Abstract
Rhes is one of several signaling molecules preferentially expressed in the striatum. This GTP-binding protein affects dopamine-mediated signaling and behavior. Denervating the striatum of its dopaminergic inputs in adulthood reduces rhes mRNA expression. Here we show that dopamine depletion in adult rats by 6-hydroxydopamine caused a significant decrease in striatal Rhes protein levels as measured by Western blotting. The role of dopamine input on rhes mRNA induction during ontogeny was also examined. Rhes mRNA was measured on postnatal days 4, 6, 8, 10, 15, and 24 with in situ hybridization to determine its normal ontogeny. Signal in striatum was detectable, but very low, on postnatal day 4 and increased gradually to peak levels at days 15 and 24. Outside of the striatum, rhes mRNA was expressed at high levels in hippocampus and cerebellum during the postnatal period. Hippocampal signal was initially highest in CA3 and dentate gyrus, but shifted to higher expression in CA1 by the late postnatal period. Several other nuclei showed low levels of rhes mRNA during ontogeny. Depletion of dopamine by 6-hydroxydopamine injection on postnatal day 4 did not affect the ontogenetic development of rhes mRNA, such that expression did not differ statistically in lesioned versus vehicle-treated animals tested in adulthood. These findings suggest that although dopamine input is not necessary for the ontogenetic development of rhes mRNA expression, changes in both rhes mRNA and Rhes protein are integral components of the response of the adult striatum to dopamine depletion.
Keywords: dopamine, mRNA, in situ hybridization, caudate-putamen, nucleus accumbens
1. Introduction
As a site of converging synaptic inputs from different brain regions, the striatum expresses many signaling molecules at uniquely high levels relative to other areas of brain (Falk et al., 1999; Usui et al., 1994). For example, DARRP-32 (Walaas et al., 1983), Regulator of G Protein Signaling (RGS) 9-2 (Rahman et al., 1999; Thomas et al., 1998a), adenylyl cyclase V (Glatt and Snyder, 1993), Gγ7 (Cali et al., 1992; Watson et al., 1994), Gαolf (Herve et al., 1993), and STEP (Lombroso et al., 1993) are highly expressed in striatum. Rhes, the Ras Homolog Enriched in Striatum, is another such signaling molecule. Rhes is a 266 amino acid GTP-binding protein that is intermediate in size between small Ras-like G proteins and heterotrimeric G protein α subunits (Falk et al., 1999). It is most closely related to AGS1/Dexras1, a GTP-binding protein that has been shown to be activated by neuronal nitric oxide synthase (Fang et al., 2000), to inhibit Gαi-mediated signaling (Graham et al., 2002; Takesono et al., 2002), and to modulate circadian rhythms through interaction with NMDA receptor signaling and Gαi/o proteins (Cheng et al., 2004). Although the full extent of the functioning of Rhes is not yet known, studies of its mechanism of action at the cellular level have shown that Rhes inhibits activation of Gαs when heterologously expressed in PC12 cells (Vargiu et al, 2004) and facilitates dopamine (DA) D2 receptor-mediated activation of Gαi/o (Errico et al., 2008). Furthermore, it is inhibitory to the expression of Gαolf and to phosphorylation of GluR1, as both of these measures are increased in rhes-/- mice (Errico et al., 2008).
Behavioral studies in rhes-/- mutant mice have provided further clues to the role of Rhes protein in striatal function. Early studies on these mice showed a decreased latency to fall from a rotarod compared with wild type littermates, in keeping with a role for the striatum in coordinated motor behavior. Rhes-/- mice showed decreased locomotor activity during the first 5 minutes in an open field, but no decrements in learning or memory. Female rhes-/- mice showed more anxiety than wild type littermates in the elevated plus maze, reversing the wild type profile of males showing more anxiety than females (Spano et al., 2004). More recently, with a strain of Rhes mutants congenic on a C57 background, a specific role in DA-mediated behavior has been demonstrated. Rhes-/- mice are more sensitive than wild type littermates to locomotor activation produced by a D1 agonist and to catalepsy produced by a D2 antagonist (Errico et al., 2008). Work in our laboratory on the same strain of Rhes mutant mice has characterized the role of Rhes in D1 and/or D2 receptor-mediated behaviors: Rhes is inhibitory to stereotypy induced by co-activation of D1 and D2 receptors or by activation of D2 receptors alone; however, it is necessary for the full expression of the D1-mediated behavior of grooming (Quintero et al., in press).
The many neurotransmitter and hormone inputs to the striatum are potential modulators of Rhes expression. For example, thyroid hormone is known to be important for striatal development. Hypothyroidism induced during development caused profound decreases in both rhes mRNA and Rhes protein, with the decrease in mRNA quickly reversed by T3 treatment (Vargiu et al., 2001). Although initial studies did not detect changes in rhes mRNA in adult hypothyroid rats (Vargiu et al., 2001), others have recently observed decreases in rhes mRNA in adult hypothyroid mice by RT-PCR (Vallortigara et al., 2008). Dopamine depletion in adult rats through either 6-hydroxydopamine (6-OHDA) lesion or reserpine treatment decreases rhes mRNA expression throughout striatum [including caudate-putamen (CPu), nucleus accumbens (NAc), and olfactory tubercle (OT)]. Furthermore, this decrease in rhes expression is strictly correlated with conditions of dopamine receptor supersensitivity, as manipulations of dopamine systems that do not result in profound receptor supersensitivity (e.g. chronic antagonist treatment) do not alter rhes expression (Harrison and LaHoste, 2006).
These behavioral and gene expression results thus suggest a two-fold relationship with DA systems: Rhes affects DA receptor function, and DA input to the striatum affects levels of Rhes expression. Here we have investigated the extent to which DA input affects Rhes expression. Although DA depletion has previously been shown to decrease rhes mRNA, its effect on expression of Rhes protein has not been measured. We have now measured Rhes protein levels after 6-OHDA lesion, which represents a stable and permanent removal of DA input. We also tested whether removal of DA input early during development, when rhes mRNA is barely detectable, would prevent its induction to adult levels. To this end, we also characterized the postnatal expression of rhes mRNA throughout brain.
2. Results
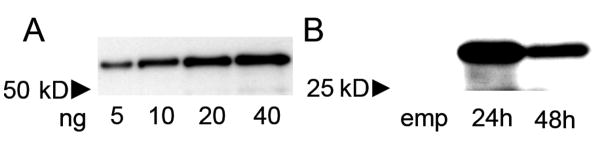
Before testing for denervation-induced changes in Rhes protein, we first tested the validity of the custom-made Rhes antibody. This antibody recognizes an 18-amino acid peptide in the C-terminal region of the protein (GDAFHPRPFCMRRTKVAG). No signal was detected with the use of pre-immune serum (not shown). In Western blots, the antibody detected a GST-tagged purified recombinant Rhes protein (Figure 1a) and detected a ∼30 kDa protein in Rhes-transfected, but not in empty vector-transfected, CHO-K1 cells (Figure 1b).
Figure 1.
An anti-peptide antibody recognizes Rhes protein. (a) Western blot of increasing amounts of a recombinant GST-tagged Rhes protein (MW = 55.5 kD). (b) CHO-K1 cells were transfected with either empty vector (pcDNA3.1; lane 1, emp) or pcDNA3.1 with an insert encoding the 30 kD form of Rhes protein, and harvested either 24h or 48h later (lanes 2 and 3). Western blot of 20 μg of total protein from cell lysates shows that the Ab recognizes a ∼30 kD band specifically in the Rhes-transfected cells.
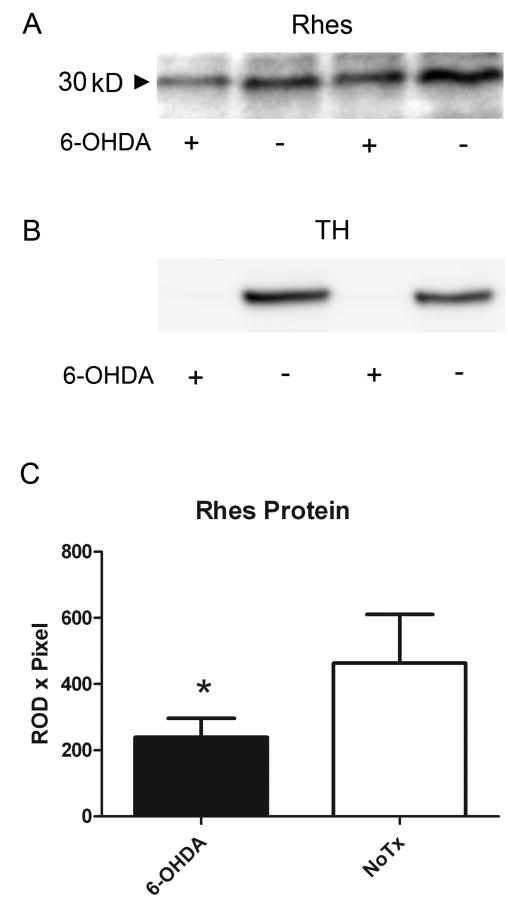
We analyzed Rhes protein expression after DA depletion by 6-OHDA in order to determine if the documented decrease in mRNA expression is accompanied by a decrease in protein expression. Three weeks post-surgery, animals fitting the criterion of contralateral rotation after apomorphine injection (0.25 mg/kg) were killed, and their left and right striata were individually analyzed for Rhes protein expression level. As shown in Figure 2, Rhes protein was significantly decreased in the 6-OHDA-treated striata relative to unlesioned striata (Figure 2a and c; p<0.05, paired t test). The same striatal tissue was subjected to Western blot analysis for tyrosine hydroxylase (TH), the rate-limiting enzyme in DA synthesis. As shown in Figure 2b, TH immunoreactivity was undetectable in the lesioned striata, indicative of loss of innervation by DA-containing neurons.
Figure 2.
Rhes protein is significantly decreased in hemispheres of adult rats receiving 6-OHDA. (a) Western blot of Rhes protein from left and right striata of two representative rats. Lanes 1 and 2 are left and right striata, respectively, of a single rat, and lanes 3 and 4 are left and right striata, respectively, of a separate rat. (b) Western blot of TH in striata of the same rats as in (a). (c) Quantification of Western blots for Rhes protein indicating a significant decrease in Rhes protein in 6-OHDA-treated striata versus untreated striata. Data were analyzed by paired t-test; n = 8. * p<0.05.
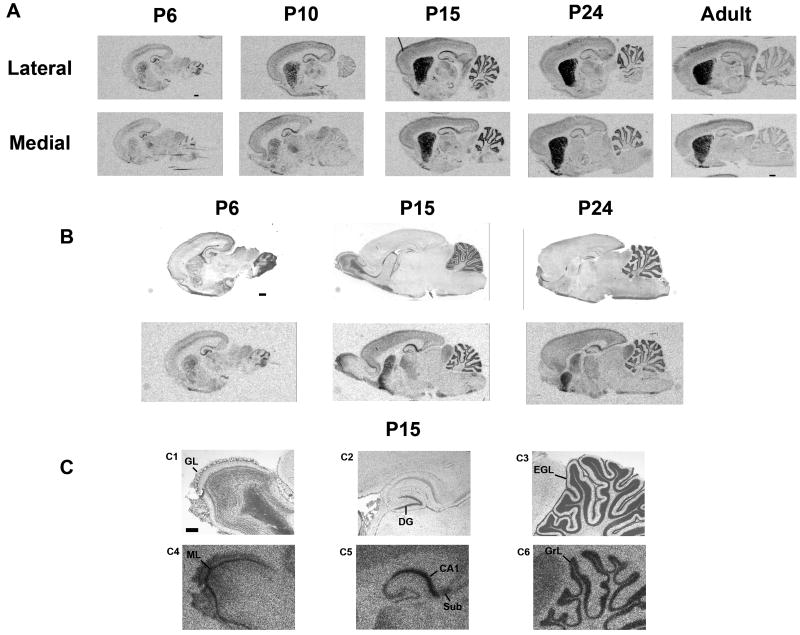
These results and our previous results measuring mRNA (Harrison and LaHoste, 2006) suggest that normal DA input is necessary in order to maintain Rhes expression. We therefore sought to test whether disruption of DA input during early ontogeny would prevent induction of rhes mRNA. Northern blotting from whole brain (Falk et al., 1999) indicated that appreciable levels of rhes mRNA emerge postnatally. Therefore, in order to determine the appropriate age at which to perform the DA depletion, we chose several postnatal time points (4, 6, 8, 10, 15, and 24 days) at which to examine the anatomical and temporal patterns of rhes mRNA induction in ontogeny. As an initial survey, we compared rhes mRNA in sagittal sections from rat pups at days 6, 10, 15, and 24, and in adults (Figure 3a). Some signal was detected in striatum at days 6 and 10, but high levels of signal were not detected until day 15. Signal increased slightly between days 15 and 24, but decreased slightly from day 24 to adulthood. The medial-to-lateral gradient of increasing signal that has been previously described in adult rats (Harrison and LaHoste, 2006) was readily apparent on all days examined. Outside of the striatum, signal in hippocampus and cerebellum was higher in neonates than in adults. The details of these extra-striatal signals were examined more closely by staining hybridized sections with cresyl violet. Within hippocampal formation, signal appeared to shift during development from being predominantly in CA3 and dentate gyrus on day 6 to CA1 and CA2 on days 15 and 24 (Figure 3b and c). Rhes mRNA is also apparent in the subiculum (Figure 3c, C2 and C5). Within the cerebellum, rhes mRNA was located in the granular layer and was absent from the external germinal layer on day 15 (3c, C3 and C6). The mitral cell layer of olfactory bulb also displays prominent signal on day 15 (Figure 3c, C1 and C4). Light signal was also detected developmentally in inferior colliculus (IC) and thalamus (Figure 3). In order to examine further all of these developmental patterns, we analyzed rhes mRNA in coronal sections throughout brain.
Figure 3.
Rhes mRNA expression in the neonatal period determined by in situ hybridization, sagittal view. Rat pups were sacrificed for analysis on postnatal days 6, 10, 15 and 24, and as adults. A, Medial and lateral sections from rats of the stated ages showing the medial-to-lateral gradient of rhes mRNA in CPu. Hippocampal and cerebellar signal is higher in neonates than in adults. B, Cresyl violet stained sections (top panel) delineate layers of rhes mRNA expression in the same sections (bottom panel). C, higher magnification of P15 from panel B. C1-C3 are cresyl violet-stained sections; C4-C6 show rhes mRNA in the same sections. scale bar = 1 mm (A, B) or 500 μM (C). GL = glomerular layer; DG = dentate gyrus; EGL = external germinal layer; ML = mitral cell layer; Sub = subiculum; GrL = granular cell layer.
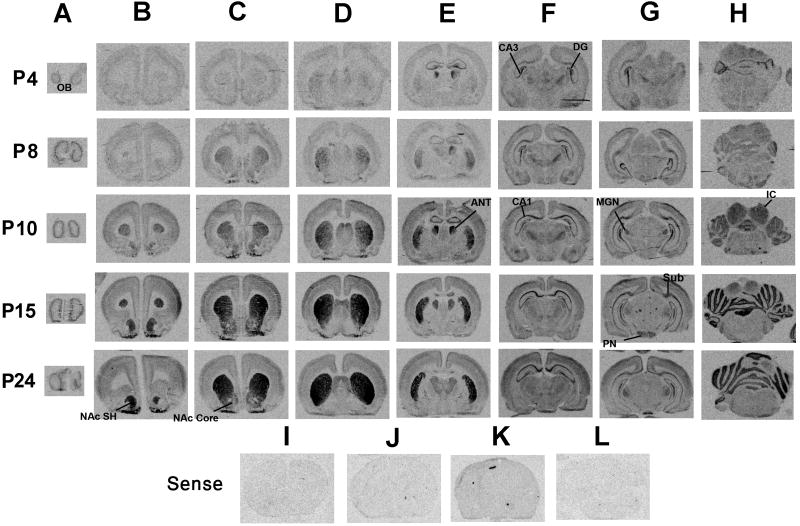
The coronal view, shown in Figure 4, confirms the observations from the sagittal view and allows the visualization of some structures not readily apparent in the sagittal plane. On day 4, the earliest age examined, there are very low levels of rhes mRNA in striatum, particularly in anterior CPu and NAc. Some light signal is detected in middle and posterior CPu, and in olfactory bulbs. As in the sagittal plane on day 6, much higher levels of mRNA expression are detected outside of the striatum, particularly in anterior thalamic nuclei, hippocampus, and cerebellum. There is also light signal in other thalamic nuclei. At later days, the pattern of striatal signal induction apparent in the sagittal plane is also detected here, with a gradual increase in signal up to day 15 and a medial-to-lateral gradient of increasing expression. The coronal plane allows visualization of a striking signal in anterior thalamic nuclei that peaks on day 10 before decreasing on days 15 and 24 (Figure 4, column E). The shift in location of hippocampal signal is again apparent (Figure 4, column F), and light signal is detected in medial geniculate nucleus and pontine nuclei (Figure 4, column G). Rhes mRNA in cortex is restricted to superficial layers, especially parietal and occipital cortices on days 10 and 15 (Figure 4, columns F and G). No signal was detected in sections hybridized with sense probe (Figure 4I-L).
Figure 4.
Rhes mRNA expression in the neonatal period as determined by in situ hybridization, coronal view. Rat pups were sacrificed for analysis on postnatal days 4, 8, 10, 15, and 24. Rhes mRNA expression increased gradually during this time in striatum (B, C, D, and E), CA1 of hippocampal formation (F), and cerebellum (H). No signal was detected with a 35S-labeled sense riboprobe in anterior CPu (I), posterior CPu (J), hippocampus (K), or cerebellum (L). OB, olfactory bulbs; NAc SH, shell of nucleus accumbens; NAc core, core of nucleus accumbens; ANT, anterior nucleus of the thalamus; DG, dentate gyrus; Sub, subiculum; MGN, medial geniculate nucleus; PN, pontine nuclei; IC inferior colliculus.
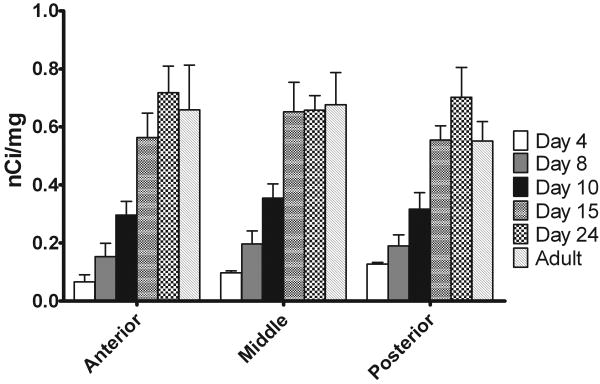
Rhes mRNA in whole striatum of coronal sections was quantified across ages with respect to standards exposed to the same film. In some hybridization runs, adult tissue was also examined for comparison. As shown in Figure 5, signal was detectable, although very low, at day 4, the earliest day examined. Increases in expression occurred up to day 15, at which point the signal generally stabilized to adult levels.
Figure 5.
Quantification of rhes mRNA during neonatal development. Rhes mRNA increased gradually until day 15, at which point it stabilized at adult levels. Autoradiographs of in situ hybridization were quantified according to 14C standards exposed to the same film. Whole striata were manually outlined, and a value of mean density was obtained. For each animal, 2-4 samples were taken from adjacent striatal sections. n = 2-3.
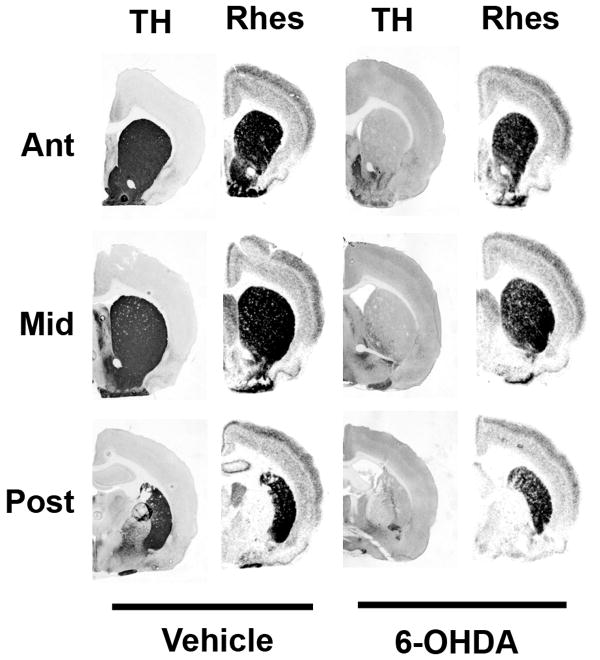
Based on this time course of ontogenetic rhes mRNA expression, we chose to perform DA depletion on Day 4, in order to test whether removal of DA input would prevent induction of striatal rhes mRNA to adult levels. Rats receiving neonatal 6-OHDA lesions showed no gross motor deficiencies and were indistinguishable from vehicle-treated littermates. Tyrosine hydroxylase immunohistochemistry confirmed the completeness of the lesions (Figure 6). Despite total or near-total loss of DA input to the striatum, 6-OHDA lesion on neonatal day 4 did not cause a decrease in rhes mRNA in adulthood in any level or region of striatum relative to vehicle-treated littermates (Figure 6). Rhes mRNA signal was quantified and expressed as a percent of vehicle-treated animals in the same hybridization run (Figure 7). Two-factor ANOVAs (region × treatment) for each level of CPu showed no significant effect of treatment [F = 0.169, p = 0.687 (anterior); F = .218, p = 0.647 (middle); F = 0.067, p = 0.799 (posterior)]. Similarly, there was no effect of treatment on rhes mRNA levels in OT or NAc (F = 1.14, p = 0.305).
Figure 6.
Dopamine depletion of neonatal rat pups does not affect rhes mRNA expression. Rat pups were administered ICV 6-OHDA or vehicle on postnatal day 4 and sacrificed at 3 months in order to determine TH immunoreactivity and rhes mRNA expression. Rats that received neonatal 6-OHDA showed severe DA depletion, as measured by TH immunoreactivity, but rhes mRNA was still expressed, as measured by in situ hybridization of adjacent sections with a 35S-labeled rhes riboprobe.
Figure 7.
Quantification of rhes mRNA levels in rats treated with 6-OHDA or vehicle on postnatal day 4. Autoradiographs from in situ hybridization were quantified according to 14C standards exposed to the same film and expressed as percent of vehicle-treated animals from the same film. DA-depleted rats showed no statistical differences in rhes mRNA expression relative to vehicle-treated controls at any level of CPu or in NAc or OT. n = 6 for vehicle-treated, n = 11 for 6-OHDA.
3. Discussion
Rhes is one of a growing number of signaling molecules with preferential expression in dopamine receptive brain areas, such as the CPu, NAc, and OT. This localization suggests an intimate relationship with DA systems. Several recent reports have demonstrated that Rhes affects DA signaling and behavior (Errico et al., 2008; Quintero et al., in press; Spano et. al., 2004; Vargiu et al., 2004), and here we have investigated the extent to which DA input affects Rhes expression. In addition to its preferential expression in striatum, we have detected rhes mRNA in other brain areas, especially during development. As with many other striatal signaling molecules, the expression of rhes mRNA is not affected by early neonatal DA depletion. However, unlike some other striatal signaling molecules thus far examined, Rhes is affected by DA depletion in adult animals. We have extended previous findings showing a decrease of rhes mRNA after DA depletion to include decrease of protein as well.
The ontogeny of rhes gene expression has previously been examined with Northern blotting of whole brain, which showed that expression is weak prenatally and postnatally before day 15 (Falk et al., 1999). Guided by these results, we chose several postnatal time points at which to examine rhes mRNA ontogeny in anatomical detail with in situ hybridization. Striatal expression is detectable but weak at postnatal days 4 and 6, is light to moderate on days 8 and 10, and peaks at days 15-24. This temporal pattern agrees with Falk et al., (1999), although others have detected a higher signal at day 10 (Vargiu et al., 2001). This ontogeny of rhes expression is distinct from that of striatal DA receptors. Both D1 and D2 receptor mRNA is present at birth at levels 75-80% of those found in adults; D1 receptor mRNA plateaus at adult levels around day 10, whereas D2 receptor mRNA reaches its highest levels 3-4 weeks postnatal and declines by approximately 5 months of age. Ligand binding to D1 or D2 receptor protein, however, is very low at birth and increases progressively to stabilize at adult levels at 2-3 weeks postnatal (Schambra et al., 1994; Srivastava et al., 1992).
The medial-to-lateral gradient of increasing striatal expression that was previously described for adult animals (Harrison and LaHoste, 2006) was apparent as early as day 6 and remained as signal increased on days 8, 10, 15, and 24. Such a medial-to-lateral gradient is reminiscent of patterns often observed with DA receptors. For example, D1 receptor binding has been shown to be highest in ventrolateral CPu, but also high in dorsomedial areas (Mansour et al., 1992; Marshall et al., 1989; Rao et al., 1991; Savasta et al., 1986). Although D1 receptor mRNA has been described as more uniformly distributed in CPu, it is expressed at highest levels ventrolaterally (Mansour et al., 1992). D2 receptor binding (Altar et al., 1984; Joyce et al., 1985; Mansour et al., 1990) and mRNA expression (Guennoun et al., 1991; Mengod et al., 1989; Szele et al., 1991) also show strong medial-to-lateral gradients of increasing expression. Another feature of striatal rhes mRNA expression in the postnatal period is the dichotomous distribution in NAc, with low levels of expression in core and higher expression in shell, including medial and lateral areas and the rostral pole. This pattern of NAc rhes expression has also been observed in adult animals (Harrison and LaHoste, 2006) and is more similar to D2 binding than to D1 binding, which is high in all areas of NAc, including core (Mansour et al., 1990; Schambra et al., 1994).
Some surprising results were found in brain areas outside of the striatum, where high levels of rhes mRNA were detected developmentally. Although others have reported rhes mRNA expression in the hippocampus and the cerebellum of adult rats (Vargiu et al., 2004), we report here that expression in these areas is higher than that in striatum early in development. Indeed, on day 4, when only very weak signal is detected in striatum, strong signal is detected in hippocampus and cerebellum. Throughout the later postnatal period examined here, signal in both hippocampus and cerebellum remains at levels comparable to those found in striatum. We observed that expression in hippocampus and cerebellum decreased in adulthood such that it is lower than striatal levels, but others have shown strong signals in these areas even in adulthood (Vargiu et al., 2004). This discrepancy could be due to differences in probe, exposure time, or age of the rats. Within the hippocampus, the distribution of rhes mRNA expression changed during postnatal development from being higher in CA3 and dentate gyrus at early ages (days 4, 6, and 8) to relatively higher expression in CA1, especially at days 15 and 24. The significance of the developmental expression of rhes mRNA in hippocampus is currently unknown, but there is a transient D1 receptor-Gαq-mediated inhibition of excitatory transmission in CA1 that decreases after day 30 (Noriyama et al., 2006). Since we have recently shown that Rhes protein is necessary for the full expression of grooming behavior (Quintero et al., in press), which has a Gαq component (Clifford et al., 1999), it is possible that Rhes can participate in D1-Gαq signaling. However, in adult animals, we have found a relatively homogenous moderate distribution in the pyramidal cell layer with lower expression in dentate gyrus. This adult pattern complements that of RGS9-2, another striatally enriched signaling molecule that is also expressed in hippocampus. RGS9-2 is expressed at moderate levels in dentate gyrus, with very low signal in CA1-CA3 (Rahman et al., 1999; Thomas et al., 1998a).
Although rhes mRNA is expressed in other brain areas developmentally, its major expression in adult animals is in the striatum, and therefore functional studies have focused on this dopaminoceptive area. Decreases in mRNA occur after manipulations of DA systems in adult animals (e.g. DA depletion by 6-OHDA or reserpine treatment) that result in DA receptor supersensitivity and loss of requisite D1/D2 receptor synergism (Harrison and LaHoste, 2006). We now show that Rhes protein is also decreased under these conditions. These findings contrast those with DARPP-32, which does not show decreases in mRNA (Ehrlich et al., 1990a) or protein (Raisman-Vozari et al., 1990) expression after adult 6-OHDA lesion. Neonatal DA depletion does not result in the same deficit syndrome as in adult-lesioned animals (reviewed in Breese et al., 2005; Bruno et al., 1998; Joyce et al., 1996), and no change in rhes mRNA expression was associated with this manipulation in the present study. Neonate-lesioned animals do display a decreased sensitivity to antagonists in that both D1 and D2 receptor antagonists are required to block motor behavior, rather than an antagonist to one receptor class alone (Bruno et al., 1998). This finding could also be interpreted as a loss of requisite D1/D2 synergism in these neonate-lesioned rats. Thus, in this model, loss of requisite D1/D2 synergism is not associated with changes in rhes expression. This finding is in agreement with recent results showing intact synergism in rhes-/- mice in that both D1 and D2 receptor stimulation was required in order to induce stereotypy (Quintero et al., in press). Thus, the major role for Rhes in DA systems defined so far is to affect the sensitivity of receptor signaling and behavior rather than D1/D2 synergism (Errico et al., 2008; Quintero et al., in press; Spano et al., 2004; Vargiu et al., 2004).
Like rhes mNA, the expression of several other striatally-enriched signaling molecules is independent of DA input during ontogeny. For example, DARPP-32 (Ehrlich et al., 1990b; Luthman et al., 1990), calcineurin (Polli et al., 1991a), CaM kinase II (Polli et al., 1991b), and DA D1 (Caboche et al., 1991; Duncan et al., 1993; Leslie et al., 1991, but see also Thomas et al., 1998b) and D2 (Chen and Weiss, 1991; Schambra et al., 1994; Thomas et al., 1998b) receptors are unaffected by loss of DA input during ontogeny. Furthermore, mice genetically engineered to lack DA nevertheless show normal D1, D2, and dopamine transporter binding levels (Kim et al., 2000) and normal striatal development (Kim et al., 2002). Collectively, these results indicate that loss of DA input to the striatum during development does not affect the expression levels of many striatal molecules, and our findings with rhes mRNA indicate that it too develops normally after removal of DA during the early neonatal period.
In conclusion, by demonstrating a decrease in Rhes protein after adult 6-OHDA lesion, the present report confirms that alterations in this signaling molecule are an integral part of the striatal response to this manipulation. Adaptation to neonatal loss of DA input does not, however, involve decreases in rhes mRNA. Furthermore, the transient expression of rhes mRNA at high levels in extra-striatal brain regions during ontogeny suggests a possible developmental function.
4. Experimental Procedure
1. Animals
For adult 6-OHDA studies, male Sprague-Dawley rats (Harlan; Indianapolis, IN) were used. Rats were housed individually in the vivarium at the University of New Orleans on a 12h light dark cycle, with lights on from 0600-1800 hours. Food and water were available ad libitum. Animals recovering from surgery were fed with a mash of Purina rat chow, sucrose, and water. For neonatal studies, pregnant Sprague-Dawley females were obtained from Harlan, and pups of both sexes were used. All procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of New Orleans. Every effort was made to minimize the suffering of the animals and to reduce the number of animals used.
2. 6-OHDA Injections
2.1 Adult surgeries
Dopamine denervation surgery was performed on male rats weighing 175-200 grams. Animals were anesthetized with a ketamine (80 mg/kg)/xylazine (8 mg/kg) mixture and placed in a stereotaxic frame. Rats were administered desmethylimipramine (DMI, 15 mg/kg, i.p.), to protect noradrenergic neurons, followed 30 min later by injection of the neurotoxin 6-OHDA (8 μg free base in a volume of 4 μl of saline in 0.1% ascorbic acid; Sigma-Aldrich; St. Louis, MO) into the medial forebrain bundle of the left hemisphere in order to cause unilateral denervation of the striatum. Three weeks post-surgery, animals were screened for contralateral rotation to apomorphine (0.25 mg/kg i.p.; Sigma-Aldrich), an indication of >95% dopamine depletion and dopamine receptor supersensitivity. Rotations were counted for 1 min at 15, 20, and 25 minutes post-apomorphine injection, with a criterion of 5 rotations/minute for each observation period required for inclusion in subsequent analysis.
2.2 Neonatal surgeries
On postnatal day 4, rat pups (male and female) were given bilateral intracerebroventricular (ICV) injections of vehicle or 6-OHDA in order to destroy dopamine input to the CPu by using a procedure adapted from Avale et al. (2004). Noradrenergic terminals were protected by administration of DMI (Sigma, 25 mg/kg, s.c.) 30 minutes prior to ICV 6-OHDA injection. Pups were then anesthetized with 4% tribromoethanol (i.p.), and skull landmarks were visualized through the skin with the aid of a dissecting microscope. A guarded, beveled cannula was inserted to a depth of 3.2 mm ventral to the skull surface at a position 0.5 mm posterior to bregma and 0.5 mm lateral to the sagittal suture. Fifty μg 6-OHDA (free base) in a volume of 5 μl saline/0.1% ascorbic acid was delivered over 4 minutes, and the cannula was removed after an additional minute. Control animals received 5 μl of vehicle. Pups were marked for identification by toe clipping and returned to the dams after complete recovery from anesthesia. At weaning, male and female rats were separated and housed in groups of 3-4 until sacrifice at 3 months of age.
3. Western blotting
3.1 Tissue Preparation
Rats were killed by rapid decapitation, and brains were quickly removed, frozen in isopentane at -40 °C, and stored at -80 °C until use. On the day of processing, brains were warmed to -14 °C in a cryostat. Landmarks on the ventral surface of the brain were used to cut a ∼1 mm slice containing the anterior CPu. Punches were taken from the CPu on each side by using a Harris Uni-Core tissue puncher with a diameter of 1mm (Electron Microscopy Sciences; Hatfield, PA). Tissue punches (2 per hemisphere) were homogenized in 20 volumes of lysis buffer consisting of 50 mM Tris-HCl (pH=8), 150 mM NaCl, 5 m MgCl2, 1% Triton X-100, 0.5% Na deoxycholate, 0.1% SDS, and Sigma protease inhibitor cocktail (Catalog # P8340). After sonication (5 pulses of 5 seconds each), samples were incubated on a rotating shaker at 4 °C for 30 minutes followed by centrifugation at 12,000 × g for 15 minutes. Aliquots of supernatants were used to determine protein concentrations with the Bio-Rad Detergent Compatible Protein Assay. Supernatants were then combined with 2× sample buffer, boiled, and stored at -20 °C.
3.2 SDS-PAGE
Proteins were separated by SDS-PAGE using 12.5% Criterion polyacrylamide gels (Bio-Rad) and transferred to PVDF membranes. For cultured cells, 20 μg total protein was used, and for brain, 40 μg total protein was used. After blocking with 5% Carnation milk in TBS-T (20 mM Tris, 150 mM NaCl, 0.1% Tween-20), membranes were incubated at 4°C overnight in primary antibody [Rhes, 1:1000, see below; TH (Chemicon/Millipore; Temecula, CA), 1:5000]. Membranes were washed and incubated for 1 hour in HRP-labeled goat anti-rabbit antibody (Rhes) or HRP-labeled goat anti-mouse antibody (TH). Bands were visualized by using the SuperSignal chemiluminescence detection kit (Pierce/Thermo Fisher; Rockford, IL).
3.3 Rhes Antibody
A rabbit polyclonal antibody was generated and affinity purified by Bethyl Laboratories (Montgomery, Texas). The antibody recognizes a peptide in the C-terminus of Rhes, the region in which Rhes shows the most divergence from other proteins. The specificity of the antibody was tested in three ways: (1) the use of preimmune serum, (2) use of purified recombinant GST-tagged Rhes protein (Abnova Corporation, Taiwan), and (3) comparing lysates from CHO-K1 cells with or without heterologously expressed Rhes. CHO-K1 cells in 12-well plates were transfected with 0.8 μg pcDNA3.1 (Invitrogen; Carlsbad, CA) containing the entire Rhes coding sequence (AF134409) or with empty pcDNA3.1 vector. Lysates were prepared 24h and 48h after transfection.
4. In Situ Hybridization
4.1 Tissue Preparation
Brains were cut in coronal or sagittal planes at 20 μm in a cryostat at -10 (neonates) or -14°C (adults), and sections were adhered to Vectabond-treated slides (Vector Laboratories; Burlingame, CA). In all cases, slide-mounted sections were fixed in 4% paraformaldehyde in phosphate buffered saline (PBS) and stored at -80°C until use.
4.2 Riboprobe and hybridization
A radiolabeled antisense riboprobe was generated by in vitro transcription as described previously (Harrison and LaHoste, 2006). A sense riboprobe was prepared in the same manner. In situ hybridization was performed as described previously (Harrison and LaHoste, 2006).
5. Tyrosine Hydroxylase Immunohistochemistry
Immunohistochemistry was performed essentially as described (Harrison and LaHoste, 2006) on slide-mounted, post-fixed coronal sections representing anterior, middle, and posterior striatum from adult rats that had received neonatal 6-OHDA lesions. The sections were adjacent to sections used for rhes in situ hybridization. Sections were pretreated with H2O2, washed in PBS, and blocked for 1 h in PBS with 5% normal horse serum (NHS) and 0.2% Triton X-100. After an overnight incubation at room temperature with mouse anti-TH monoclonal antibody (1:5000; Chemicon) in PBS with 1% NHS and 0.1% Triton X-100, sections were washed and incubated for 1 h in horse anti-mouse biotinylated IgG (1:200; Vector Laboratories) in PBS with 1% NHS. After a final set of washes in PBS, immunoreactivity was visualized by incubation of sections with ABC reagent (Vector) followed by diaminobenzidine.
6. Data Analysis
Films of Western blots were digitized, and a measure of relative optical density (ROD) × pixels was obtained with MCID Elite software (Cambrigde, UK). Data were analyzed with GraphPad Prism by paired t-test (left and right hemispheres of a given rat paired), with p<0.05 considered significant.
In situ hybridization autoradiographs were digitized and analyzed with MCID Elite software. For each hybridization run, optical density readings were taken from the images of the 14C standards, and a standard curve was established based on these values. Caudate-putamen (CPu) was divided into anterior (11.08-10.08, AP coordinates in mm anterior to IA, from Paxinos and Watson, 1998), middle (10.08-9.08), and posterior (9.08-8.08) segments, which were sampled separately. Anatomical areas to be measured were outlined manually. For some studies, the CPu was divided into four quadrants [dorsomedial (DM), dorsolateral (DL), ventromedial (VM), and ventrolateral (VL)], which were measured separately. In the posterior CPu, VM is replaced by globus pallidus, an area with negligible rhes mRNA signal which was not measured. A value representing the mean density of each area was obtained and was converted to nCi/mg tissue equivalent based on the 14C standard curve. For neonate-lesion studies, since multiple hybridization runs were performed, data were expressed as percent of the vehicle-treated control samples from the same film. Data were analyzed by two factor (treatment × region) ANOVA with GraphPad Prism software.
Acknowledgments
We thank Dr. YouE He for expert assistance with cell culture. Supported by NIH grant RR016816.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altar CA, Walter RJ, Neve KA, Marshall JF. Computer assisted video analysis of [3H] spiroperidol binding autoradiographs. J Neurosci Meth. 1984;10:173–188. doi: 10.1016/0165-0270(84)90054-2. [DOI] [PubMed] [Google Scholar]
- Avale ME, Falzone TL, Gelman DM, Low MJ, Grandy DK, Rubenstein M. The dopamine D4 receptor is essential for hyperactivity and impaired behavioral inhibition in a mouse model of attention deficit/hyperactivity disorder. Mol Psychiatry. 2004;9:718–726. doi: 10.1038/sj.mp.4001474. [DOI] [PubMed] [Google Scholar]
- Breese GR, Knapp DJ, Criswell HE, Moy SS, Papadeas ST, Blake BL. The neonate-6-hydroxydopamine-lesioned rat: a model for clinical neuroscience and neurobiological principles. Brain Res Rev. 2005;48:57–73. doi: 10.1016/j.brainresrev.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Bruno JP, Sandstrom MI, Arnold HM, Nelson CL. Age-dependent neurobehavioral plasticity following forebrain dopamine depletions. Dev Neurosci. 1998;20:164–179. doi: 10.1159/000017311. [DOI] [PubMed] [Google Scholar]
- Caboche J, Rogard M, Besson MJ. Comparative development of D1-dopamine and mu opiate receptors in normal and in 6-hydroxydopamine-lesioned neonatal rat striatum: dopaminergic fibers regulate mu but not D1 receptor distribution. Dev Brain Res. 1991;58:111–122. doi: 10.1016/0165-3806(91)90243-c. [DOI] [PubMed] [Google Scholar]
- Cali JJ, Balcueva EA, Rybalkin I, Robishaw JD. Selective tissue distribution of G protein γ subunits, including a new form of the γ subunits identified by cDNA cloning. J Biol Chem. 1992;267:24023–24027. [PubMed] [Google Scholar]
- Chen JF, Weiss B. Ontogenetic expression of D2 dopamine receptor mRNA in rat corpus striatum. Dev Brain Res. 1991;63:95–104. doi: 10.1016/0165-3806(91)90070-y. [DOI] [PubMed] [Google Scholar]
- Cheng HYM, Obrietan K, Cain SW, Lee BY, Agostino PV, Joza NA, Harrington ME, Ralph MR, Penninger JM. Dexras1 potentiates photic and suppresses nonphotic responses of the circadian clock. Neuron. 2004;43:715–728. doi: 10.1016/j.neuron.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Clifford JJ, Tighe O, Croke DT, Kinsella A, Sibley DR, Drago J, Waddington JL. Conservation of behavioral topography to dopamine D1-like receptor agonists in mutant mice lacking the D1A receptor implicates a D1-like receptor not coupled to adenylyl cyclase. Neuroscience. 1999;93:1483–1489. doi: 10.1016/s0306-4522(99)00297-3. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Breese GR, Criswell HE, Johnson KB, Schambra UB, Mueller RA, Caron MG, Fremeau RT. D1 dopamine receptor binding and mRNA levels are not altered after neonatal 6-hydroxydopamine treatment: evidence against dopamine-mediated induction of D1 dopamine receptors during postnatal development. J Neurochem. 1993;61:1255–1262. doi: 10.1111/j.1471-4159.1993.tb13616.x. [DOI] [PubMed] [Google Scholar]
- Ehrlich ME, Kurihara T, Greengard P. Rat DARPP-32: cloning, sequencing, and characterization of the cDNA. J Mol Neurosci. 1990a;2:1–10. doi: 10.1007/BF02896920. [DOI] [PubMed] [Google Scholar]
- Ehrlich ME, Rosen NL, Kurihara T, Shalaby IA, Greengard P. DARPP-32 development in the caudate nucleus is independent of afferent input from the substantia nigra. Dev Brain Res. 1990b;54:257–263. doi: 10.1016/0165-3806(90)90148-r. [DOI] [PubMed] [Google Scholar]
- Errico F, Santini E, Migliarini S, Borgkvist A, Centonze D, Nasti V, Carta M, De Chiara V, Prosperetti C, Spano D, Herve D, Pasqualetti M, Di Lauro R, Fisone G, Usiello A. The GTP-binding protein Rhes modulates dopamine signalling in striatal medium spiny neurons. Mol Cell Neurosci. 2008;37:335–345. doi: 10.1016/j.mcn.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Falk JD, Vargiu P, Foye PE, Usui H, Perez J, Danielson PE, Lerner DL, Bernal J, Sutcliffe JG. Rhes: A striatal-specific ras homolog related to Dexras1. J Neurosci Res. 1999;57:782–788. [PubMed] [Google Scholar]
- Fang M, Jaffrey SR, Sawa A, Ye K, Luo X, Snyder SH. Dexras1: A G protein specifically coupled to neuronal nitric oxide synthase via CAPON. Neuron. 2000;28:183–193. doi: 10.1016/s0896-6273(00)00095-7. [DOI] [PubMed] [Google Scholar]
- Glatt CE, Snyder SH. Cloning and expression of adenylyl cyclase localized to the corpus striatum. Nature. 1993;361:536–538. doi: 10.1038/361536a0. [DOI] [PubMed] [Google Scholar]
- Graham TE, Prossnitz ER, Dorin RI. Dexras1/AGS-1 inhibits signal transduction from the Gi-coupled formyl peptide receptor to Erk-1/2 MAP kinases. J Biol Chem. 2002;277:10876–10882. doi: 10.1074/jbc.M110397200. [DOI] [PubMed] [Google Scholar]
- Guennoun R, Bloch B. D2 dopamine receptor gene expression in the rat striatum during ontogeny: an in situ hybridization study. Dev Brain Res. 1991;60:79–87. doi: 10.1016/0165-3806(91)90157-e. [DOI] [PubMed] [Google Scholar]
- Harrison LM, LaHoste GJ. Rhes, the ras homolog enriched in striatum, is reduced under conditions of dopamine supersensitivity. Neuroscience. 2006;137:483–492. doi: 10.1016/j.neuroscience.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Herve D, Levi-Strauss M, Marey-Semper I, Verney C, Tassin JP, Glowinski J, Girault JA. Golf and Gs in rat basal ganglia: possible involvement of Golf in the coupling of dopamine D1 receptor with adenylyl cyclase. J Neurosci. 1993;13:2237–2248. doi: 10.1523/JNEUROSCI.13-05-02237.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce JN, Loeschen SK, Marshall JF. Dopamine D-2 receptors in rat caudate-putamen: the lateral to medial gradient does not correspond to dopaminergic innervation. Brain Res. 1985;338:209–218. doi: 10.1016/0006-8993(85)90149-0. [DOI] [PubMed] [Google Scholar]
- Joyce JN, Frohna PA, Neal-Beliveau BS. Functional and molecular differentiation of the dopamine system induced by neonatal denervation. Neurosci Biobehav Rev. 1996;20:453–486. doi: 10.1016/0149-7634(95)00025-9. [DOI] [PubMed] [Google Scholar]
- Kim DS, Szczypka MS, Palmiter RD. Dopamine-deficient mice are hypersensitive to dopamine receptor agonists. J Neurosci. 2000;20:4405–4413. doi: 10.1523/JNEUROSCI.20-12-04405.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DS, Froelick GJ, Palmiter RD. Dopamine-dependent desensitization of dopaminergic signaling in the developing mouse striatum. J Neurosci. 2002;22:9841–9849. doi: 10.1523/JNEUROSCI.22-22-09841.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie CA, Robertson MW, Cutler AJ, Bennett JP. Postnatal development of D1 dopamine receptors in the medial prefrontal cortex, striatum and nucleus accumbens of normal and neonatal 6-hydroxydopamine treated rats: a quantitative autoradiographic analysis. Dev Brain Res. 1991;62:109–114. doi: 10.1016/0165-3806(91)90195-o. [DOI] [PubMed] [Google Scholar]
- Lombroso PJ, Naegele JR, Sharma E, Lerner M. A protein tyrosine phosphatase expressed within dopaminoceptive neurons of the basal ganglia and related structures. J Neurosci. 1993;13:3064–3074. doi: 10.1523/JNEUROSCI.13-07-03064.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthman J, Lindqvist E, Young D, Cowburn R. Neonatal dopamine lesion in the rat results in enhanced adenylate cyclase activity without altering dopamine receptor binding or dopamine- and adenosine 3′:5′-monophosphate-regulated phosphoprotein (DARPP-32) immunoreactivity. Exp Brain Res. 1990;83:85–95. doi: 10.1007/BF00232196. [DOI] [PubMed] [Google Scholar]
- Mansour A, Meador-Woodruff JH, Bunzow JR, Civelli O, Akil H, Watson SJ. Localization of dopamine D2 receptor mRNA and D1 and D2 receptor binding in the rat brain and pituitary: An in situ hybridization-receptor autoradiographic analysis. J Neurosci. 1990;10:2587–2600. doi: 10.1523/JNEUROSCI.10-08-02587.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Meador-Woodruff JH, Zhou Q, Civelli O, Akil H, Watson SJ. A comparison of D1 receptor binding and mRNA in rat brain using receptor autoradiographic and in situ hybridization techniques. Neuroscience. 1992;46:959–971. doi: 10.1016/0306-4522(92)90197-a. [DOI] [PubMed] [Google Scholar]
- Marshall JF, Navarrete R, Joyce JN. Decreased striatal binding density following mesotelencephalic 6-hydroxydopamine injections: an autoradiographic analysis. Brain Res. 1989;493:247–257. doi: 10.1016/0006-8993(89)91160-8. [DOI] [PubMed] [Google Scholar]
- Mengod G, Martinez-Mir MI, Vilaro MT, Palacios JM. Localization of the mRNA for the dopamine D2 receptor in the rat brain by in situ hybridization histochemistry. Proc Natl Acad Sci USA. 1989;86:8560–8564. doi: 10.1073/pnas.86.21.8560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriyama Y, Ogawa Y, Yoshino H, Yamashita M, Kishimoto T. Dopamine profoundly suppresses excitatory transmission in neonatal rat hippocampus via phosphatidylinositol-linked D1-like receptor. Neuroscience. 2006;138:475–485. doi: 10.1016/j.neuroscience.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; New York: 1998. [DOI] [PubMed] [Google Scholar]
- Polli JW, Billingsley ML, Kincaid RL. Expression of the calmodulin-dependent protein phosphatase, calcineurin, in rat brain: Developmental patterns and the role of nigrostriatal innervation. Dev Brain Res. 1991a;63:105–119. doi: 10.1016/0165-3806(91)90071-p. [DOI] [PubMed] [Google Scholar]
- Polli JW, Kincaid RL, Torris J, Billingsley ML. Expression of calmodulin-dependent enzymes in developing rat striatum is not affected by perturbation of dopaminergic systems. Synapse. 1991b;9:136–143. doi: 10.1002/syn.890090208. [DOI] [PubMed] [Google Scholar]
- Quintero GC, Spano D, LaHoste GJ, Harrison LM. The ras homolog rhes affects dopamine D1 and D2 receptor-mediated behavior in mice. NeuroReport. doi: 10.1097/WNR.0b013e3283118434. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman Z, Gold SJ, Potenza MN, Cowan CW, Ni YG, He W, Wensel TG, Nestler EJ. Cloning and characterization of RGS9-2: A striatal-enriched alternatively spliced product of the RGS9 gene. J Neurosci. 1999;19:2016–2026. doi: 10.1523/JNEUROSCI.19-06-02016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisman-Vozari R, Girault JA, Moussaoui S, Feuerstein C, Jenner P, Marsden CD, Agid Y. Lack of change in striatal DARPP-32 levels following nigrostriatal dopaminergic lesions in animals and in parkinsonian syndromes in man. Brain Res. 1990;507:45–50. doi: 10.1016/0006-8993(90)90520-l. [DOI] [PubMed] [Google Scholar]
- Rao PA, Molinoff PB, Joyce JN. Ontogeny of dopamine D1 and D2 receptor subtypes in rat basal ganglia: a quantitative autoradiographic study. Dev Brain Res. 1991;60:161–177. doi: 10.1016/0165-3806(91)90045-k. [DOI] [PubMed] [Google Scholar]
- Savasta M, Dubois A, Scatton B. Autoradiographic localization of D1 dopamine receptors in the rat brain with [3H]SCH 23390. Brain Res. 1986;375:291–301. doi: 10.1016/0006-8993(86)90749-3. [DOI] [PubMed] [Google Scholar]
- Schambra UB, Duncan GE, Breese GR, Fornaretto MG, Caron MG, Fremeau RT. Ontogeny of D1A and D2 dopamine receptor subtypes in rat brain using in situ hybridization and receptor binding. Neuroscience. 1994;62:65–85. doi: 10.1016/0306-4522(94)90315-8. [DOI] [PubMed] [Google Scholar]
- Spano D, Branchi I, Roscia A, Pirro MT, Riccio A, Mithbaokar P, Affuso A, Arra C, Campolongo P, Terracciano D, Macchia V, Bernal J, Alleva E, Di Lauro R. Rhes is involved in striatal function. Mol Cell Biol. 2004;24:5788–5796. doi: 10.1128/MCB.24.13.5788-5796.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava LK, Morency MA, Mishra RK. Ontogeny of dopamine D2 receptor mRNA in rat brain. Eur J Pharmacol. 1992;225:143–150. doi: 10.1016/0922-4106(92)90094-c. [DOI] [PubMed] [Google Scholar]
- Szele FG, Artymyshyn R, Molinoff PB, Chesselet MF. Heterogeneous distribution of dopamine D2 receptor mRNA in the rat striatum: a quantitative analysis with in situ hybridization histochemistry. Anat Rec. 1991;231:548–558. doi: 10.1002/ar.1092310416. [DOI] [PubMed] [Google Scholar]
- Takesono A, Nowak MW, Cismowski M, Duzic E, Lanier SM. Activator of G-protein signaling 1 blocks GIRK channel activation by a G-protein-coupled receptor: Apparent disruption of receptor signaling complexes. J Biol Chem. 2002;277:13827–13830. doi: 10.1074/jbc.M201064200. [DOI] [PubMed] [Google Scholar]
- Thomas EA, Danielson PE, Sutcliffe JG. RGS9: A regulator of G-protein signaling with specific expression in rat and mouse striatum. J Neurosci Res. 1998a;52:118–124. doi: 10.1002/(SICI)1097-4547(19980401)52:1<118::AID-JNR11>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Thomas WS, Neal-Beliveau BS, Joyce JN. There is a limited critical period for dopamine's effects on D1 receptor expression in the developing rat neostriatum. Dev Brain res. 1998b;111:99–106. doi: 10.1016/s0165-3806(98)00126-6. [DOI] [PubMed] [Google Scholar]
- Usui H, Falk JD, Dopazo A, de Lecea L, Erlander MG, Sutcliffe JG. Isolation of clones of rat striatum-specific mRNAs by directional tag PCR subtraction. J Neurosci. 1994;14:4915–4926. doi: 10.1523/JNEUROSCI.14-08-04915.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallortigara J, Alfos S, Micheau J, Higueret P, Enderlin V. T3 administration in adult hypothyroid mice modulates expression of proteins involved in striatal synaptic plasticity and improves motor behavior. Neurobiol Dis. 2008 doi: 10.1016/j.nbd.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Vargiu P, Morte B, Manzano J, Perez J, de Abajo R, Sutcliffe JG, Bernal J. Thyroid hormone regulation of rhes, a novel Ras homolog gene expressed in the striatum. Mol Brain Res. 2001;94:1–8. doi: 10.1016/s0169-328x(01)00140-1. [DOI] [PubMed] [Google Scholar]
- Vargiu P, De Abajo R, Garcia-Ranea JA, Valencia A, Santisteban P, Crespo P, Bernal J. The small GTP-binding protein, Rhes, regulates signal transduction from G protein-coupled receptors. Oncogene. 2004;23:559–568. doi: 10.1038/sj.onc.1207161. [DOI] [PubMed] [Google Scholar]
- Walaas SI, Aswad DW, Greengard P. A dopamine- and cyclic AMP-regulated phosphoprotein enriched in dopamine-innervated brain regions. Nature. 1983;301:69–71. doi: 10.1038/301069a0. [DOI] [PubMed] [Google Scholar]
- Watson JB, Coulter PM, Margulies JE, de Lecea L, Danielson PE, Erlander MG, Sutcliff JG. G protein gamma 7 subunit is selectively expressed in medium-sized neurons and dendrites of the rat neostriatum. J Neurosci Res. 1994;39:108–116. doi: 10.1002/jnr.490390113. [DOI] [PubMed] [Google Scholar]