite (Chong and Maiese, 2007b). Interestingly in disorders that involve diabetes and impaired cellular metabolism, it has been shown that both prolonged duration as well as short periods of hyperglycemia can generate ROS (Yano, et al., 2004), signifying the importance to develop therapeutic strategies that can ameliorate the debilitating effects of ROS during impaired cellular metabolism.
We therefore first examined the effect of exposure to oxidative stress through different NO donors on neuronal survival. NO exposure in mammalian SH-SY5Y neuronal cells resulted in progressive cell injury and apoptotic death with significant loss of cell survival observed at 50 µM and increasing to approximately 40% and 60% cell loss at clinically relevant toxic NO exposure concentrations of 100 µM and 300 µM (Thomas, et al., 2008). DN overexpression in mammalian neuronal cells significantly enhanced neuronal survival and reduced cellular membrane breakdown as assessed through trypan blue staining (Balan et al., 2008). Recent studies suggesting a cytoprotective role during nicotinamidase activity support our observations by demonstrating that overexpression of the nicotinamidase pnc-1 gene in the nematode C. elegans leads to increased survival of worms exposed to the pesticide paraquat (van der Horst, et al., 2007).
Our work further extends these observations by illustrating that neuronal protection by DN during oxidative stress involves the specific prevention of both late and early apoptotic cell injury programs. Apoptotic neuronal degeneration that involves genomic DNA fragmentation may represent a significant component for neuronal cell loss during multiple disorders such as diabetes, ischemia, and general cognitive loss (Chong, et al., 2005c, Harris, et al., 2007, Okouchi, et al., 2007). The cleavage of genomic DNA into fragments is considered to be a delayed event that occurs late during apoptosis (Maiese, et al., 2005, Maiese, et al., 2008f). DN overexpression in our neuronal cell line significantly prevented apoptotic nuclear DNA fragmentation during NO donor administration. Yet, the protective capacity of DN against NO induced oxidative stress extends beyond the prevention of genomic DNA destruction and encompasses the prevention of cellular membrane PS externalization. Blockade of neuronal membrane PS exposure can be critical for the prevention of clinical disability since neurons expressing externalized PS may be removed by microglia (Chong, et al., 2005a, Li, et al., 2006b, Lin and Maiese, 2001) and membrane PS externalization on platelets has been associated with clot formation in the vascular cell system (Thiagarajan and Tait, 1990). We illustrate that exposure to NO donors results in the significant externalization of PS in wildtype and vector transfected neurons. However, neurons that overexpress DN significantly prevent membrane PS externalization during oxidative stress.
Enhanced nicotinamidase activity appears to be necessary to foster neuronal cell protection during oxidative stress. In worms that overexpress pnc-1, the benefits against paraquat administration with increased survival are significantly reduced during excessive nicotinamide administration (van der Horst, et al., 2007), indicating that nicotinamidase protection may require the conversion of nicotinamide. In a similar manner, we show that administration of nicotinamide in a series of concentrations from 0.25 µM to 1.00 µM is not toxic and does not worsen neuronal cell survival in wildtype cells. However, these same nicotinamide concentrations administered to neurons overexpressing DN significantly blocked the neuroprotective effects of DN, supporting the premise that DN leads to increased neuronal survival during oxidative stress through pathways that involve the reduction in nicotinamide concentrations.
Since nicotinamide also can function as an inhibitor of sirtuins (Chong, et al., 2005d, Porcu and Chiarugi, 2005) and block Sir2 protein by intercepting an ADP-ribosyl-enzyme-acetyl peptide intermediate with the regeneration of NAD+ (transglycosidation) (Jackson, et al., 2003), we extended our studies to investigate the role of sirtuin activation during DN neuronal protection. Resveratrol is a natural polyphonic phytoalexin in the skin of grapes that not only activates SIRT1 (Tang and Chua, 2008), but also has been shown to be neuroprotective in models of Alzheimer's disease (Kim, et al., 2007) and against ROS (Bastianetto, et al., 2000). Furthermore, SIRT1 has been associated with endothelial nitric oxide synthase expression (Nisoli, et al., 2005). In our neuronal SH-SY5Y model, resveratrol increased cell survival during oxidative stress either alone or in conjunction with the overexpression of DN to a similar degree, suggesting that DN may rely upon SIRT1 activation to foster neuronal protection. This work was further extended by illustrating that SIRT1 overexpression in SH-SY5Y neurons prevented cell injury during oxidative stress to a similar degree to neurons overexpressing DN. In our subsequent studies that assessed either SIRT1 or DN overexpression with the onset of apoptotic DNA degradation, both SIRT1 and DN overexpression protected these neurons specifically expressing these proteins against apoptotic injury, also advancing the premise that DN and SIRT1 may employ similar pathways for neuronal protection during oxidative stress. In our final studies, we demonstrate that inhibition of sirtuin activity with sirtinol administration in a series of increasing concentrations was detrimental to neuronal survival during NO exposure, suggesting that an endogenous level of sirtuins may be necessary to block oxidative stress induced cell injury. In addition, sirtinol administration during overexpression of DN or SIRT1 abrogated protection by these proteins to suggest in combination with our prior data that SIRT1 activity may be necessary and sufficient for DN neuroprotection during oxidative stress.
Although DN appears to rely upon sirtuin mediated pathways to confer neuronal protection during oxidative stress, additional pathways linked to either nicotinamide or sirtuins also may be essential for the results we observed with DN overexpression in neurons. For example, nicotinamide at higher concentrations than investigated in the present studies can protect neurons against oxidative stress that involve modulation of multiple pathways such as caspases, mitochondrial permeability, energy metabolism, p21WAF1, BAD, hormonal receptor modulation, inflammation, and protein kinase B (Akt) (Aoyagi and Archer, 2008, Biedron, et al., 2008, Chong, et al., 2005d, Chong, et al., 2002b, Feng, et al., 2006, Ieraci and Herrera, 2006, Lee, et al., 2008a, Lee, et al., 2008b, Li, et al., 2006a, Lin, et al., 2001, Lin, et al., 2000, Maiese, 2008b, Maiese and Chong, 2003). In addition, pathways such as Akt can enhance cell survival during several injury models such as during free radical exposure (Chong, et al., 2003, Matsuzaki, et al., 1999), hyperglycemia (Anitha, et al., 2006), hypoxia (Chong, et al., 2002a, Li, et al., 2006c, Nakka, et al., 2008), β-amyloid toxicity (Chong, et al., 2005b, Du, et al., 2004, Nakagami, et al., 2002), and oxidative stress (Chong, et al., 2004a, Kang, et al., 2003a, Kang, et al., 2003b). Akt also can prevent cellular apoptosis through the phosphorylation of forkhead transcription factors of the "O" subclass, FoxO (Maiese, et al., 2008c, Maiese, et al., 2008e). Protein inhibition or gene knockdown of FoxO proteins can enhance neuronal survival by estradiol (Won, et al., 2006), mediate the protective effects of metabotropic glutamate receptors (Chong, et al., 2006), and provides trophic factor protection with erythropoietin (Chong and Maiese, 2007a). Interestingly, nicotinamide not only can maintain phosphorylation of FoxO3a and inhibit its activity, but also can preserve the integrity of the FoxO3a protein to block FoxO3a proteolysis that can yield proapoptotic amino-terminal fragments (Chong, et al., 2004b). FoxO proteins also have been associated with cell longevity through SIRT1. However, this relationship sometimes varies among experimental models. Some studies suggest that stimulation of SIRT1 during starvation is dependent upon FoxO3a activity as well as p53 (Nemoto, et al., 2004). In contrast, other work has shown in cell culture that SIRT1 may repress the activity of FoxO1, FoxO3a, and FoxO4, suggesting that increased cellular survival and lifespan may benefit from reduction in FoxO proteins (Motta, et al., 2004). Other studies indicate that SIRT1 binds to FoxO proteins, such as FoxO4, to catalyze its deacetylation and enhance FoxO4 activity while acetylation of FoxO4 by cyclic-AMP responsive element binding (CREB)-binding protein serves to inhibit FoxO4 transcriptional activity (Maiese, et al., 2008c, Maiese, et al., 2007b). Therefore, modulation of nicotinamidase activity in mammalian cells may rely, at least in part, upon sirtuin mediated pathways to foster neuronal protection, but it is clear that additional work is warranted to further elucidate the cellular mechanisms that govern nicotinamidase activity in neuronal cells during oxidative stress.
ACKNOWLEDGEMENTS
This research was supported by the following grants (KM): American Diabetes Association, American Heart Association (National), Bugher Foundation Award, Janssen Neuroscience Award, LEARN Foundation Award, MI Life Sciences Challenge Award, Nelson Foundation Award, NIH NIEHS (P30 ES06639), and NIH NINDS/ NIA.
REFERENCES
- Anderson RM, Bitterman KJ, Wood JG, Medvedik O, Cohen H, Lin SS, Manchester JK, Gordon JI, Sinclair DA. Manipulation of a nuclear NAD+ salvage pathway delays aging without altering steady-state NAD+ levels. J Biol Chem. 2002;277:18881–18890. doi: 10.1074/jbc.M111773200. [DOI] [PubMed] [Google Scholar]
- Anitha M, Gondha C, Sutliff R, Parsadanian A, Mwangi S, Sitaraman SV, Srinivasan S. GDNF rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. J Clin Invest. 2006;116:344–356. doi: 10.1172/JCI26295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyagi S, Archer TK. Nicotinamide uncouples hormonedependent chromatin remodeling from transcription complex assembly. Mol Cell Biol. 2008;28:30–39. doi: 10.1128/MCB.01158-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balan V, Miller GS, Kaplun L, Balan K, Chong ZZ, Faqi L, Kaplun A, VanBerkum MFA, Arking R, Freeman DC, Maiese K, Tzivion G. Lifespan extension and neuronal cell protection by Drosophila nicotinamidase. J Biol Chem. 2008 doi: 10.1074/jbc.M804681200. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastianetto S, Zheng WH, Quirion R. Neuroprotective abilities of resveratrol and other red wine constituents against nitric oxide-related toxicity in cultured hippocampal neurons. Br J Pharmacol. 2000;131:711–720. doi: 10.1038/sj.bjp.0703626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedron R, Ciszek M, Tokarczyk M, Bobek M, Kurnyta M, Slominska EM, Smolenski RT, Marcinkiewicz J. 1-Methylnicotinamide and nicotinamide: two related anti-inflammatory agents that differentially affect the functions of activated macrophages. Arch Immunol Ther Exp (Warsz) 2008;56:127–134. doi: 10.1007/s00005-008-0009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitterman KJ, Anderson RM, Cohen HY, Latorre-Esteves M, Sinclair DA. Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1. J Biol Chem. 2002;277:45099–45107. doi: 10.1074/jbc.M205670200. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang J, Li F, Maiese K. mGluRI Targets Microglial Activation and Selectively Prevents Neuronal Cell Engulfment Through Akt and Caspase Dependent Pathways. Curr Neurovasc Res. 2005a;2:197–211. doi: 10.2174/1567202054368317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Akt1 drives endothelial cell membrane asymmetry and microglial activation through Bcl-x(L) and caspase 1, 3, and 9. Exp Cell Res. 2004a;296:196–207. doi: 10.1016/j.yexcr.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Erythropoietin fosters both intrinsic and extrinsic neuronal protection through modulation of microglia, Akt1, Bad, and caspase-mediated pathways. Br J Pharmacol. 2003;138:1107–1118. doi: 10.1038/sj.bjp.0705161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002a;106:2973–2979. doi: 10.1161/01.cir.0000039103.58920.1f. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Cellular demise and inflammatory microglial activation during beta-amyloid toxicity are governed by Wnt1 and canonical signaling pathways. Cell Signal. 2007;19:1150–1162. doi: 10.1016/j.cellsig.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Erythropoietin requires NF-kappaB and its nuclear translocation to prevent early and late apoptotic neuronal injury during beta-amyloid toxicity. Curr Neurovasc Res. 2005b;2:387–399. doi: 10.2174/156720205774962683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Group I Metabotropic Receptor Neuroprotection Requires Akt and Its Substrates that Govern FOXO3a, Bim, and beta-Catenin During Oxidative Stress. Curr Neurovasc Res. 2006;3:107–117. doi: 10.2174/156720206776875830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: Novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol. 2005c;75:207–246. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Lin SH, Li F, Maiese K. The sirtuin inhibitor nicotinamide enhances neuronal cell survival during acute anoxic injury through Akt, Bad, PARP, and mitochondrial associated "antiapoptotic" pathways. Curr Neurovasc Res. 2005d;2:271–285. doi: 10.2174/156720205774322584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Lin SH, Maiese K. Nicotinamide Modulates Mitochondrial Membrane Potential and Cysteine Protease Activity during Cerebral Vascular Endothelial Cell Injury. J Vasc Res. 2002b;39:131–147. doi: 10.1159/000057762. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Lin SH, Maiese K. The NAD+ precursor nicotinamide governs neuronal survival during oxidative stress through protein kinase B coupled to FOXO3a and mitochondrial membrane potential. J Cereb Blood Flow Metab. 2004b;24:728–743. doi: 10.1097/01.WCB.0000122746.72175.0E. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Maiese K. Erythropoietin involves the phosphatidylinositol 3-kinase pathway, 14-3-3 protein and FOXO3a nuclear trafficking to preserve endothelial cell integrity. Br J Pharmacol. 2007a;150:839–850. doi: 10.1038/sj.bjp.0707161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Maiese K. The Src homology 2 domain tyrosine phosphatases SHP-1 and SHP-2: diversified control of cell growth, inflammation, and injury. Histol Histopathol. 2007b;22:1251–1267. doi: 10.14670/hh-22.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresto JC, Fabiano de Bruno LE, Cao GF, Pastorale CF, Confalonieri N, del Carmen Camberos M, Basabe JC. The association of acetyl-l-carnitine and nicotinamide remits the experimental diabetes in mice by multiple low-dose streptozotocin. Pancreas. 2006;33:403–411. doi: 10.1097/01.mpa.0000236740.07854.b1. [DOI] [PubMed] [Google Scholar]
- Crino A, Schiaffini R, Ciampalini P, Suraci MC, Manfrini S, Visalli N, Matteoli MC, Patera P, Buzzetti R, Guglielmi C, Spera S, Costanza F, Fioriti E, Pitocco D, Pozzilli P. A two year observational study of nicotinamide and intensive insulin therapy in patients with recent onset type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2005;18:749–754. doi: 10.1515/jpem.2005.18.8.749. [DOI] [PubMed] [Google Scholar]
- Dabelea D, Bell RA, D'Agostino RB, Jr, Imperatore G, Johansen JM, Linder B, Liu LL, Loots B, Marcovina S, Mayer-Davis EJ, Pettitt DJ, Waitzfelder B. Incidence of diabetes in youth in the United States. JAMA. 2007;297:2716–2724. doi: 10.1001/jama.297.24.2716. [DOI] [PubMed] [Google Scholar]
- Du B, Ohmichi M, Takahashi K, Kawagoe J, Ohshima C, Igarashi H, Mori-Abe A, Saitoh M, Ohta T, Ohishi A, Doshida M, Tezuka N, Takahashi T, Kurachi H. Both estrogen and raloxifene protect against beta-amyloid-induced neurotoxicity in estrogen receptor alpha-transfected PC12 cells by activation of telomerase activity via Akt cascade. J Endocrinol. 2004;183:605–615. doi: 10.1677/joe.1.05775. [DOI] [PubMed] [Google Scholar]
- Feng Y, Paul IA, LeBlanc MH. Nicotinamide reduces hypoxic ischemic brain injury in the newborn rat. Brain Res Bull. 2006;69:117–122. doi: 10.1016/j.brainresbull.2005.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye RA. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys Res Commun. 2000;273:793–798. doi: 10.1006/bbrc.2000.3000. [DOI] [PubMed] [Google Scholar]
- Gallo CM, Smith DL, Jr, Smith JS. Nicotinamide clearance by Pnc1 directly regulates Sir2-mediated silencing and longevity. Mol Cell Biol. 2004;24:1301–1312. doi: 10.1128/MCB.24.3.1301-1312.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozinger CM, Chao ED, Blackwell HE, Moazed D, Schreiber SL. Identification of a class of small molecule inhibitors of the sirtuin family of NAD-dependent deacetylases by phenotypic screening. J Biol Chem. 2001;276:38837–38843. doi: 10.1074/jbc.M106779200. [DOI] [PubMed] [Google Scholar]
- Harris SE, Fox H, Wright AF, Hayward C, Starr JM, Whalley LJ, Deary IJ. A genetic association analysis of cognitive ability and cognitive ageing using 325 markers for 109 genes associated with oxidative stress or cognition. BMC Genet. 2007;8:43. doi: 10.1186/1471-2156-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoane MR, Pierce JL, Holland MA, Anderson GD. Nicotinamide treatment induces behavioral recovery when administered up to 4 hours following cortical contusion injury in the rat. Neuroscience. 2008;154:861–868. doi: 10.1016/j.neuroscience.2008.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Wang Y, Wang L, Zhang H, Zhao B, Zhang A, Li Y. Effects of nicotinamide on prevention and treatment of streptozotocin-induced diabetes mellitus in rats. Chin Med J (Engl) 1996;109:819–822. [PubMed] [Google Scholar]
- Ieraci A, Herrera DG. Nicotinamide protects against ethanolinduced apoptotic neurodegeneration in the developing mouse brain. PLoS Med. 2006;3:e101. doi: 10.1371/journal.pmed.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MD, Schmidt MT, Oppenheimer NJ, Denu JM. Mechanism of nicotinamide inhibition and transglycosidation by Sir2 histone/protein deacetylases. J Biol Chem. 2003;278:50985–50998. doi: 10.1074/jbc.M306552200. [DOI] [PubMed] [Google Scholar]
- Jacobson AM, Musen G, Ryan CM, Silvers N, Cleary P, Waberski B, Burwood A, Weinger K, Bayless M, Dahms W, Harth J. Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med. 2007;356:1842–1852. doi: 10.1056/NEJMoa066397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JQ, Chong ZZ, Maiese K. Akt1 protects against inflammatory microglial activation through maintenance of membrane asymmetry and modulation of cysteine protease activity. J Neurosci Res. 2003a;74:37–51. doi: 10.1002/jnr.10740. [DOI] [PubMed] [Google Scholar]
- Kang JQ, Chong ZZ, Maiese K. Critical role for Akt1 in the modulation of apoptotic phosphatidylserine exposure and microglial activation. Mol Pharmacol. 2003b;64:557–569. doi: 10.1124/mol.64.3.557. [DOI] [PubMed] [Google Scholar]
- Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, Puigserver P, Sinclair DA, Tsai LH. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer's disease and amyotrophic lateral sclerosis. EMBO J. 2007;26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HI, Cho HJ, Han JA, Jang SY, Wang KM, Kang HT, Hwan ES. Transient downregulation of protein O-N-acetylglucosaminylation by treatment of high-dose nicotinamide in human cells. Exp Mol Med. 2008a;40:246–253. doi: 10.3858/emm.2008.40.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HI, Jang SY, Kang HT, Hwang ES. p53-, SIRT1-, and PARP-1-independent downregulation of p21WAF1 expression in nicotinamide-treated cells. Biochem Biophys Res Commun. 2008b;368:298–304. doi: 10.1016/j.bbrc.2008.01.082. [DOI] [PubMed] [Google Scholar]
- Li F, Chong ZZ, Maiese K. Cell Life Versus Cell Longevity: The Mysteries Surrounding the NAD(+) Precursor Nicotinamide. Curr Med Chem. 2006a;13:883–895. doi: 10.2174/092986706776361058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Chong ZZ, Maiese K. Microglial integrity is maintained by erythropoietin through integration of Akt and its substrates of glycogen synthase kinase-3beta, beta-catenin, and nuclear factorkappaB. Curr Neurovasc Res. 2006b;3:187–201. doi: 10.2174/156720206778018758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Chong ZZ, Maiese K. Navigating novel mechanisms of cellular plasticity with the NAD+ precursor and nutrient nicotinamide. Front Biosci. 2004;9:2500–2520. doi: 10.2741/1412. [DOI] [PubMed] [Google Scholar]
- Li F, Chong ZZ, Maiese K. Winding through the WNT pathway during cellular development and demise. Histol Histopathol. 2006c;21:103–124. doi: 10.14670/hh-21.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SH, Chong ZZ, Maiese K. Nicotinamide: A Nutritional Supplement that Provides Protection Against Neuronal and Vascular Injury. J Med Food. 2001;4:27–38. doi: 10.1089/10966200152053686. [DOI] [PubMed] [Google Scholar]
- Lin SH, Maiese K. The metabotropic glutamate receptor system protects against ischemic free radical programmed cell death in rat brain endothelial cells. J Cereb Blood Flow Metab. 2001;21:262–275. doi: 10.1097/00004647-200103000-00010. [DOI] [PubMed] [Google Scholar]
- Lin SH, Vincent A, Shaw T, Maynard KI, Maiese K. Prevention of nitric oxide-induced neuronal injury through the modulation of independent pathways of programmed cell death. J Cereb Blood Flow Metab. 2000;20:1380–1391. doi: 10.1097/00004647-200009000-00013. [DOI] [PubMed] [Google Scholar]
- Liu HK, Green BD, Flatt PR, McClenaghan NH, McCluskey JT. Effects of long-term exposure to nicotinamide and sodium butyrate on growth, viability, and the function of clonal insulin secreting cells. Endocr Res. 2004;30:61–68. doi: 10.1081/erc-120028485. [DOI] [PubMed] [Google Scholar]
- Maiese K. Diabetic stress: new triumphs and challenges to maintain vascular longevity. Expert Rev Cardiovasc Ther. 2008a;6:281–284. doi: 10.1586/14779072.6.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K. Triple play: Promoting neurovascular longevity with nicotinamide, WNT, and erythropoietin in diabetes mellitus. Biomed Pharmacother. 2008b;62:218–232. doi: 10.1016/j.biopha.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ. Nicotinamide: necessary nutrient emerges as a novel cytoprotectant for the brain. Trends Pharmacol Sci. 2003;24:228–232. doi: 10.1016/S0165-6147(03)00078-6. [DOI] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Hou J, Shang YC. Erythropoietin and oxidative stress. Curr Neurovasc Res. 2008a;5:125–142. doi: 10.2174/156720208784310231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Li F, Shang YC. Erythropoietin: Elucidating new cellular targets that broaden therapeutic strategies. Prog Neurobiol. 2008b;85:194–213. doi: 10.1016/j.pneurobio.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Shang YC. Mechanistic insights into diabetes mellitus and oxidative stress. Curr Med Chem. 2007a;14:1729–1738. doi: 10.2174/092986707781058968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Shang YC. OutFOXOing disease and disability: the therapeutic potential of targeting FoxO proteins. Trends Mol Med. 2008c;14:219–227. doi: 10.1016/j.molmed.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Shang YC. Raves and risks for erythropoietin. Cytokine Growth Factor Rev. 2008d;19:145–155. doi: 10.1016/j.cytogfr.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Shang YC. "Sly as a FOXO": New paths with Forkhead signaling in the brain. Curr Neurovasc Res. 2007b;4:295–302. doi: 10.2174/156720207782446306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Shang YC, Hou J. Rogue proliferation versus restorative protection: where do we draw the line for Wnt and forkhead signaling? Expert opinion on therapeutic targets. 2008e;12:905–916. doi: 10.1517/14728222.12.7.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. JAMA. 2005;293:90–95. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Li F, Chong ZZ, Shang YC. The Wnt signaling pathway: Aging gracefully as a protectionist? Pharmacol Ther. 2008f;118:58–81. doi: 10.1016/j.pharmthera.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Morhan SD, Chong ZZ. Oxidative stress biology and cell injury during type 1 and type 2 diabetes mellitus. Curr Neurovasc Res. 2007c;4:63–71. doi: 10.2174/156720207779940653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallat M, Marin-Teva JL, Cheret C. Phagocytosis in the developing CNS: more than clearing the corpses. Curr Opin Neurobiol. 2005;15:101–107. doi: 10.1016/j.conb.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Matsuzaki H, Tamatani M, Mitsuda N, Namikawa K, Kiyama H, Miyake S, Tohyama M. Activation of Akt kinase inhibits apoptosis and changes in Bcl-2 and Bax expression induced by nitric oxide in primary hippocampal neurons. J Neurochem. 1999;73:2037–2046. [PubMed] [Google Scholar]
- Memisogullari R, Bakan E. Levels of ceruloplasmin, transferrin, and lipid peroxidation in the serum of patients with Type 2 diabetes mellitus. J Diabetes Complications. 2004;18:193–197. doi: 10.1016/S1056-8727(03)00032-1. [DOI] [PubMed] [Google Scholar]
- Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–563. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- Nakagami Y, Nishimura S, Murasugi T, Kubo T, Kaneko I, Meguro M, Marumoto S, Kogen H, Koyama K, Oda T. A novel compound RS-0466 reverses beta-amyloid-induced cytotoxicity through the Akt signaling pathway in vitro. Eur J Pharmacol. 2002;457:11–17. doi: 10.1016/s0014-2999(02)02657-2. [DOI] [PubMed] [Google Scholar]
- Nakka VP, Gusain A, Mehta SL, Raghubir R. Molecular mechanisms of apoptosis in cerebral ischemia: multiple neuroprotective opportunities. Mol Neurobiol. 2008;37:7–38. doi: 10.1007/s12035-007-8013-9. [DOI] [PubMed] [Google Scholar]
- Nemoto S, Fergusson MM, Finkel T. Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science. 2004;306:2105–2108. doi: 10.1126/science.1101731. [DOI] [PubMed] [Google Scholar]
- Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, Moncada S, Carruba MO. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- Okouchi M, Ekshyyan O, Maracine M, Aw TY. Neuronal apoptosis in neurodegeneration. Antioxid Redox Signal. 2007;9:1059–1096. doi: 10.1089/ars.2007.1511. [DOI] [PubMed] [Google Scholar]
- Olmos PR, Hodgson MI, Maiz A, Manrique M, De Valdes MD, Foncea R, Acosta AM, Emmerich MV, Velasco S, Muniz OP, Oyarzun CA, Claro JC, Bastias MJ, Toro LA. Nicotinamide protected first-phase insulin response (FPIR) and prevented clinical disease in first-degree relatives of type-1 diabetics. Diabetes Res Clin Pract. 2006;71:320–333. doi: 10.1016/j.diabres.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcu M, Chiarugi A. The emerging therapeutic potential of sirtuin-interacting drugs: from cell death to lifespan extension. Trends Pharmacol Sci. 2005;26:94–103. doi: 10.1016/j.tips.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Reddy S, Bibby NJ, Wu D, Swinney C, Barrow G, Elliott RB. A combined casein-free-nicotinamide diet prevents diabetes in the NOD mouse with minimum insulitis. Diabetes Res Clin Pract. 1995a;29:83–92. doi: 10.1016/0168-8227(95)01109-9. [DOI] [PubMed] [Google Scholar]
- Reddy S, Salari-Lak N, Sandler S. Long-term effects of nicotinamide-induced inhibition of poly(adenosine diphosphateribose) polymerase activity in rat pancreatic islets exposed to interleukin-1 beta. Endocrinology. 1995b;136:1907–1912. doi: 10.1210/endo.136.5.7720637. [DOI] [PubMed] [Google Scholar]
- Ruifrok WP, de Boer RA, Westenbrink BD, van Veldhuisen DJ, van Gilst WH. Erythropoietin in cardiac disease: new features of an old drug. Eur J Pharmacol. 2008;585:270–277. doi: 10.1016/j.ejphar.2008.01.054. [DOI] [PubMed] [Google Scholar]
- Saunders LR, Verdin E. Sirtuins: critical regulators at the crossroads between cancer and aging. Oncogene. 2007;26:5489-–5504. doi: 10.1038/sj.onc.1210616. [DOI] [PubMed] [Google Scholar]
- Slomka M, Zieminska E, Lazarewicz J. Nicotinamide and 1-methylnicotinamide reduce homocysteine neurotoxicity in primary cultures of rat cerebellar granule cells. Acta Neurobiol Exp (Wars) 2008a;68:1–9. doi: 10.55782/ane-2008-1666. [DOI] [PubMed] [Google Scholar]
- Slomka M, Zieminska E, Salinska E, Lazarewicz JW. Neuroprotective effects of nicotinamide and 1-methylnicotinamide in acute excitotoxicity in vitro. Folia Neuropathol. 2008b;46:69–80. [PubMed] [Google Scholar]
- Tang BL, Chua CE. SIRT1 and neuronal diseases. Mol Aspects Med. 2008;29:187–200. doi: 10.1016/j.mam.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Thiagarajan P, Tait JF. Binding of annexin V/placental anticoagulant protein I to platelets. Evidence for phosphatidylserine exposure in the procoagulant response of activated platelets. J Biol Chem. 1990;265:17420–17423. [PubMed] [Google Scholar]
- Thomas DD, Ridnour LA, Isenberg JS, Flores-Santana W, Switzer CH, Donzelli S, Hussain P, Vecoli C, Paolocci N, Ambs S, Colton CA, Harris CC, Roberts DD, Wink DA. The chemical biology of nitric oxide: implications in cellular signaling. Free Radic Biol Med. 2008;45:18–31. doi: 10.1016/j.freeradbiomed.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Horst A, Schavemaker JM, Pellis-van Berkel W, Burgering BM. The Caenorhabditis elegans nicotinamidase PNC-1 enhances survival. Mech Ageing Dev. 2007;128:346–349. doi: 10.1016/j.mad.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- Won CK, Ji HH, Koh PO. Estradiol prevents the focal cerebral ischemic injury-induced decrease of forkhead transcription factors phosphorylation. Neurosci Lett. 2006;398:39–43. doi: 10.1016/j.neulet.2005.12.060. [DOI] [PubMed] [Google Scholar]
- Yano M, Hasegawa G, Ishii M, Yamasaki M, Fukui M, Nakamura N, Yoshikawa T. Short-term exposure of high glucose concentration induces generation of reactive oxygen species in endothelial cells: implication for the oxidative stress associated with postprandial hyperglycemia. Redox Rep. 2004;9:111–116. doi: 10.1179/135100004225004779. [DOI] [PubMed] [Google Scholar]