Abstract
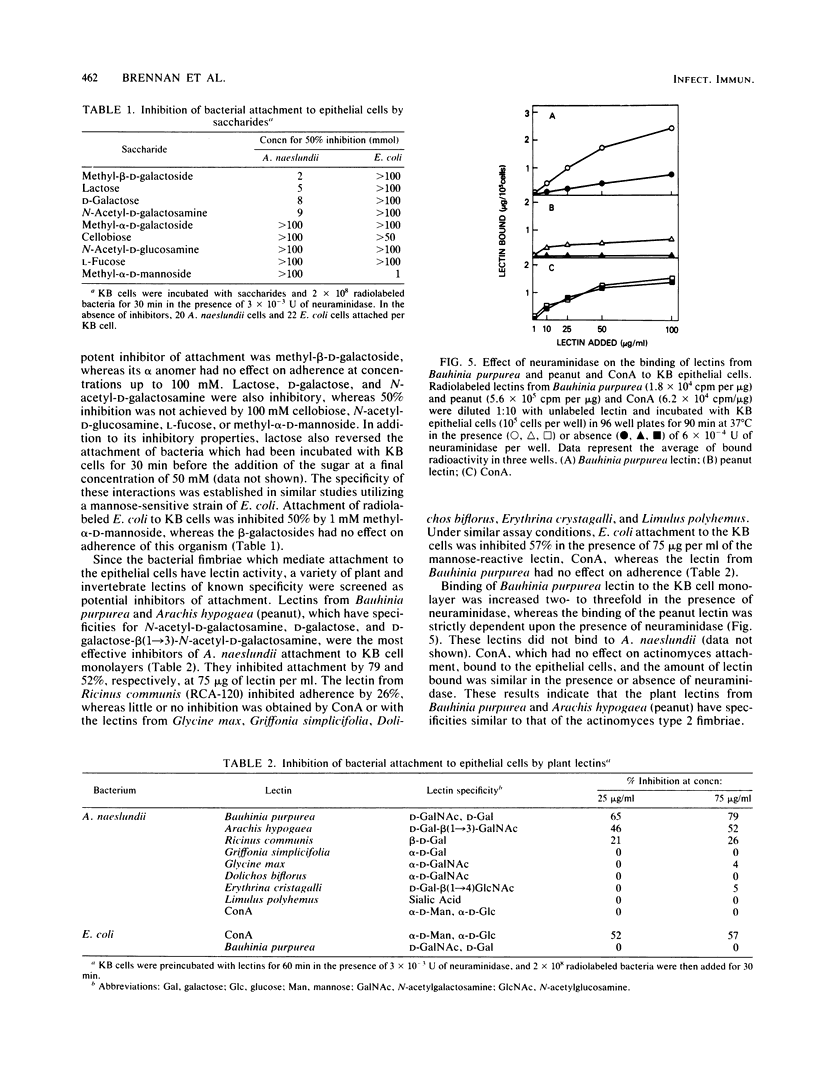
The adherence of Actinomyces naeslundii WVU45 to monolayer cultures of human epithelial cell lines was mediated by the lactose-sensitive fimbriae (type 2) of strain WVU45. The attachment of Actinomyces viscosus T14V, which has both types 1 and 2 fimbriae, was approximately half that of A. naeslundii, and only minimal attachment of A. naeslundii and A. viscosus mutants lacking type 2 fimbriae was detected. The adherence of strain WVU45 was enhanced two- to threefold by neuraminidase treatment of the epithelial cells. The Fab fragments of antibodies which recognize the type 2 fimbriae inhibited the adherence of A. naeslundii WVU45 to the epithelial cells. The bacterial interaction with epithelial cells was inhibited by lactose, methyl-beta-D-galactoside, and N-acetyl-D-galactosamine, but not by methyl-alpha-D-galactoside, cellobiose, N-acetyl-D-glucosamine, L-fucose, or D-mannose. To further characterize the epithelial cell receptors for the bacterial lectin, we utilized several plant and invertebrate lectins as potential inhibitors of bacterial adherence. Lectins from Bauhinia purpurea and Arachis hypogaea which recognize N-acetyl-D-galactosamine, D-galactose, and D-galactose-beta-(1----3)-N-acetyl-D-galactosamine inhibited bacterial attachment, and binding of these lectins to epithelial cells was enhanced by the addition of neuraminidase. Lectins reacting with alpha-linked D-galactose, alpha-linked N-acetyl-D-galactosamine, D-mannose, or sialic acid were not inhibitory. Under similar assay conditions, adherence of a mannose-sensitive strain of Escherichia coli was inhibited by concanavalin A but not by the lectin from Bauhinia purpurea. These results indicate that certain plant lectins have specificities similar to that of the actinomyces fimbrial lectin and are, therefore, useful probes for identifying receptors on epithelial cells for certain bacteria.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashwell G., Harford J. Carbohydrate-specific receptors of the liver. Annu Rev Biochem. 1982;51:531–554. doi: 10.1146/annurev.bi.51.070182.002531. [DOI] [PubMed] [Google Scholar]
- Cisar J. O., Barsumian E. L., Curl S. H., Vatter A. E., Sandberg A. L., Siraganian R. P. Detection and localization of a lectin on Actinomyces viscosus T14V by monoclonal antibodies. J Immunol. 1981 Oct;127(4):1318–1322. [PubMed] [Google Scholar]
- Cisar J. O., Curl S. H., Kolenbrander P. E., Vatter A. E. Specific absence of type 2 fimbriae on a coaggregation-defective mutant of Actinomyces viscosus T14V. Infect Immun. 1983 May;40(2):759–765. doi: 10.1128/iai.40.2.759-765.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar J. O., David V. A., Curl S. H., Vatter A. E. Exclusive presence of lactose-sensitive fimbriae on a typical strain (WVU45) of Actinomyces naeslundii. Infect Immun. 1984 Nov;46(2):453–458. doi: 10.1128/iai.46.2.453-458.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar J. O., Kolenbrander P. E., McIntire F. C. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979 Jun;24(3):742–752. doi: 10.1128/iai.24.3.742-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar J. O., Vatter A. E., McIntire F. C. Identification of the virulence-associated antigen on the surface fibrils of Actinomyces viscosus T14. Infect Immun. 1978 Jan;19(1):312–319. doi: 10.1128/iai.19.1.312-319.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Wheeler T. T., Cisar J. O. Specific inhibition of adsorption of Actinomyces viscosus T14V to saliva-treated hydroxyapatite by antibody against type 1 fimbriae. Infect Immun. 1984 Feb;43(2):497–501. doi: 10.1128/iai.43.2.497-501.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello A. H., Cisar J. O., Kolenbrander P. E., Gabriel O. Neuraminidase-dependent hamagglutination of human erythrocytes by human strains of Actinomyces viscosus and Actinomyces naeslundii. Infect Immun. 1979 Nov;26(2):563–572. doi: 10.1128/iai.26.2.563-572.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P. Establishment and distribution of Actinomyces viscosus and Actinomyces naeslundii in the human oral cavity. Infect Immun. 1976 Nov;14(5):1119–1124. doi: 10.1128/iai.14.5.1119-1124.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Fillery E. D., Chan K. H., Grove D. A. Sialidase-enhanced lectin-like mechanism for Actinomyces viscosus and Actinomyces naeslundii hemagglutination. Infect Immun. 1980 Feb;27(2):335–343. doi: 10.1128/iai.27.2.335-343.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Walker D. L., Chan K. H. Association of long surface appendages with adherence-related functions of the gram-positive species Actinomyces naeslundii. J Bacteriol. 1978 Jun;134(3):1171–1175. doi: 10.1128/jb.134.3.1171-1175.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentoft N., Dearborn D. G. Labeling of proteins by reductive methylation using sodium cyanoborohydride. J Biol Chem. 1979 Jun 10;254(11):4359–4365. [PubMed] [Google Scholar]
- Kaifu R., Osawa T. Syntheses of O-beta-D-galactopyranosyl-(1 leads to 3)-0-(2-acetamido-2-deoxy-alpha(and -beta)-D-galactopyranosyl)-N-tosyl-L-serine and their interaction with D-galactose-binding lectins. Carbohydr Res. 1979 Mar;69:79–88. doi: 10.1016/s0008-6215(00)85753-5. [DOI] [PubMed] [Google Scholar]
- Kaladas P. M., Kabat E. A., Iglesias J. L., Lis H., Sharon N. Immunochemical studies on the combining site of the D-galactose/N-acetyl-D-galactosamine specific lectin from Erythrina cristagalli seeds. Arch Biochem Biophys. 1982 Sep;217(2):624–637. doi: 10.1016/0003-9861(82)90544-6. [DOI] [PubMed] [Google Scholar]
- Lotan R., Skutelsky E., Danon D., Sharon N. The purification, composition, and specificity of the anti-T lectin from peanut (Arachis hypogaea). J Biol Chem. 1975 Nov 10;250(21):8518–8523. [PubMed] [Google Scholar]
- Ofek I., Mirelman D., Sharon N. Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors. Nature. 1977 Feb 17;265(5595):623–625. doi: 10.1038/265623a0. [DOI] [PubMed] [Google Scholar]
- Pereira M. E., Kabat E. A., Lotan R., Sharon N. Immunochemical studies on the specificity of the peanut (Arachis hypogaea) agglutinin. Carbohydr Res. 1976 Oct;51(1):107–118. doi: 10.1016/s0008-6215(00)84040-9. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revis G. J., Vatter A. E., Crowle A. J., Cisar J. O. Antibodies against the Ag2 fimbriae of Actinomyces viscosus T14V inhibit lactose-sensitive bacterial adherence. Infect Immun. 1982 Jun;36(3):1217–1222. doi: 10.1128/iai.36.3.1217-1222.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J. M., Miller C. H. Attachment of Actinomyces naeslundii to human buccal epithelial cells. Infect Immun. 1980 Sep;29(3):981–989. doi: 10.1128/iai.29.3.981-989.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J. M., Miller C. H. Neuraminidase-activated attachment of Actinomyces naeslundii ATCC 12104 to human buccal epithelial cells. J Dent Res. 1983 Oct;62(10):1038–1040. doi: 10.1177/00220345830620100501. [DOI] [PubMed] [Google Scholar]
- Wheeler T. T., Clark W. B. Fibril-mediated adherence of Actinomyces viscosus to saliva-treated hydroxyapatite. Infect Immun. 1980 May;28(2):577–584. doi: 10.1128/iai.28.2.577-584.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A. M., Kabat E. A., Gruezo F. G., Allen H. J. Immunochemical studies on the combining site of the D-galactopyranose and 2-acetamido-2-deoxy-D-galactopyranose specific lectin isolated from Bauhinia purpurea alba seeds. Arch Biochem Biophys. 1980 Oct 15;204(2):622–639. doi: 10.1016/0003-9861(80)90074-0. [DOI] [PubMed] [Google Scholar]



