Abstract
We describe the development of cell-penetrating inhibitors of Ras and study their ability to inhibit T cell activation. The inhibitors transduced T cells in a time and concentration-dependent manner and interacted with endogenous Ras. Anti-CD3/CD28-activated cells when treated with the inhibitors, exhibited a notable reduction in cell size, diminished proliferative capacity, and were more prone to apoptosis. Similarly, lymphocytes activated by antigen in vivo, exhibited accelerated apoptosis when treated with the inhibitors ex vivo. Our data reveal a pro-survival role for Ras in activated primary T cells and describe a new methodology for regulating its activity.
Structured summary
MINT-6802882: RAF1 (uniprotkb:P04049) physically interacts (MI:0218) with RAS (uniprotkb:P01112) by anti tag co-immunoprecipitation (MI:0007)
Abbreviations: RBD, Ras-binding domain of Raf-1; PTD, protein transduction domain; TCR, T cell antigen receptor; mBSA, methylated BSA; DTH, delayed type hypersensitivity
Keywords: Ras, Signalling inhibitor, Protein transduction domain, T cell activation, Apoptosis, T cell antigen receptor
1. Introduction
Ras proteins are small GTPases that control cell growth, differentiation, and apoptosis [1,2]. The family comprises three members, H-Ras, K-Ras and N-Ras with similar function. They regulate intracellular signalling such as the Raf-1/ERK [3] and PI3 kinase [4,5] cascades which are essential for survival and proliferation.
Many studies have demonstrated a role for Ras in immune cells. In T lymphocytes stimulation of the T cell antigen receptor (TCR) causes rapid accumulation of the active GTP-bound form of Ras [6], which in combination with other signals leads to cytokine gene expression and clonal expansion [7–9]. Recent reports have linked impaired Ras activation to induction of T cell anergy [10,11] highlighting the crucial role of this GTPase in determining the final outcome following TCR stimulation. However, the role of Ras during the different stages of activation of primary human T cells, or its role in animal models of inflammatory disease, has not been fully delineated.
In the present study, we describe the generation and testing of novel protein inhibitors of Ras, which contain the Ras-binding domain of Raf-1 (RBD), linked to the TAT protein transduction domain (PTD). RBD specifically binds to Ras, while TAT PTD enables heterogeneous proteins and other biological agents to enter cells [12,13]. We also test the effect of the Ras neutralizing mAb, Y13-259 [14], when linked to TAT PTD. Our data show that these reagents readily enter cells and have a dual function; they diminish growth and increase apoptosis of lymphocytes stimulated in vitro, although with varying efficiency, suggesting a pro-survival role for Ras in activated T cells. Furthermore, using a model of T cell mediated inflammation, we show that lymphocytes activated physiologically in vivo are similarly susceptible to apoptosis when exposed to the TAT-coupled Ras inhibitors.
2. Materials and methods
2.1. Cells, Abs, and reagents
Human PBMCs were isolated from heparinized venous blood by centrifugation over Ficoll-Hypaque (ICN Biomedicals, Aurora, OH) and cultured in RPMI 1640 medium containing 5% FCS, 2 mM l-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin. Splenocytes from C57Blk/6 mice were obtained by pushing spleens through a 70 μm cell strainer (BD Biosciences, Bedford, MA) and mononuclear cells were purified by Ficoll-Hypaque. The human leukemic T cell line Jurkat was maintained in the same medium as PBMCs and COS-7 cells were cultured in DMEM/10% FCS. All phosphor-specific antibodies were from Cell Signaling Technology (Beverly, MA), to Ras (Y13-259) from Santa Cruz Biotechnology (Santa Cruz, CA), and to anti-HA tag (mAb 12CA5) from Babco (Lakeside, CA). For stimulation of human and mouse T cells, the following combination of mAbs were used; anti-human CD3 (clone HIT3a)/CD28 (clone CD28.2), and anti-mouse CD3 (clone 145-2C11)/CD28 (clone 37.51) from eBioscience (San Diego, CA). Dynabeads coated with sheep anti-rat IgG and sheep anti-mouse IgG were from Dynal (Oslo, Norway). PD098059 and LY294002 were obtained from Calbiochem (La Jolla, CA) and farnesylthiosalicylic acid (FTS) from Biomol (Exeter, UK).
2.2. Expression constructs and purification of TAT-fusion proteins
The RBD domain of human Raf-1 gene (amino acids 50–130) was amplified with PCR using the forward primer 5′-GGAGGTACCCCTTCTAAGACAAGCAACA-3′ and the reverse primer 5′-GAGCATGCTCACAGGAAATCTACTTGAAGT-3′. For RBD-CRD (RCRD) (amino acids 50–220 of Raf-1 which contains the cysteine-rich domain adjacent to RBD) the same forward primer was used with the reverse primer 5′-GAGCATGCTCAAGACTCTCGCATACGACG-3′. PCR products were digested with KpnI/SphI and subcloned in frame into the corresponding sites of the pRSET-TAT-HA vector. This vector, a kind gift from S. Dowdy (UCSD, CA), has been described previously and contains an 6xHis epitope for protein purification, the TAT PTD, and the HA tag [15]. TAT-RHA, which contains the HA2 fusogenic peptide from influenza haemmaglutinin (it is different from the HA tag) upstream of the RBD domain, was constructed by commercially synthesizing the HA2-6xHis-TAT segment (GenScript, Piscataway, NJ). The HA2-6xHis-TAT was digested with XbaI/KpnI and inserted into the corresponding sites of pRSET-TAT-RBD to generate pRSET-TAT-RHA. DNA sequencing verified the accuracy of all expression constructs.
Purification of TAT-fusion proteins was performed under denaturing conditions using 8 M urea as previously described [15,16]. The eluted proteins were adjusted to 10% glycerol, and stored at −70 °C. To prevent aggregation proteins were kept at concentrations of 0.5 mg/ml. Y13-259 rat IgG1 was purified from culture supernatant using protein G-Sepharose (Amersham Biosciences, Uppsala, Sweden). Synthetic TAT peptide was commercially synthesized (Advanced Biotechnology Centre, Imperial College London) and covalently attached to the mAb by sulpho-SMCC cross-linking (Pierce, Rockford, IL) according to manufacturers instructions.
2.3. Cell stimulation, immunoprecipitation and Western blotting
For T cell stimulation, human PBMCs were incubated on ice for 10 min with 1 μg/ml each of anti-CD3 and anti-CD28 mAbs, followed by 5 min incubation at 37 °C after the addition of 1 μg/ml of anti-mouse cross-linking antibody. Stimulation of splenocytes was performed as above with mAbs specific for murine CD3 and CD28 receptors. Cell lysis, gel electrophoresis and Western blotting are described in the Supplementary text. For immunoprecipitation experiments, cells were incubated with TAT-reagents for 90 min, washed and stimulated with anti-CD3/CD28 mAbs as above. Alternatively, COS-7 cells, transfected with a G12V-Ras expressing plasmid were cultured for 24 h before incubation with TAT-proteins. Pre-cleared lysates were incubated with either anti-rat IgG, or anti-HA mAb and anti-mouse coated Dynabeads. Immune complexes were immunoblotted with Y13-259-HRP to visualize co-immunoprecipitated Ras.
2.4. Flow cytometry and apoptosis assay
TAT-reagents were labeled with FITC (NHS-Fluorescein, Pierce, Rockford, IL) and dialyzed extensively against PBS. Cells were incubated with FITC-TAT-reagents as indicated in figure legends, washed and treated with trypsin for 5 min at 37 °C to eliminate membrane bound proteins. After washing, cells were fixed and analyzed on a FACS Calibur. Apoptosis of T lymphocytes stimulated with anti-CD3/CD28 coated T cell expander Dynabeads (Dynal Biotech, Oslo, Norway) for 24 and 84 h, was measured by flow cytometry after staining with FITC-conjugated Annexin V and propidium iodide (BD Biosciences, Oxford, UK). Ex vivo apoptosis of murine lymphocytes isolated from inguinal lymph nodes of mice at 48 h post-challenge of a delayed type hypersensitivity (DTH) reaction, was measured after incubation with TAT-GFP control (20 μg/ml) or TAT-RBD (20 μg/ml) for 4 h. Cell size was assessed by the analysis of SSC versus FSC flow cytometry plots gated on the live cell population.
2.5. Carboxyfluorescein diacetate succinimidyl ester (CFSE) proliferation assay
Suspensions of PBMCs (1 × 106 cells/ml) in warm PBS/0.1% BSA were labeled with 2 μM CFSE (Molecular Probes, Eugene, OR) for 10 min at 37 °C. Staining was quenched with five volumes of ice-cold media and incubated on ice for 5 min. After washings CFSE-labeled cells were stimulated with anti-CD3/CD28 coated Dynabeads for 84 h and analyzed by flow cytometry, gating on the live cells.
2.6. Statistics
Statistical analysis was performed using ANOVA, followed by Dunnet post-hoc test. Data are expressed as mean ± S.E. of the mean.
3. Results
3.1. Generation of cell-transducing Ras inhibitors
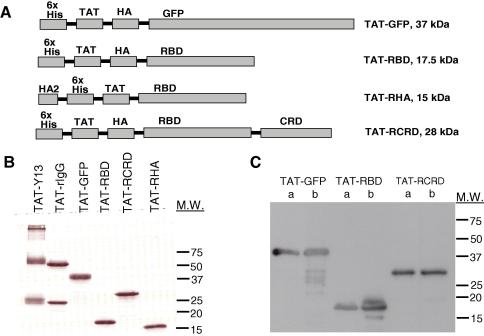
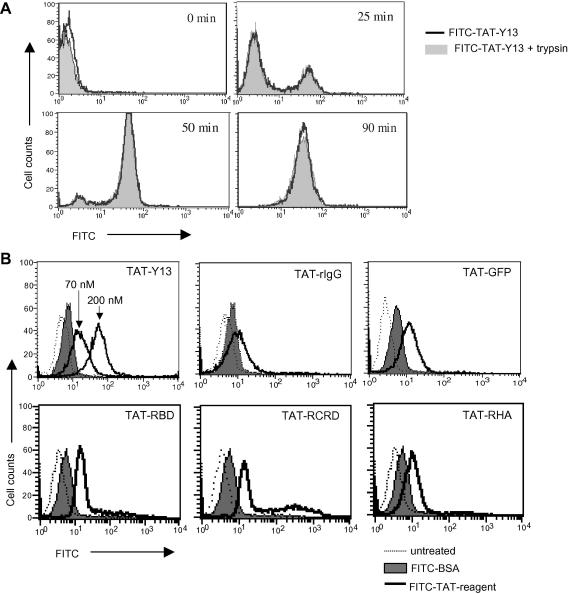
To develop a strategy for inhibition of Ras function in primary cells, we generated protein cell-transducing inhibitors specific for Ras. These were the neutralizing Y13-259 mAb [14] chemically crosslinked to the TAT peptide (TAT-Y13), and TAT-fusion proteins containing the RBD of Raf-1 expressed and purified from bacteria (Fig. 1A). TAT-GFP, TAT-RBD and TAT-RCRD also contained the HA tag; the HA epitope was not included in the TAT-RHA protein (Fig. 1B and C). Instead TAT-RHA contained at the N-terminus the fusogenic HA2 peptide from haemagglutinin influenza. The ability of FITC labeled TAT-Y13 or TAT-rat IgG control, and TAT-fusion proteins to transduce Jurkat T cells was confirmed by flow cytometry (Fig. 2A and B). Transduction by TAT-Y13 was observed within 25 min after protein addition to the culture medium reaching maximal transduction after 90 min (Fig. 2A). Treatment of cells with trypsin prior to analysis to eliminate surface bound protein (or heparin to dislodge proteins bound via charge; not shown), demonstrated that TAT-Y13 was internalized (Fig. 2A). The transduction ability of TAT-Y13 (Fig. 2B) and of TAT-fusion proteins (not shown) was concentration dependent. FITC-conjugated BSA was used as control for non-specific fluorescence.
Fig. 1.
Characterization of purified TAT-proteins. (A) Schematic representation of the TAT-linked inhibitors. (B) Purity of TAT-linked proteins established by Coomassie blue stain. (C) Purified proteins (a-0.25 μg, b-0.5 μg) probed with the anti-HA mAb, 12CA5. The migration distance of molecular weight (M.W.) markers is shown.
Fig. 2.
Cell transduction by purified TAT-linked proteins. (A) Kinetics of cell transduction was established by culturing Jurkat T cells with 20 μg/ml of FITC-conjugated TAT-Y13. At the indicated times, cells were collected and treated briefly with or without trypsin to eliminate non-specific cell surface binding. (B) Jurkat cells were incubated with 20 μg/ml of FITC-conjugated TAT-fusion proteins for 90 min. FITC-TAT-Y13 used at 70 and 200 nM confirmed the dose dependent manner of cell transduction. 20 μg/ml of FITC-conjugated BSA was used as control for non-specific binding.
3.2. Cell-penetrating proteins interact with endogenous Ras
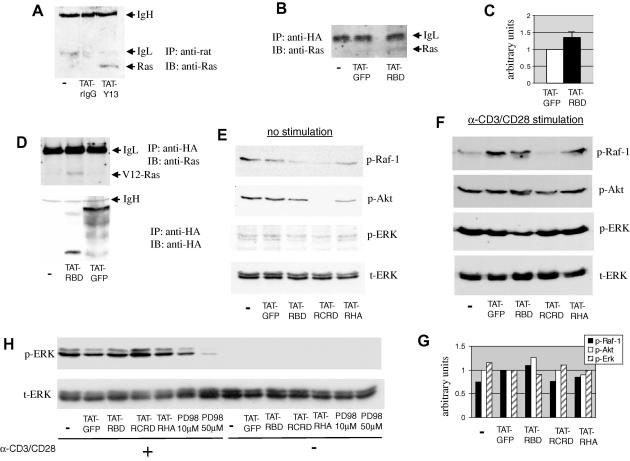
We next investigated whether internalized TAT-Y13 and TAT-RBD proteins could interact with intracellular Ras. Jurkat cells were incubated with TAT-Y13 or TAT-rIgG control, lysed and internalized mAb was immunoprecipitated with anti-rat mAb. TAT-Y13 was able to precipitate endogenous Ras as opposed to control antibody treated cells (Fig. 3A). Similarly, immunoprecipitation of internalized TAT-RBD with anti-HA mAb, showed a small but consistent increase in co-immunoprecipitated Ras as compared to the TAT-GFP control (Fig. 3B and C). The weaker co-immunoprecipitation of Ras with TAT-RBD could reflect the smaller pool of accessible RasGTP, as opposed to total Ras available to TAT-Y13. In additional experiments, to increase the cell pool of GTP-bound Ras, COS-7 cells were transfected with the G12V-Ras mutant which is deficient in GTP hydrolysis, before incubation with TAT-RBD. Fig. 3D again shows that internalized TAT-RBD associated with more Ras compared to TAT-GFP control.
Fig. 3.
Interaction of TAT proteins with endogenous Ras and inhibition of the homeostatic, but not the anti-CD3/CD28-induced, phosphorylation of Ras effectors. Jurkat cells were left untreated (−) or incubated with 20 μg/ml TAT-linked mAbs (A) or TAT-fusion proteins (B) for 90 min. In (A) internalized Abs were immunoprecipitated with anti-rat IgG, while in (B) cells were immunoprecipitated with anti-HA mAb. Co-immunoprecipitated endogenous Ras was detected by Western blotting. Representative data are shown from three similar observations. IgH and IgL denote the heavy and light chain of the immunoprecipitating antibody, respectively. (C) Cumulative data from three experiments of the intensity of the bands that correspond to co-immunoprecipitated Ras from cells incubated with TAT-GFP and TAT-RBD. P < 0.06. (D) COS-7 cells expressing the G12V-Ras mutant were incubated with 20 μg/ml of the indicated TAT-fusion proteins and internalized recombinant proteins were precipitated with anti-HA. Co-immunoprecipitated Ras was detected by Western blotting. Splenocytes cultured with 20 μg/ml of the indicated TAT-fusion proteins were left unstimulated (E) or were stimulated with anti-CD3/CD28 coated beads for 5 min (F). Equivalent amount of lysates were sequentially immunoblotted with antibodies specific for the phosphorylated/activated forms of c-Raf-1, Akt, and ERK1/2. Protein loading was assessed with an anti-ERK2 antibody. (G) Densitometric analysis of the blots shown in (F) normalized for protein loading according to the intensity of the bands in the t-ERK blot. Results are expressed in arbitrary units in which the TAT-GFP treatment control was set at the value of 1. (H) Jurkat cells were incubated with 20 μg/ml of TAT-fusion proteins and then stimulated for 5 min with anti-CD3/CD28 antibodies. ERK1/2 phosphorylation was determined as above. p-ERK, phosphorylated ERK; t-ERK, total ERK; PD98, MEK inhibitor PD098059. The blots in panel (E) were exposed considerably longer compared to blots in (F) and (G).
3.3. Ras inhibitors reduce the homeostatic phosphorylation of c-Raf-1, Akt, and ERK in T cells
To see if the cell-transducing inhibitors have an effect on the activity of Ras effectors, murine splenocytes cultured with TAT-fusion proteins and cell lysates were Western blotted with phospho-specific antibodies to Raf-1, Akt and ERK. In non-stimulated cells the RBD-containing proteins were able to reduce to varying degrees phosphorylation of all three signalling molecules (Fig. 3E). However, in anti-CD3/CD28 stimulated splenocytes the TAT-coupled inhibitors produced variable, albeit not consistent, inhibition of Raf-1, Akt and ERK1/2 phosphorylation (Fig. 3F). Densitometric analysis of the intensity of the bands in Fig. 3F, normalized for protein loading according to the t-ERK blot (4th panel), showed that TAT-RBD treatment reduced ERK phosphorylation but not p-Raf-1 or p-Akt, while TAT-RCRD reduced Raf-1 phosphorylation, which however was not translated into a measurable reduction in the phosphorylation of the downstream molecules Akt and ERK (Fig. 3G). Similarly, TAT-RHA treatment resulted in small reduction in p-Raf-1 and p-Akt, but not of p-ERK (Fig. 3G). Therefore, although some level of inhibition was produced by TAT-RBD-containing proteins in activated T cells, this reduction was not consistent possibly reflecting the strong nature of stimulation caused by the anti-CD3/CD28 beads. Similarly, no inhibition of ERK1/2 phosphorylation was seen in Jurkat cells following TCR stimulation (Fig. 3H), in contrast to the MEK inhibitor PD098059, used as positive control, which was inhibitory as expected [17]. These results could suggest that the TAT-RBD proteins are potent enough to reduce the homeostatic activity of Ras-controlled pathways in T cells, but not the strong burst of activation that follows in vitro stimulation of cells with anti-TCR antibodies.
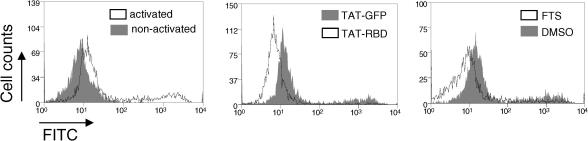
3.4. TAT-coupled proteins inhibit the increase in size and proliferation of activated T cells
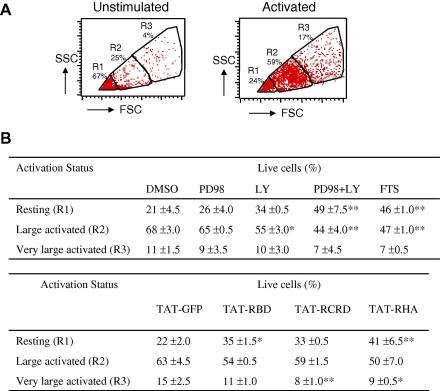
The strong but transient burst of activation after TCR triggering is followed by a low but sustained level of activation over a period of hours. This phase is required for T cell growth, as indicated by the substantial increase in cell size [18], and for proliferation [19]. To assess potential effects of the inhibitors over the extended period of stimulation, cell size was determined by flow cytometry using FSC versus SSC dot plot analysis. Live cells were sub-divided into three groups indicative of small non-activated (R1), large activated (R2), and very large activated cells (R3) (Fig. 4A). Treatment of anti-CD3/CD28-stimulated cells with PD098059 showed a small but measurable trend towards reduced cell size (Fig. 4B). However the reduction was much greater in cells treated with the pan-PI3K inhibitor, LY294002. The combination of the two inhibitors produced an even greater reduction in size with approximately 50% of the cells concentrating in the R1 gate (Fig. 4B). This reduction was equivalent to that produced by farnesylthiosalicylic acid (FTS), which delocalizes Ras from the plasma membrane [20], suggesting that the ERK and PI3K pathways cooperate to induce T cell growth. When cells were stimulated in the presence of TAT-fusion proteins, a reduction in cell size was noted in the RBD-containing chimeras as compared to GFP control, with an increase in the number of cells remaining in the R1 gate (Fig. 4B).
Fig. 4.
Ras regulates cell growth of TCR-activated lymphocytes. (A) Human PBMCs were left unstimulated or stimulated for 84 h with anti-CD3/CD28 and live cells were analyzed by flow cytometry for changes in their cell size. The percentage of resting (R1), large activated (R2) and very large activated (R3) cells were calculated by defining threshold gates around populations that changed after stimulation. (B) PBMCs were stimulated with anti-CD3/CD28 in the presence of the indicated chemical inhibitors or 20 μg/ml of TAT-fusion proteins for 84 h. Cells were sub-divided as in (A) and the relative percentage of cells in each gate was calculated. PD98, MEK inhibitor PD098059; LY, pan-PI3K inhibitor LY294002. Each percentage is the mean of triplicate readings. Significant differences from respective controls are indicated as ∗∗P < 0.01 and ∗P < 0.05. This experiment was repeated two additional times with similar results.
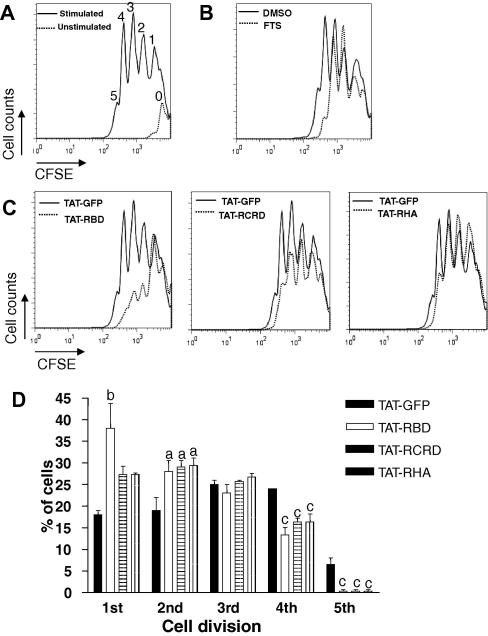
To see if the TAT-fusion proteins inhibit cell proliferation, human PBMCs were labeled with CFSE, stimulated with anti-CD3/CD28 mAbs, and 84 h later live cells were analyzed by flow cytometry. In the experiment shown in Fig. 5, up to five cell divisions are visible in activated cells, while the vast majority of non-activated cells fall within a single peak (Fig. 5A). FTS treatment reduced the number of cell divisions, particularly peaks that correspond to 4 and 5 cell divisions (Fig. 5B). Similarly, in cultures containing cell-permeable Ras inhibitors, a reduction in the number of cell divisions was clearly visible compared to TAT-GFP treated cultures, with the most notable reduction in the peaks that represent 3, 4 and 5 cell divisions (Fig. 5C). Statistical analysis of the data shows that incubation with TAT-RBD-containing proteins reduces the capacity of cells to undergo multiple divisions (Fig. 5D). The same result was obtained when BrdU incorporation was used as readout to assess the effect of TAT-RBD on murine splenocyte proliferation (Supplementary Fig. 1).
Fig. 5.
Ras inhibition reduces TCR-induced proliferation. CFSE-labeled human PBMCs were incubated with (A) TAT-GFP control, (B) FTS (50 μM), or (C) TAT-linked fusion proteins (20 μg/ml) prior to stimulation with anti-CD3/CD28 for 84 h. The numbers appearing above each peak in (A) indicate each cell division population, with fluorescence halving with each division. (D) Compilation of data, each bar showing the percentage of cells in each cell division ± S.E.M. of three triplicate experiments. Significant differences from respective TAT-GFP control are indicated as a, P < 0.05; b, P < 0.01, and c, P < 0.001.
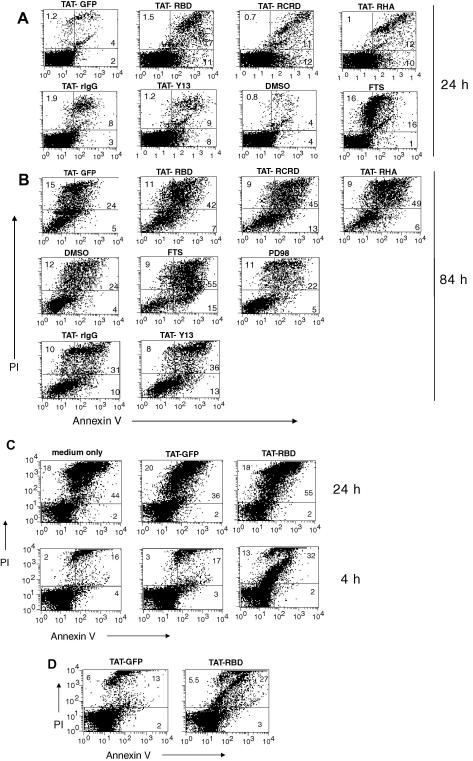
3.5. Increased apoptosis of activated T cells treated with cell-penetrating inhibitors of Ras
Ras may have a role in preventing apoptosis of stimulated T cells [21]. Therefore, we investigated whether the TAT-RBD proteins and TAT-T13 were able to increase apoptosis in stimulated T cells. At 24 h post-stimulation, in cells treated with TAT-RBD-containing proteins there was ∼5-fold increase in early apoptotic cells (positive only for annexin V) and 3–4-fold increases in late apoptotic/necrotic cells (double positive) compared to that in PBMCs treated with the TAT-GFP control (Fig. 6A). At 84 h there were still clear differences between RBD and control proteins, although the overall cell death had also increased (Fig. 6B). In cells treated with TAT-Y13 there was a small increase in early apoptotic cells versus control antibody both at 24 and 84 h (Fig. 6A and B), although overall this inhibitor was the least potent. FTS was most effective while the MEK inhibitor PD098059 did not increase apoptosis (Fig. 6B). Similar results were obtained when murine splenocytes were used instead of human PBMCs (data not shown). In cells that were left unstimulated, incubation with TAT-RBD for 24 h resulted in somewhat higher apoptosis as compared to TAT-GFP treatment, however, the overall apoptosis of non-stimulated cells significantly increased even without any treatment (Fig. 6C, top row). To bypass this problem we measured apoptosis of non-stimulated cells after 4 h treatment. At this time point while the overall cell apoptosis remained low, the TAT-RBD-treated culture showed higher apoptosis compared to TAT-GFP control, suggesting that Ras activity is required for homeostatic T cell survival (Fig. 6C, lower row).
Fig. 6.
TAT-RBD proteins increase apoptosis of activated lymphocytes. Human PBMCs were stimulated for 24 (A) or 84 (B) h with anti-CD3/CD28, stained with annexin V-FITC plus PI and analyzed by flow cytometry. Percentages indicate early apoptotic (annexin V+; PI−), late apoptotic/necrotic (annexin V+; PI+) and necrotic (annexin V−; PI+) cell populations. Data are representative of three separate experiments. (C) Non-stimulated human PBMCs were incubated with or without 20 μg/ml TAT-RBD or TAT-GFP proteins for 24 or 4 h after which cell apoptosis was measured as in (A). (D) Lymphocytes isolated from inguinal lymph nodes of mice at 48 h post-challenge of a DTH reaction were incubated with TAT-GFP or TAT-RBD for 4 h and then analyzed as in (A). Data are representative of two separate experiments. Statistical analysis of Annexin V+ cells from the two experiments revealed that the increase in apoptosis of TAT-RBD versus TAT-GFP-treated cells is significant, P < 0.002.
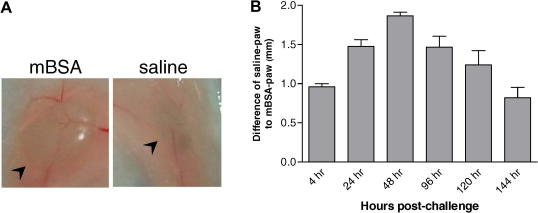
To see if the cell-penetrating Ras inhibitors accelerate apoptosis of cells activated in vivo, we used a model of DTH, which is T cell-mediated, antigen-driven inflammation. Following intradermal injection of methylated BSA (mBSA) in the paw in previously sensitized mice, swelling occurs as early as 4 h and peaks at 48 h before resolving after 6–7 days (Supplementary Fig. 2 and 3). Lymphocytes isolated from inguinal draining LNs of mice challenged with mBSA for 48 h were placed in culture in the presence of TAT-RBD or TAT-GFP control for 4 h after which they were double stained with annexin V and PI to measure apoptosis. As shown in Fig. 6D, cells cultured ex vivo with TAT-RBD were 2-fold more apoptotic as compared to TAT-GFP-treated cultures corroborating the results obtained with the in vitro activation of human PBMCs.
4. Discussion
Ras inhibitors suitable for in vivo application will be useful in down-regulating immune responses and inhibiting growth of certain types of cancer. Although pharmacologic inhibitors of post-translational processing of Ras are available, doubts have been raised as to their specificity [22]. We utilized protein transduction to introduce Ras-inhibitory molecules into cells by linking them to TAT PTD. We previously used this method to down-modulate NF-κB signalling in vivo [23]. Protein transduction has been used to introduce TAT-dominant negative Ras into cells and was shown to ameliorate airway inflammation [24]. Also, transduction of eoshinophils with TAT-dominant negative Ras, down-modulated IL5-mediated ERK activity and cell survival [25], and decreased cell adhesion mediated by beta-2 integrin binding to intracellular adhesion molecule-1 [26]. We chose inhibitors known for their high specificity; the neutralizing Y13-259 mAb [14] and the RBD of Raf-1. The RBD is the minimum region of Raf-1 that binds to active Ras with high affinity (Kd of 20 nM), whereas its affinity for RasGDP is 3 orders of magnitude lower [27]. We also investigated whether addition of CRD (residues 139–184) [28], or of the fusogenic peptide HA2 from influenza haemagglutinin [29] could increase potency over RBD alone.
The TAT-linked inhibitors transduced Jurkat cells and interacted with endogenous Ras (Fig. 2). RBD-containing TAT-fusion proteins were less effective in precipitating Ras compared to TAT-Y13 (Fig. 3). A possible explanation for this difference could be that while the Y13-259 mAb binds to both RasGTP and RasGDP, RBD-containing proteins interact with high affinity only with RasGTP. In agreement with this explanation, TAT-RBD precipitated more Ras when added to cells expressing the constitutively active G12V-Ras mutant (Fig. 3D).
In non-stimulated splenocytes, TAT-fusion inhibitors diminished phosphorylation of the Ras effectors Raf-1, Akt, and ERK (Fig. 3D), but only marginally inhibited the strong, although transient, phosphorylation after TCR stimulation. An explanation for this result might have to do with the strength of the anti-CD3/CD28 signal as delivered during in vitro activation. The sustained activation of the PI3K and ERK pathways determines proliferation and cell cycle progression [17,30]. In our experiments we confirmed the importance of these pathways in the activation of human T lymphocytes using pharmacological inhibitors (Fig. 4B). Similarly, the cell-penetrating Ras inhibitors diminished T cell activation as demonstrated by the reduction in cell size (Fig. 4B) and the decrease in the number of cell divisions as monitored by CFSE staining (Fig. 5) and BrdU incorporation (Supplement Fig. 1).
Next we tested whether the cell-penetrating inhibitors affect survival of activated lymphocytes. TCR stimulation in the presence of TAT-RBD proteins resulted in increased apoptosis (Fig. 6), as was ex vivo apoptosis of lymphocytes activated physiologically during a DTH reaction (Fig. 6C). The Y13-259 mAb has been shown to inhibit Ras signalling in Jurkat cells [14] however, in our study TAT-Y13 was the least effective among the TAT-coupled Ras inhibitors tested. A possible explanation may have to do with the different method of delivery employed. While in the published study a single chain Fv version of the antibody was expressed endogenously most likely resulting in high levels of intracellular antibody, in our study it was chemically coupled to TAT peptide and added exogenously. At present, we do not know the fate of the internalized mAb or how much of it targets to activated Ras as this may be a critical step determining its potency. Also, some variability between the 3 TAT-fusion proteins has been observed. For example, TAT-RHA was more effective in inhibiting cell growth (Fig. 4B) while TAT-RBD was more potent in reducing the number of cell divisions (Fig. 5C). It is possible that the presence of the hydrophobic HA2 peptide in TAT-RHA, which may destabilize endosomes, has an effect on cell growth. Overall, however, during the course of these experiments, all the RBD-containing fusion proteins were inhibitory compared to TAT-GFP control.
Ras can be either pro-apoptotic or anti-apoptotic depending on cell type, context and signal strength. For example, over-expression of active Ras in Jurkat cells leads to the expression of Fas ligand (FasL), whereas dominant negative Ras inhibits FasL expression in response to TCR stimulation [31]. In contrast, other studies have shown that Ras can protect cells from apoptosis [32], one mechanism being via activation of NF-κB and the subsequent expression of anti-apoptotic proteins [33]. Our results argue for a pro-survival role for Ras in activated T cells. Blocking the ERK and PI3K Ras effector pathways with chemical inhibitors shows that both cascades contribute to activation-induced T cell growth (Fig. 4). However, lack of complete inhibition may indicate that either these inhibitors were not entirely stable over the course of the experiment (84 h), or that additional independent signalling pathways exist that are capable of sustaining some T cell growth during activation. A K-Ras/Raf pathway that operates independent of MEK/ERK to induce growth during colon cancer progression has been recently reported [34].
The long-term goal of establishing the protein transduction technology is to regulate Ras signalling in vivo to tackle diseases such as autoimmunity and cancer. However, the difficulties of translating this technology into a viable therapeutic option in the clinic should not be underestimated. Potential obstacles could be that the protein inhibitors elicit an immune response preventing their repeated administration but also limiting their usefulness as down-modulators of immune responses. Another potential problem could be unwanted side effects since the inhibitors would be capable of penetrating a variety of cell types and tissues. Also, our incomplete understanding regarding the fate of internalized TAT-coupled proteins may limit its in vivo applications. Although association with the endogenous protein target has been observed, part of the internalized TAT-fusion inhibitors may be sequestered into intracellular compartments and become inaccessible [35]. Nevertheless, the testing of the TAT-coupled Ras inhibitors in animal models of disease will help us in addressing several critical questions about their in vivo usefulness.
In summary, we demonstrate here the feasibility of using a cell-permeable version of RBD to regulate the activity of Ras signalling in activated lymphocytes. TAT-RBD regulates T cell function by down-modulating TCR-mediated cell growth and proliferation, but also by inducing apoptosis of activated cells.
Acknowledgements
This work was supported by the Arthritis Research Campaign (ARC) UK, Grant ID 16018 to P.S.K. and D.W.G. The authors thank Nathan Blackwell for help with cell transduction experiments, Richard Marais for providing the Raf-1 cDNA and the G12V-Ras mutant.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.febslet.2008.11.042.
Appendix A. Supplementary data
Fig. S1.
TAT-RBD inhibits T cell proliferation in vitro. Murine splenocytes were activated in vitro for 72 h, in the presence of the indicated inhibitors or appropriate controls, and proliferation was assessed by BrdU incorporation.
Fig. S2.
Methylated-BSA model of DTH in the paw. (A) Images of inguinal lymph nodes (arrowhead) taken from sites draining mBSA (left panel) and saline (right panel) injections. (B) Time-course of inflammation in the right hind paw of mice challenged with mBSA (n=6).
Supplementary materials and methods.
References
- 1.Shields J.M., Pruitt K., McFall A., Shaub A., Der C.J. Understanding Ras: ‘it ain’t over’ ‘til it’s over’. Trends Cell Biol. 2000;10:147–154. doi: 10.1016/s0962-8924(00)01740-2. [DOI] [PubMed] [Google Scholar]
- 2.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat. Rev. Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 3.Marais R., Light Y., Paterson H.F., Marshall C.J. Ras recruits Raf-1 to the plasma membrane for activation by tyrosine phosphorylation. EMBO J. 1995;14:3136–3145. doi: 10.1002/j.1460-2075.1995.tb07316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez-Viciana P., Warne P.H., Dhand R., Vanhaesebroeck B., Gout I., Fry M.J., Waterfield M.D., Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 5.Gupta S. Binding of ras to phosphoinositide 3-kinase p110alpha is required for ras-driven tumorigenesis in mice. Cell. 2007;129:957–968. doi: 10.1016/j.cell.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 6.Downward J., Graves J.D., Warne P.H., Rayter S., Cantrell D.A. Stimulation of p21ras upon T-cell activation. Nature. 1990;346:719–723. doi: 10.1038/346719a0. [DOI] [PubMed] [Google Scholar]
- 7.Izquierdo M., Leevers S.J., Marshall C.J., Cantrell D. P21ras couples the T cell antigen receptor to extracellular signal-regulated kinase 2 in T lymphocytes. J. Exp. Med. 1993;178:1199–1208. doi: 10.1084/jem.178.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodrow M.A., Rayter S., Downward J., Cantrell D.A. P21ras function is important for T cell antigen receptor and protein kinase C regulation of nuclear factor of activated T cells. J. Immunol. 1993;150:3853–3861. [PubMed] [Google Scholar]
- 9.Baldari C.T., Heguy A., Telford J.L. Ras protein activity is essential for T-cell antigen receptor signal transduction. J. Biol. Chem. 1993;268:2693–2698. [PubMed] [Google Scholar]
- 10.Fields P.E., Gajewski T.F., Fitch F.W. Blocked Ras activation in anergic CD4+ T cells. Science. 1996;271:1276–1278. doi: 10.1126/science.271.5253.1276. [DOI] [PubMed] [Google Scholar]
- 11.Zha Y. T cell anergy is reversed by active Ras and is regulated by diacylglycerol kinase-alpha. Nat. Immunol. 2006;7:1166–1173. doi: 10.1038/ni1394. [DOI] [PubMed] [Google Scholar]
- 12.Schwarze S.R., Hruska K.A., Dowdy S.F. Protein transduction: unrestricted delivery into all cells? Trends Cell Biol. 2000;10:290–295. doi: 10.1016/s0962-8924(00)01771-2. [DOI] [PubMed] [Google Scholar]
- 13.Kabouridis P.S. Biological applications of protein transduction technology. Trends Biotechnol. 2003;21:498–503. doi: 10.1016/j.tibtech.2003.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Werge T.M., Baldari C.T., Telford J.L. Intracellular single chain Fv antibody inhibits Ras activity in T-cell antigen receptor stimulated Jurkat cells. FEBS Lett. 1994;351:393–396. doi: 10.1016/0014-5793(94)00892-2. [DOI] [PubMed] [Google Scholar]
- 15.Nagahara H. Transduction of full-length TAT fusion proteins into mammalian cells: TAT-p27Kip1 induces cell migration. Nat. Med. 1998;4:1449–1452. doi: 10.1038/4042. [DOI] [PubMed] [Google Scholar]
- 16.Kabouridis P.S., Hasan M., Newson J., Gilroy D.W., Lawrence T. Inhibition of NF-kB activity by a membrane-transducing mutant of IkBa. J. Immunol. 2002;169:2587–2593. doi: 10.4049/jimmunol.169.5.2587. [DOI] [PubMed] [Google Scholar]
- 17.Dumont F.J., Staruch M.J., Fischer P., DaSilva C., Camacho R. Inhibition of T cell activation by pharmacologic disruption of the MEK1/ERK MAP kinase or calcineurin signaling pathways results in differential modulation of cytokine production. J. Immunol. 1998;160:2579–2589. [PubMed] [Google Scholar]
- 18.Frauwirth K.A. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–777. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 19.Iezzi G., Karjalainen K., Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- 20.Haklai R., Weisz M.G., Elad G., Paz A., Marciano D., Egozi Y., Ben-Baruch G., Kloog Y. Dislodgment and accelerated degradation of Ras. Biochemistry. 1998;37:1306–1314. doi: 10.1021/bi972032d. [DOI] [PubMed] [Google Scholar]
- 21.Gomez J., Martinez A.C., Fernandez B., Garcia A., Rebollo A. Critical role of Ras in the proliferation and prevention of apoptosis mediated by IL-2. J. Immunol. 1996;157:2272–2281. [PubMed] [Google Scholar]
- 22.Pan J., Yeung S.C. Recent advances in understanding the antineoplastic mechanisms of farnesyltransferase inhibitors. Cancer Res. 2005;65:9109–9112. doi: 10.1158/0008-5472.CAN-05-2635. [DOI] [PubMed] [Google Scholar]
- 23.Blackwell N.M., Sembi P., Newson J.S., Lawrence T., Gilroy D.W., Kabouridis P.S. Reduced infiltration and increased apoptosis of leukocytes at sites of inflammation by systemic administration of a membrane-permeable IkappaBalpha repressor. Arthritis Rheum. 2004;50:2675–2684. doi: 10.1002/art.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Myou S., Zhu X., Myo S., Boetticher E., Meliton A.Y., Liu J., Munoz N.M., Leff A.R. Blockade of airway inflammation and hyperresponsiveness by HIV-TAT-dominant negative Ras. J. Immunol. 2003;171:4379–4384. doi: 10.4049/jimmunol.171.8.4379. [DOI] [PubMed] [Google Scholar]
- 25.Hall D.J., Cui J., Bates M.E., Stout B.A., Koenderman L., Coffer P.J., Bertics P.J. Transduction of a dominant-negative H-Ras into human eosinophils attenuates extracellular signal-regulated kinase activation and interleukin-5-mediated cell viability. Blood. 2001;98:2014–2021. doi: 10.1182/blood.v98.7.2014. [DOI] [PubMed] [Google Scholar]
- 26.Myou S., Zhu X., Boetticher E., Myo S., Meliton A., Lambertino A., Munoz N.M., Leff A.R. Blockade of focal clustering and active conformation in beta 2-integrin-mediated adhesion of eosinophils to intercellular adhesion molecule-1 caused by transduction of HIV TAT-dominant negative Ras. J. Immunol. 2002;169:2670–2676. doi: 10.4049/jimmunol.169.5.2670. [DOI] [PubMed] [Google Scholar]
- 27.de Rooij J., Bos J.L. Minimal Ras-binding domain of Raf1 can be used as an activation-specific probe for Ras. Oncogene. 1997;14:623–625. doi: 10.1038/sj.onc.1201005. [DOI] [PubMed] [Google Scholar]
- 28.Bondeva T., Balla A., Varnai P., Balla T. Structural determinants of Ras–Raf interaction analyzed in live cells. Mol. Biol. Cell. 2002;13:2323–2333. doi: 10.1091/mbc.E02-01-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wadia J.S., Stan R.V., Dowdy S.F. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat. Med. 2004;10:310–315. doi: 10.1038/nm996. [DOI] [PubMed] [Google Scholar]
- 30.Appleman L.J., van Puijenbroek A.A., Shu K.M., Nadler L.M., Boussiotis V.A. CD28 costimulation mediates down-regulation of p27kip1 and cell cycle progression by activation of the PI3K/PKB signaling pathway in primary human T cells. J. Immunol. 2002;168:2729–2736. doi: 10.4049/jimmunol.168.6.2729. [DOI] [PubMed] [Google Scholar]
- 31.Latinis K.M., Carr L.L., Peterson E.J., Norian L.A., Eliason S.L., Koretzky G.A. Regulation of CD95 (Fas) ligand expression by TCR-mediated signaling events. J. Immunol. 1997;158:4602–4611. [PubMed] [Google Scholar]
- 32.Khwaja A., Rodriguez-Viciana P., Wennstrom S., Warne P.H., Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grumont R., Lock P., Mollinari M., Shannon F.M., Moore A., Gerondakis S. The mitogen-induced increase in T cell size involves PKC and NFAT activation of Rel/NF-kappaB-dependent c-myc expression. Immunity. 2004;21:19–30. doi: 10.1016/j.immuni.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 34.Haigis K.M. Differential effects of oncogenic K-Ras and N-Ras on proliferation, differentiation and tumor progression in the colon. Nat. Genet. 2008;40:600–608. doi: 10.1038/ngXXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dietz G.P.H., Bähr M. Delivery of bioactive molecules into the cell: the Trojan horse approach. Mol. Cell Neurosci. 2004;27:85–131. doi: 10.1016/j.mcn.2004.03.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials and methods.