Abstract
Prostaglandin E2 (PGE2) is reported to play an important role in tumor development. We explored the differential expression of genes governing production of, and response to, PGE2 during development of invasive bladder cancer. N-butyl-N-(4-hydroxybutyl)-nitrosamine (BBN) or vehicle-treated mice (n = 4–5) were euthanized after 4–8 weeks (period 1, P1), 12–16 weeks (P2), and 20–23 weeks (P3). Half of each bladder was analyzed histologically and the other half extracted for mRNA analysis by quantitative real-time PCR. Bladders from BBN-treated mice showed progression from submucosal inflammation (P1) to squamous metaplasia/focal CIS (P2) to poorly differentiated, invasive cancer (P3). mRNA levels for the inducible cyclooxygenase, COX-2, were elevated three to fourfold at all time points in BBN-treated mice compared to controls. In contrast, mRNA levels for constitutive COX-1 and cytosolic phospholipase A2 (cPLA2), which releases substrate for COX, were either unchanged or decreased in BBN-treated mice relative to controls. Downstream of COX, mRNA levels of membrane-bound PGE2 synthase (mPGES-1) were increased 1.7-fold at P1 in BBN bladders but returned to control levels at P2 and P3. mRNA levels for 15-prostaglandin dehydrogenase (PGDH), which inactivates PGE2, were reduced 50–80% in BBN-treated bladders at all time points. mRNA levels for EP2R and EP4R, receptors for PGE2, were two to threefold increased at P1, but returned to control levels or below at P3. Hence, increased COX-2 and decreased PDGH expression occurred throughout tumor development, while mPGES-1, EP2R and EP4R were elevated only before development of invasive cancer. We compared expression of these genes in the malignant human urothelial cell lines, HTB-5 and HT-1376, with expression in a benign urothelial cell line, UROtsa. Neither malignant cell line reproduced the complete in vivo pattern, relative to benign cells, but each showed abnormal basal expression of several of the genes downstream of COX-2, but not COX-2 itself. We conclude that components involved in PGE2 synthesis and activity are differentially regulated during bladder tumor development and the therapeutic efficacy of targeting the various components may vary with stage of tumor development.
Keywords: Bladder cancer, Cyclooxygenase, Prostaglandin
1. Introduction
Bladder cancer is the fourth most common solid malignancy in men and the ninth most common in women, representing 7% and 3% of all cancers, respectively. In 2008 there will be an estimated 68,810 new cases with 14,100 deaths [1]. Bladder cancer almost never presents as an incidental finding at autopsy, indicating that at some point during the natural history of the tumor it manifests clinically. Stage at diagnosis is fundamental to outcome. High-grade or muscle invasive tumors tend to progress and metastasize with up to 50% of muscle invasive tumors having occult metastatic disease at the time of diagnosis. Invasive and/or metastatic disease carries a relatively poor prognosis with 50% of those with metastatic disease dying within 2 years of diagnosis. Five-year survival rates are as low as 6%. There are no feasible tumor markers capable of stratifying bladder cancer patients with regard to progression, prognosis or treatment. Current therapies for advanced disease are disappointing. Even with aggressive surgical and medical treatment most patients with advanced bladder cancer ultimately succumb to their disease.
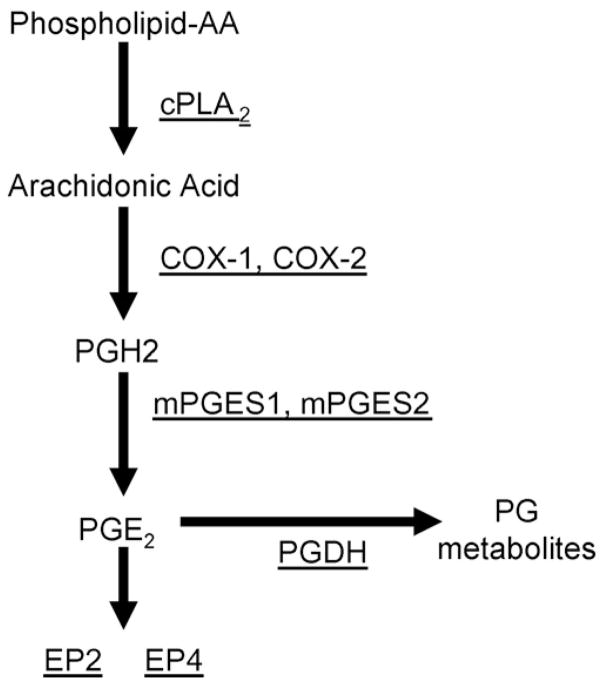
Prostaglandins (PGs) and inducible cyclooxygenase-2 (COX-2) have been implicated in the pathogenesis of many tumors including bladder. COX-2 is overexpressed in bladder cancer [2–7], and a polymorphism in the NFkB-binding region of the COX-2 promoter was shown to be associated with an increased risk of bladder cancer [8]. Overexpression of COX-2 is also found in bladder cancer that develops in N-butyl-N-(4-hydroxybutyl)-nitrosamine (BBN)-treated mice and treatment with both non-selective (indomethacin) and selective (Celecoxib) COX inhibitors was shown to inhibit tumor development [3,9,10]. Arguments for COX-2 selective inhibitors in the prevention and treatment of bladder cancer have been summarized in a recent review [11]. Although inhibition of COX-2 activity with selective NSAIDs has been the most common approach to inhibiting the PG pathway, there are other potential targets (Fig. 1), such as cPLA2, which releases arachodonic acid (AA) from cell membranes to be acted on by COX. Prostaglandin dehydrogenase (PGDH), which degrades PGE2, has recently been implicated in multiple types of cancer as well [12,13]. Given the concerns about the safety of long-term inhibition of COX-2 activity, selective targeting of individual PGE2 receptors is also being considered [14].
Fig. 1.
PG pathway.
We explored the expression of the components of the PG pathway during development of high-grade invasive bladder cancer in a BBN murine model. In addition, we examined the expression of these components in an immortalized benign urothelial (UROtsa) and two high-grade bladder cancer (HTB-5, HT-1376) cell lines at basal conditions.
2. Materials and methods
2.1. BBN animal model
All animal protocols were approved by the University of Connecticut Center for Laboratory Animal Care. Five-month-old male mice in a C57Bl/6 background were started on BBN 0.05% in water (treatment) or water only (control). BBN is an N-nitrosamine compound whose metabolites are known to cause bladder cancer. These cause a significant inflammatory reaction in the bladder and also lead to similar genetic alterations as seen in human disease. There were 12 control and 13-treated mice. Two mice from each group were euthanized at 4, 8, 12, 16 and 20 weeks after starting treatment. The last mice were euthanized at 23 weeks, 1 week earlier than planned, due to the presence of carcinoma in the 20-week group. At 23 weeks there were three mice in the treated group and two in the control group.
2.2. Bladder pathology
Bladders were halved along the midline saggital plane and then each half was sectioned into three levels (inner, mid, outer) at 250 μm intervals. If a tumor was identified on gross inspection, bladders were halved to include a portion of tumor. Three-micron sections from each level were stained with H&E and all sections examined for tumor. Characteristics such as number of cell layers, nuclear size, nuclear membrane irregularity, chromatin pattern, nuclear:cytoplasmic ratio, presence of nucleoli and mitosis were evaluated in order to provide a final diagnosis. Diagnoses were: (1) normal: 3–7 cell layers with normal maturation from basal to luminal levels which includes large/flat umbrella cells; (2) metaplasia: nontransitional appearance with squamous changes but no atypia; (3) atypia: nuclear (pleomorphism) or architectural distortion without increased number of cells; (4) carcinoma in situ (CIS): flat lesion within the urothelial layer displaying loss of polarity/differentiation with marked nuclear pleomorphism, high nuclear/cytoplasmic ratio and mitotic figures; and (5) carcinoma: nodular/sessile lesions with increased number of cells displaying characteristics as noted in CIS. The term carcinoma is applied to all cancers since lesions showed mixed histology with both transitional and squamous cell elements. Invasion was diagnosed if tumor cells were seen infiltrating the muscle layers of the bladder. A single pathologist (P.H.) reviewed all slides in a blinded fashion.
2.3. Measurement of mRNA
Gene expression was analyzed by quantitative real time (qPCR) using RNA extracted from half of each bladder. RNA extraction from mouse tissue has been previously described [15,16]. In brief, tissue was homogenized in a Polytron with TRIzol® Reagent (Invitrogen Life Technologies, Carlsbad, CA). After quantitation at 260 nm, total RNA was converted to cDNA by ABI High Capacity cDNA Archive Kit (Applied Biosystems, Foster city, CA) following the manufacturer’s protocol. Real-time PCR was performed for different gene expression in separate wells (singleplex assay) of 96-well plate in reaction volume of 20 μl. GAPDH was used as endogenous control. Two replicates of each sample were amplified using Assays-on-Demand Gene Expression (Applied Biosytems, Foster City, CA), which contains predesigned unlabeled gene-specific PCR primers and TaqMan MGB FAM dye-labeled probe. The PCR reaction mixture (including 2X TaqMan Universal PCR Master Mix, 20X Assays-on-Demand Gene Expression Assay Mix, 50 ng of cDNA) was run in ABI Prism 7300 Sequence Detection System instrument utilizing universal thermal cycling parameters. For the genes for which the efficiencies of target and endogenous control amplification were approximately equal, the relative quantification expression in a test sample to a reference calibrator sample (ΔΔCt Method) was used for data analysis. For the genes which were not amplified with the same efficiency as the endogenous control, the relative standard curve method in which target quantity is determined from the standard curve and divided by the target quantity of the endogenous control, was used. For analysis, we grouped the samples into periods according to time bladder pathology at time of euthanasia: period 1, 4–8 weeks; period 2, 12–16 weeks; and period 3, 20–23 weeks. Statistical analysis of control versus treated samples was by t-test or ANOVA where appropriate.
2.4. Cell culture
UROtsa a cells were generously donated by Dr. Brian Philips (University of Pittsburg). HTB-5 and HT-1376 cells were obtained from ATCC (Manassas, VA). All cells were grown in media containing 100 U/ml penicillin, 50 μg/ml streptomycin and 10% fetal calf serum (FCS) in a humidified atmosphere of 5% CO2 at 37 °C. HT-1376 cells were maintained in MEM (Eagle) in Earle’s BSS 90%. HTB-5 cells were maintained in MEM with Earle’s BSS, nonessential amino acids and 1 mM sodium pyruvate 90%. UROtsa cells were maintained in DMEM without phenol red supplemented with L-glutamine up to 1.5 nM.
For experiments, cells were plated in six-well dishes at 5 × 104 cells/ml and grown to 80% confluence. Three separate experiments were performed. Prior to takedown serum level in the media was reduced to 0.5% to avoid the effect of media on the induction of COX-2. RNA was extracted, quantified and reverse transcribed as previously noted. Real-time PCR was performed as above with GAPDH used as endogenous control. The relative standard curve method was used for data analysis. ANOVA and a post hoc test (t-test) were used to compare the groups.
2.5. Statistical analysis
Statistical analyses were done using SigmStat (Chicago, IL).
3. Results
3.1. Bladder pathology
Pathologic results for the different time periods are summarized in Table 1. Pathologic findings in our BBN model have previously been reported [17]. In brief, all bladders from control animals have normal findings. All bladders from animals receiving BBN displayed equal amounts of submucosal edema and inflammatory cell infiltrates (not shown). Varying degrees of atypia and metaplasia were noted through week 12. Due to the lack of dysplastic elements, these changes are benign in nature. Carcinoma was not noted until 16 weeks in the treatment group. These were focal lesions representing areas of CIS (not shown). Animals euthanized at weeks 20 and 23 showed poorly differentiated, high-grade, muscle invasive carcinoma.
Table 1.
Pathologic diagnosis in mice treated with BBN.
| Period | Pathologic diagnosis of BBN-treated mice |
|---|---|
| 1 (Weeks 4–8) | Atypia (n = 4/4) |
| 2 (Weeks 12–16) | Squamous metaplasia (n = 3/4) Focal CIS (n = 1/4) |
| 3 (Weeks 20–23) | Invasive carcinoma (n = 5/5) |
There were two mice in each group (except for week 23, which had five). Diagnosis represents most significant findings.
3.2. Bladder gene expression
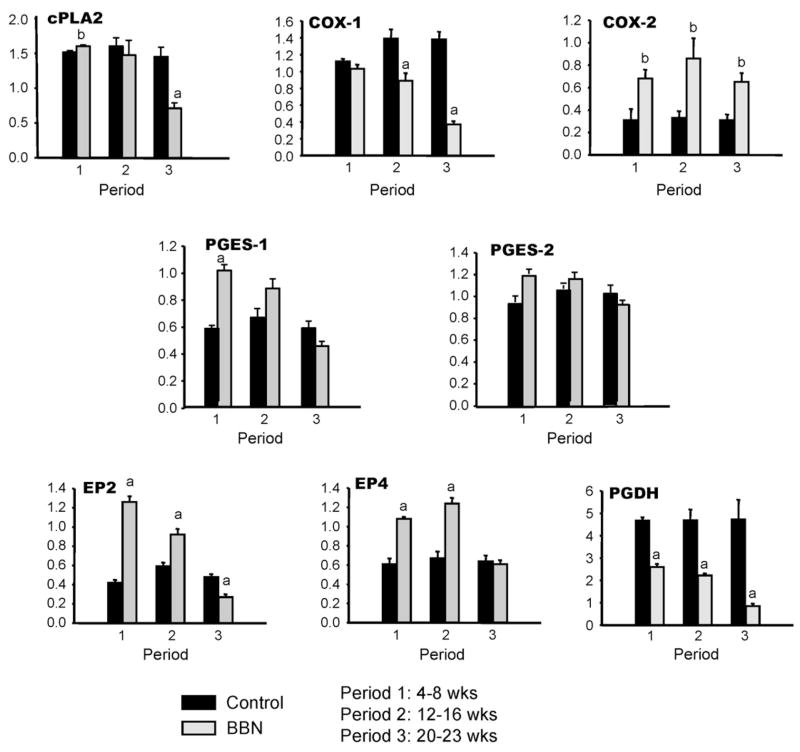
We evaluated mRNA for factors in these samples that might affect the production or action of PGs. These included; COX-1, COX-2, cytoplasmic phospholipase A2 (cPLA2), PG synthases 1 and 2 (PGES-1, PGES-2), PG receptors EP2 and EP4 and PGDH (Fig. 1). To test for reproducibility over time in our model COX-2 levels for control bladders, which are not expected to change with time, were evaluated from periods 1–3. Mean ± S.E. mRNA levels calculated by qPCR relative to a calibrator pool as described in methods were 0.31 ± 0.10, 0.33 ± 0.06 and 0.31 ± 0.05, respectively. Likewise, mRNA levels were analyzed in control bladders for other factors across all time periods with very good consistency.
COX-2 expression was three to fourfold increased in BBN-treated bladders over all time periods (Fig. 2). PGDH expression was reduced 45–55% in treated bladders in the first 2 time periods and 80% at period 3 when invasive tumor was present. Both have been previously implicated in the pathogenesis of bladder cancer. COX-1 mRNA expression was not upregulated at any timepoint and decreased 80% by the time of invasive tumor development. Similar results were noted for cPLA2, the enzyme responsible for release of substrate for COX. There was a progressive decrease over the course of tumor development to 50% of its original expression level by period 3.
Fig. 2.
Prostaglandin pathway gene expression by qPCR in BBN mouse model of bladder cancer. Black bars are means ± S.E. for n = 4 control bladders. Gray bars are means ± S.D. for n = 3–4 BBN-treated bladders. aSignificant difference relative to control at same period, P < 0.01; bP < 0.05.
As expected, the synthases and receptors were increased early, and therefore might contribute to PG production. PGES-1 was elevated approximately twofold and PGES-2 1.5-fold in period 1 and returned to baseline during periods 2 and 3. The mRNA for PG receptors EP2R and EP4R was increased three and twofold in period 1, respectively. The levels remained elevated in period 2 but by the time of invasive tumor were reduced 40% or were back to control levels, respectively.
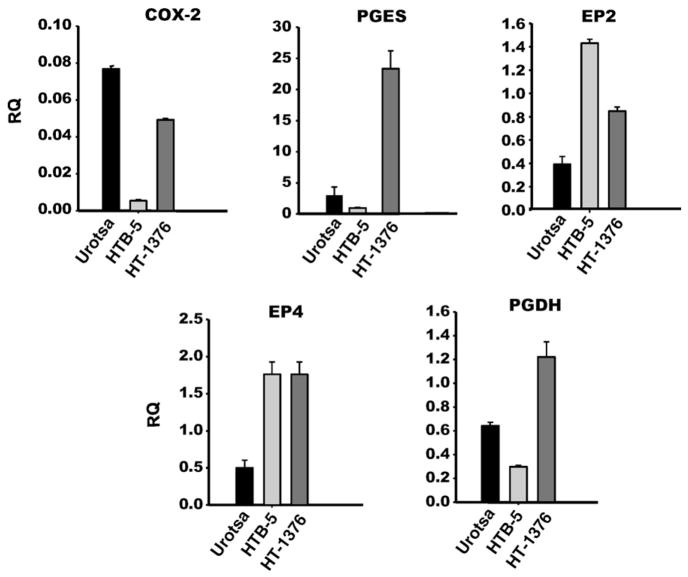
3.3. Benign and bladder cancer cell line gene expression
These cell lines have been used to study regulatory mechanisms involved in bladder cancer. We compared the cancer lines to a benign urothelial line to examine the expression pattern of genes involved in PG synthesis in attempt to identify a cell culture model that has a similar pattern of change as seen in our animal model. Levels of COX-2, PTGES-1, EP2, EP4 and PGDH mRNA were measured in each cell line as these were noted to change significantly at one or more timepoints in our in vivo model (Fig. 3). Unexpectedly, COX-2 levels were approximately 90% reduced in all three experiments in the HTB-5 cancer lines as compared to the UROtsa cell line. In two of three experiments, COX-2 levels in HT-1376 cells were the same as seen in UROtsa cells with one experiment showing a 40% reduction. PGDH, the terminal enzyme for PG degradation, was 50% lower in the HTB-5 cells and twofold higher in the HT-1376 cells when compared to UROtsa cells. Whereas PGES-1 showed as much as a 14-fold increase in the HT-1376 cell line it was 40% less in the HTB-5 line as compared to UROtsa cells. The receptors for PGE2 both showed significant increase in levels in the cancer lines compared to UROtsa. EP2 was 3.6-fold and 1.6-fold higher and EP4 was 3.5-fold and 2.2-fold higher in the HTB-5 and HT-1376 lines, respectively.
Fig. 3.
Prostaglandin pathway gene expression by qPCR in benign (UROtsa) and malignant (HTB-5, HT-1376) cell lines. All differences between cancer cell lines and UROtsa are statistically significant (p < 0.05). RQ = relative quantification.
4. Discussion
A role for PGs in cancer was first suggested by studies showing that aspirin use decreased colorectal cancer [18]. Subsequently, studies found that COX-2, which is normally expressed at low levels until induced, is overexpressed in colon adenocarcinomas as well as many other types of tumors including breast, gastric, pancreatic and prostate [19–24]. Additional studies have demonstrated inhibitory effects of COX-2 selective NSAIDs on carcinogenesis [25–27]. An initial clinical trial examining the use of the COX-2-specific inhibitor Celecoxib in the prevention of colon cancer reported significant reduction in the number of colon polyps in patients with familial adenomatous polyposis (FAP) and led to FDA approval for this use [28]. Based in part on these results, clinical trials using Celecoxib to prevent many other cancer types including bladder, breast, cervical, colorectal, esophageal, head and neck, skin, lung, oral and prostate cancers as well as multiple myeloma were initiated. However, concern was raised regarding the long-term use of COX-2 inhibitors after the report of a twofold increased risk of cardiovascular events in patients taking the COX-2 inhibitor rofecoxib in a colon adenoma prevention trial [29].
Although inhibition of COX-2 activity and subsequent PG production with selective NSAIDs has been the most common approach to inhibiting the PG pathway, other potential targets exist. Early studies have shown promise at varying points along the pathway of PG production to receptors and degrading enzymes (Fig. 1). Downregulation of cPLA2 has been reported to lead to growth inhibition and apoptosis in colon cancer cells [30]. Selective targeting of individual PGE2 receptors is also being explored in a model of hormone-refractory, metastatic breast cancer [14]. Additionally, decreased levels of PGDH, which degrades PGE2, has recently been implicated in colon cancer and has been reported in high-grade bladder cancer [12,13,31]. We sought to characterize the role of components of the PG pathway in a model covering the time-course of progression from dysplasia through high-grade, invasive bladder cancer. In addition, we evaluated the expression of these factors in cell lines representing benign and high-grade disease in attempt to identify a viable model for exploration of mechanisms involved.
In our study the components of the pathway involved in PG production showed variable expression during the timecourse of development of invasive cancer. Two genes, COX-2 and PGDH, were consistently different than control throughout the study. COX-2 was elevated and PGDH was decreased, both consistent with increased PGs. Both have been previously reported to be involved in the development of bladder cancer. Consistent with previous studies, our data indicate that elevated expression of COX-2 might be involved in the pathogenesis of bladder cancer in the BBN model [3,9,10]. COX-2 mRNA levels were increased threefold at the earliest timepoint and remained essentially unchanged through development of invasive cancer. A recent study has reported a progressive increase in the protein expression of COX-2 by immunohistochemistry (IHC) in tissue from normal urothelium to invasive and metastatic disease [32]. An important difference between the studies is that they examined protein expression, whereas we evaluated mRNA levels. It is possible that there may in fact be increased expression of COX-2 in tumor cells in our model however this may have been lost in sampling which included adjacent tissue with high levels of inflammation. Future studies could utilize in situ hybridization or laser capture microdissection to determine the exact cells in which COX-2 mRNA expression is increased. PGDH levels decrease progressively over the development of cancer. This reciprocal drop in PGDH at the time of COX-2 elevation has been reported recently in lung cancer tissue [33]. Additionally, evaluation of PGDH in bladder cancer specimens reported a significant correlation between the degree of immunohistochemical staining for PGDH with tumor stage and grade [31]. Decreased staining was associated with higher stage/grade tumors. The above would suggest that the impact of change occurs early and inhibition would therefore also need to be early in the development of tumor.
COX-1 levels decreased throughout the course of the study. cPLA2 levels were also decreased at the time of invasive disease. It is possible that these represent a negative feedback from the presumed production of PGs which would be present with the sustained elevation of COX-2 and decrease in PGDH. One other study reported elevated cPLA2 levels at the time of cancer presence in a rat model of bladder cancer but did not comment on the stage or grade of tumor [34]. Bladder cancer is known to exhibit two distinct phenotypes, low-grade and high-grade, which have distinct genetic and epigenetic alterations as well as clinical courses [35]. Treatment of rats with BBN is known to cause papillomas and low-grade disease, whereas the BBN model in mice causes high-grade invasive tumors which could potentially account for these differences.
As expected both the synthases and receptors for PGs were increased early. Although the synthases showed early increases they returned to baseline levels by the time of tumor development which could also be in response to elevated PGs. Increased mPGES-1 and normal mPGES-2 levels have been reported in bladder cancer in rats [34] and increased mPGES-1 levels noted in precancerous skin lesions [36]. Our data would be consistent with these findings in that elevated mPGES-1 was noted during the time of inflammation and dysplasia and mPGES-2 was not seen to be elevated in the presence of cancer. We chose to evaluate two of the four receptors based on prior literature indicating potential roles for EP2 and EP4 in cancer [36,37]. Increased PG receptor levels would indicate increased sensitivity to PG. We found the expression of EP2 to be markedly elevated at the time of predominant inflammation and EP4 levels to be elevated through early cancer presentation (CIS). In both cases the levels gradually declined to basal or lower levels as invasive cancer developed. Contrary to our study, PG receptor levels have been reported to increase across the spectrum of normal to adenoma to adenocarcinoma in colon (EP4) [37] and be increased in skin papillomas (EP2) [36]. It is possible that this represents a response to the sustained presence of inflammation and increase in COX-2 and presumed PG production in our model.
Our cell culture model displayed high variability with regard to expression levels of the components of PG synthesis. Contrary to prior studies that reported high levels of COX-2 expression in malignant urothelial cell lines [38,39], we saw a statistically significant reduction in COX-2 expression in the cancer lines compared to the benign UROtsa cells. One difference between these studies and ours which may account for this disparity is the use of a benign cell line. The studies noted above did not use such a line for comparison. However, in agreement with these studies we found the level of expression to be highly variable across the lines. Conversely, the PG receptor levels were found to be significantly higher in the cancer cells as was the synthase in at least one of the cell lines (HT-1376). PGDH was noted to be highest in this line as well. Overall we did not see a trend in expression when comparing the benign to cancer cells. The variability seen in our study as well as others would suggest that in vitro models of PG production in bladder cancer cell lines are less reliable than an in vivo model of de novo cancer development. The latter would also allow for study of the impact of selective inhibitor use or specific gene deletion.
Our study confirms prior observations made regarding elements of the PG pathway in other cancers at static levels of tumor development. However, our report suggested that there is differential regulation of the components involved in PG synthesis and activity during bladder tumor development and that the therapeutic efficacy of targeting the various components will vary with stage of tumor development. Cell culture modeling does not appear to be as reliable a method of study of the role of the PG pathway given the high variability of component expression between benign and cancer cell lines.
Acknowledgments
This work was supported by IRG-06-002-01 from the American Cancer Society (JAT), R01DK48361 (CP) and R01AG028657 (GAK, JAT, CP).
Footnotes
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues.
Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited.
In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier's archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright
References
- 1.American Cancer Society; 2008.
- 2.Ristimaki A, Nieminen O, Saukkonen K, Hotakainen K, Nordling S, Haglund C. Expression of cyclooxygenase-2 in human transitional cell carcinoma of the urinary bladder. Am J Pathol. 2001;158(3):849–53. doi: 10.1016/S0002-9440(10)64033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grubbs CJ, Juliana MM, Eto I, et al. Chemoprevention by indomethacin of N-butyl-N-(4-hydroxybutyl)-nitrosamine-induced urinary bladder tumors. Anticancer Res. 1993;13(1):33–6. [PubMed] [Google Scholar]
- 4.Eschwege P, Ferlicot S, Droupy S, et al. A histopathologic investigation of PGE (2) pathways as predictors of proliferation and invasion in urothelial carcinomas of the bladder. Eur Urol. 2003;44(4):435–41. doi: 10.1016/s0302-2838(03)00313-0. [DOI] [PubMed] [Google Scholar]
- 5.Shirahama T. Cyclooxygenase-2 expression is up-regulated in transitional cell carcinoma and its preneoplastic lesions in the human urinary bladder. Clin Cancer Res. 2000;6(6):2424–30. [PubMed] [Google Scholar]
- 6.Shariat SF, Kim JH, Ayala GE, Kho K, Wheeler TM, Lerner SP. Cyclooxygenase-2 is highly expressed in carcinoma in situ and T1 transitional cell carcinoma of the bladder. J Urol. 2003;169(3):938–42. doi: 10.1097/01.ju.0000043638.89552.ed. [DOI] [PubMed] [Google Scholar]
- 7.Shariat SF, Matsumoto K, Kim J, et al. Correlation of cyclooxygenase-2 expression with molecular markers, pathological features and clinical outcome of transitional cell carcinoma of the bladder. J Urol. 2003;170(3):985–9. doi: 10.1097/01.ju.0000080401.85145.ee. [DOI] [PubMed] [Google Scholar]
- 8.Kang S, Kim YB, Kim MH, et al. Polymorphism in the nuclear factor kappa-B-binding promoter region of cyclooxygenase-2 is associated with an increased risk of bladder cancer. Cancer Lett. 2005;217(1):11–6. doi: 10.1016/j.canlet.2004.06.053. [DOI] [PubMed] [Google Scholar]
- 9.Kitayama W, Denda A, Okajima E, Tsujiuchi T, Konishi Y. Increased expression of cyclooxygenase-2 protein in rat urinary bladder tumors induced by N-butyl-N-(4-hydroxybutyl) nitrosamine. Carcinogenesis. 1999;20(12):2305–10. doi: 10.1093/carcin/20.12.2305. [DOI] [PubMed] [Google Scholar]
- 10.Grubbs CJ, Lubet RA, Koki AT, et al. Celecoxib inhibits N-butyl-N-(4-hydroxybutyl)-nitrosamine-induced urinary bladder cancers in male B6D2F1 mice and female Fischer-344 rats. Cancer Res. 2000;60(20):5599–602. [PubMed] [Google Scholar]
- 11.Pruthi RS, Derksen E, Gaston K, Wallen EM. Rationale for use of cyclooxygenase-2 inhibitors in prevention and treatment of bladder cancer. Urology. 2004;64(4):637–42. doi: 10.1016/j.urology.2004.04.047. [DOI] [PubMed] [Google Scholar]
- 12.Backlund MG, Mann JR, Holla VR, et al. 15-Hydroxyprostaglandin dehydrogenase is down-regulated in colorectal cancer. J Biol Chem. 2005;280(5):3217–23. doi: 10.1074/jbc.M411221200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mann JR, Backlund MG, Buchanan FG, et al. Repression of prostaglandin dehydrogenase by epidermal growth factor and snail increases prostaglandin E2 and promotes cancer progression. Cancer Res. 2006;66(13):6649–56. doi: 10.1158/0008-5472.CAN-06-1787. [DOI] [PubMed] [Google Scholar]
- 14.Fulton AM, Ma X, Kundu N. Targeting prostaglandin E EP receptors to inhibit metastasis. Cancer Res. 2006;66(20):9794–7. doi: 10.1158/0008-5472.CAN-06-2067. [DOI] [PubMed] [Google Scholar]
- 15.Chikazu D, Li X, Kawaguchi H, et al. Bone morphogenetic protein 2 induces cyclo-oxygenase 2 in osteoblasts via a Cbfal binding site: role in effects of bone morphogenetic protein 2 in vitro and in vivo. J Bone Miner Res. 2002;17(8):1430–40. doi: 10.1359/jbmr.2002.17.8.1430. [DOI] [PubMed] [Google Scholar]
- 16.Okada Y, Lorenzo JA, Freeman AM, et al. Prostaglandin G/H synthase-2 is required for maximal formation of osteoclast-like cells in culture. J Clin Invest. 2000;105(6):823–32. doi: 10.1172/JCI8195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor JA, 3rd, Kuchel GA, Hegde P, et al. Null mutation for macrophage migration inhibitory factor (MIF) is associated with less aggressive bladder cancer in mice. BMC Cancer. 2007;7:135. doi: 10.1186/1471-2407-7-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams CS, Smalley W, DuBois RN. Aspirin use and potential mechanisms for colorectal cancer prevention. J Clin Invest. 1997;100(6):1325–9. doi: 10.1172/JCI119651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ristimaki A, Honkanen N, Jankala H, Sipponen P, Harkonen M. Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer Res. 1997;57(7):1276–80. [PubMed] [Google Scholar]
- 20.Chang SH, Liu CH, Conway R, et al. Role of prostaglandin E2-dependent angiogenic switch in cyclooxygenase 2-induced breast cancer progression. Proc Natl Acad Sci USA. 2004;101(2):591–6. doi: 10.1073/pnas.2535911100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trifan OC, Hla T. Cyclooxygenase-2 modulates cellular growth and promotes tumorigenesis. J Cell Mol Med. 2003;7(3):207–22. doi: 10.1111/j.1582-4934.2003.tb00222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sano H, Kawahito Y, Wilder RL, et al. Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Res. 1995;55(17):3785–9. [PubMed] [Google Scholar]
- 23.Tucker ON, Dannenberg AJ, Yang EK, et al. Cyclooxygenase-2 expression is up-regulated in human pancreatic cancer. Cancer Res. 1999;59(5):987–90. [PubMed] [Google Scholar]
- 24.Kirschenbaum A, Klausner AP, Lee R, et al. Expression of cyclooxygenase-1 and cyclooxygenase-2 in the human prostate. Urology. 2000;56(4):671–6. doi: 10.1016/s0090-4295(00)00674-9. [DOI] [PubMed] [Google Scholar]
- 25.Wang D, Dubois RN. Prostaglandins and cancer. Gut. 2006;55(1):115–22. doi: 10.1136/gut.2004.047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pruthi RS, Wallen EM. Cyclooxygenase-2: a therapeutic target for prostate cancer. Clin Genitourin Cancer. 2005;4(3):203–11. doi: 10.3816/CGC.2005.n.034. [DOI] [PubMed] [Google Scholar]
- 27.Pereg D, Lishner M. Non-steroidal anti-inflammatory drugs for the prevention and treatment of cancer. J Intern Med. 2005;258(2):115–23. doi: 10.1111/j.1365-2796.2005.01519.x. [DOI] [PubMed] [Google Scholar]
- 28.Steinbach G, Lynch PM, Phillips RK, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342(26):1946–52. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 29.Bresalier RS, Sandler RS, Quan H, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352(11):1092–102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- 30.Yu HG, Huang JA, Yang YN, et al. Inhibition of cytosolic phospholipase A2 mRNA expression: a novel mechanism for acetylsalicylic acid-mediated growth inhibition and apoptosis in colon cancer cells. Regul Pept. 2003;114(2–3):101–7. doi: 10.1016/s0167-0115(03)00084-3. [DOI] [PubMed] [Google Scholar]
- 31.Gee JR, Montoya RG, Khaled HM, Sabichi AL, Grossman HB. Cytokeratin 20, AN43, PGDH, and COX-2 expression in transitional and squamous cell carcinoma of the bladder. Urol Oncol. 2003;21(4):266–70. doi: 10.1016/s1078-1439(02)00271-5. [DOI] [PubMed] [Google Scholar]
- 32.Margulis V, Shariat SF, Ashfaq R, et al. Expression of cyclooxygenase-2 in normal urothelium, and superficial and advanced transitional cell carcinoma of bladder. J Urol. 2007;177(3):1163–8. doi: 10.1016/j.juro.2006.10.033. [DOI] [PubMed] [Google Scholar]
- 33.Tai HH, Tong M, Ding Y. 15-hydroxyprostaglandin dehydrogenase (15-PGDH) and lung cancer. Prostaglandins Other Lipid Mediat. 2007;83(3):203–8. doi: 10.1016/j.prostaglandins.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi Y, Cui L, Dai G, et al. Elevated prostaglandin E2 level via cPLA2–COX-2–mPGES-1 pathway involved in bladder carcinogenesis induced by terephthalic acid-calculi in Wistar rats. Prostaglandins Leukot Essent Fatty Acids. 2006;74(5):309–15. doi: 10.1016/j.plefa.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 35.Wu XR. Urothelial tumorigenesis: a tale of divergent pathways. Nat Rev Cancer. 2005;5(9):713–25. doi: 10.1038/nrc1697. [DOI] [PubMed] [Google Scholar]
- 36.Neumann M, Dulsner E, Furstenberger G, Muller-Decker K. The expression pattern of prostaglandin E synthase and EP receptor isoforms in normal mouse skin and preinvasive skin neoplasms. Exp Dermatol. 2007;16(5):445–53. doi: 10.1111/j.1600-0625.2007.00549.x. [DOI] [PubMed] [Google Scholar]
- 37.Chell SD, Witherden IR, Dobson RR, et al. Increased EP4 receptor expression in colorectal cancer progression promotes cell growth and anchorage independence. Cancer Res. 2006;66(6):3106–13. doi: 10.1158/0008-5472.CAN-05-3702. [DOI] [PubMed] [Google Scholar]
- 38.Gee J, Lee IL, Jendiroba D, Fischer SM, Grossman HB, Sabichi AL. Selective cyclooxygenase-2 inhibitors inhibit growth and induce apoptosis of bladder cancer. Oncol Rep. 2006;15(2):471–7. [PubMed] [Google Scholar]
- 39.Bostrom PJ, Uotila P, Rajala P, Nurmi M, Huhtaniemi I, Laato M. Interferon-alpha inhibits cyclooxygenase-1 and stimulates cyclooxygenase-2 expression in bladder cancer cells in vitro. Urol Res. 2001;29(1):20–4. doi: 10.1007/s002400000149. [DOI] [PubMed] [Google Scholar]