Abstract
We identified and characterized four different recombination mechanisms involved in the cointegrative transfer of the Neisseria gonorrhoeae beta-lactamase plasmid pSJ5.2 by the gonococcal 41 kb tet(M) and the Gram negative self-transmissible plasmids N3 and R64 drd-33 using an Escherichia coli recA- background. Mobilization of pSJ5.2 by the tet(M) plasmid occurred by cointegration through a replicative transposition of two IS1 elements inserted upstream from the beta-lactamase gene of pSJ5.2 and creating a IS1::beta-lactamase hybrid promoter. Two types of recombinational events occurred within the 1.8 kb BamH1-HindIII fragment of pSJ5.2 with the N3 and R64 plasmids. A non-homologous recombination was found at coordinates 1817 and 2849 of pSJ5.2 with sequences from R64. A non-homologous recombination combined with an IS26-mediated one-ended transposition was found at coordinates 1817 and 3010 of pSJ5.2 with N3. In both recombinational events, a deletion of over 1 kb of pSJ5.2 occurred. The fourth recombination event was detected in the 1.0 kb BamH1-HindIII fragment of pSJ5.2 by homologous recombination between DNA from the truncated Tn3 resolvase gene of pSJ5.2 and the resolvase sequences from R64 and N3.
Keywords: Neisseria gonorrhoeae, beta-lactamase plasmids, mobilization, IS1, IS26, cointegration, non-homologous recombination
1. Introduction
The 5.2 kb beta-lactamase plasmid from Neisseria gonorrhoeae (the gonococcus) is one of the three small broad-host range and mobilizable resistant (R)-plasmids associated with the worldwide spread and prevalence of Pennicillinase-Producing Neisseria gonorrhoeae (PPNG) infections (Phillips, 1976; Ray et al., 2005; Scharbaai-Vázquez et al., 2007). The 5.2 kb R-plasmid is a mob/oriT deletion derivative of the parental 7.4 kb R-plasmid (Dillon et al., 1991; Pagotto et al., 2000; Candelas, 2000). These R-plasmids in the pathogenic gonococci have the potential to be transferred by conjugation to commensal Neisseria spp. and other endogenous flora, creating a pool of resistance genes in healthy hosts (Kirvin and Thornsberry, 1977; Sox et al., 1978; Flett et al., 1981; Guinney and Ito, 1982; Mc Nichol et al., 1983; Piffareti et al., 1988; Roberts and Knapp, 1988; Van Passel et al. 2006).
The conjugal transfer of the gonococcal 7.4 kb and the 5.6 kb R-plasmids has been extensively studied (Roberts and Falkow, 1977; Sox et al., 1978; Guinney and Ito, 1982; McNichol et al., 1983; Tenover et al., 1985; Ikeda et al., 1986; Pifaretti et al., 1988; Roberts and Knapp, 1988; Biswas et al., 1989; Dillon and Yeung, 1989; Gauthier, 1990; Dillon et al., 1991; Rodríguez-Bonano and Torres-Bauzá, 2004). These two R-plasmids carry a mobilization (mob) region encoding a specific endonuclease (relaxase) and an origin of transfer (oriT) that allow them to be efficiently mobilized with the help of either the co-resident gonococcal 38 kb or the 41 kb tetracyclineencoding tet(M) plasmid among gonococci and by the Incompatibility Group P (IncP-type) conjugative plasmids in Escherichia coli (Sox et al., 1978; Roberts and Knapp, 1988; Guinney and Ito, 1982; Rodríguez-Bonano and Torres-Bauzá, 2004). In the 5.2 kb R-plasmid, which has no known oriT, conjugal transfer has been reported in both a recA- E. coli and in the gonococcal natural host (Dillon and Yeung, 1989; Marquez et al., 1999). These observations suggest that the gonococcal R-plasmids may employ alternative mechanisms for plasmid conjugal spread.
We previously demonstrated the cointegration of the 5.2 kb R-plasmid isolated in San Juan, Puerto Rico (pSJ5.2) with the genomes of the Enterobacteria-derived R64 and N3 and the gonococcal 41 kb tet(M) conjugative plasmids in recA- E. coli (Scharbaai-Vázquez, et al., 2007). Stable R64::pSJ5.2 and N3::pSJ5.2 plasmid cointegrates lost pSJ5.2-derived DNA within the 1.8 kb BamH1-HindIII fragment of pSJ5.2 indicating that cointegrates formed by recombination within that fragment (Scharbaai-Vazquez, et al ., 2007). E. coli-to-E. coli mobilization of pSJ5.2 using N. gonorrhoeae providing the tet(M) conjugative plasmid was observed to occur by transposomal insertion of IS1 in the 2.4 kb BamH1-BamH1 fragment of pSJ5.2 (Scharbaai-Vázquez, et al., 2007). In addition, the 1.0 kb BamH1-HindIII fragment of pSJ5.2 was capable of mobilizing a pUC19 vector (pUC1.0) at frequencies of 1.9 × 10-7 and 2.2 × 10-7 transconjugants per donor cell in the presence of R64 and N3, respectively (unpubl. obs. from our laboratory). Therefore, each of the three BamH1-HindIII restriction fragments of pSJ5.2 contained active sites for cointegrative mobilization (Scharbaai-Vazquez, et al. 2007). E.coli transconjugants containing stable R64::pSJ5.2, N3::pSJ5.2, R64::pUC1.0 and N3::pUC1.0 cointegrates were isolated for further study.
The gonococci mobilize R-plasmids DNA through cointegration with self-transmisible plasmids (Sox et al., 1978; Dillon et al., 1991). Moreover, conjugative transfer of gonococcal plasmid DNA by cointegration and stable integration into the gonococcal genome was reported for the cryptic 4.2 kb plasmid (Johnson et al., 1983; Hagblom et al., 1986). It is well known that the gonococcus take up and integrate DNA from related species by transformation and homologous recombination, respectively (Reviewed in Kline, et al., 2003; Hamilton and Dillard, 2006). However, the study of the mechanisms of recombination during conjugation has been hindered by the very low frequency of conjugal mobilization of plasmids by cointegration. In addition, plasmid cointegrates often dissociate (resolve) rapidly into their components after conjugative transfer.
Plasmid cointegrates have a profound significance in genetic diversity and evolution, as a novel combination of genes is available for transfer (Brom et al., 2004). Some R-plasmids rely on cointegration with self-transmissible plasmids to circumvent limitations of spread, such as: (1) incompatibility of the transferred plasmids with those plasmids already present in the new host cells, (2) replication deficiency of plasmids in new hosts, (3) presence of restriction-modification systems in the new hosts, (3) functional inactivity of the resistance gene product in new hosts and (4) reduced expression of resistance genes in new hosts. The study of plasmid oriT-independent transfer is interesting due to the discovery of strategies to inhibit the relaxase enzyme required for oriT-mediated plasmid transfer (Fernández-López et al., 2005; Lujón et al., 2007).
We report here that pSJ5.2 utilizes four different mechanisms for oriT-independent cointegrative mobilization with the self-transmissible plasmids tested, and present evidence of several hot-spots for pSJ5.2 recombination with self-transmissible plasmids for its dissemination. In this paper we describe the sequence analysis of each of the recombination junctions leading to the cointegrative intermediates. Two non-homologous recombination events occurring within the 1.8 kb BamH1-HindIII fragment of pSJ5.2 with the N3 and R64 self-transmissible plasmids are described. An intermediate of a homologous recombination between the resolvase gene from the 1.0 kb BamH1-HindIII fragment of pSJ5.2 and the resolvases of N3 and R64 was identified. The insertion of approximately 800 bp comprising the IS1 transposon observed in the pSJ5.2-insertion derivatives was cloned and used as a probe to elucidate the source of the transposon. Therefore, pSJ5.2 provides a unique model to study alternative mechanisms for gonococcal R-plasmid transfer. Given that no functional oriT has been identified so far in the 5.2 kb R-plasmid, it is probable that transfer mechanisms by cointegration have evolved to ensure its survival.
2. Materials and Methods
2.1 DNA isolation and cloning procedures
The plasmid constructs used in this work are listed in Table 1. Plasmid and chromosomal DNAs were purified from E. coli and N. gonorrhoeae using the Strataprep plasmid Miniprep Kit® (Stratagene, La Jolla, CA) and QIAamp DNA Mini kit® (Qiagen, Valencia, CA), respectively. Restriction digests, ligations and transformations to E. coli DH10B were performed as specified by the manufacturers (New England Biolabs, Beverly, MA; Epicentre, Madison, WI; and Invitrogen Corp., Carlsbad, CA). Isolation and screening of plasmids from E. coli transconjugant colonies was done using a chemical lysis and boiling plasmid extraction method (Colony Fast-Screen Kit ®; Epicentre) followed by agarose gel electrophoresis.
Table 1.
Plasmids used in the study
| Plasmids | Relevant characteristics | Reference or source |
|---|---|---|
| Plasmid vectors | ||
| pUC19 | E. coli cloning vector; ampR, lacZα | Invitrogen, CA |
| Recombinant plasmids | ||
| pUC3.6 | 2.4 kb BamH1-BamH1 fragment plus an insert isolated from a pSJ5.2 insertion derivative and cloned into pUC19 |
This study |
| pUC1.0 | 1.0 kb BamH1-HindIII fragment of pSJ5.2 cloned into pUC19 |
Our laboratory |
| Clones derived from plasmid rescue experiments and subclones | ||
| pN5.2 | Miniplasmid obtained by rescue of pSJ5.2 from a N3::pSJ5.2 cointegrate |
This study |
| pUC3.0 | 3,010 bp BamH1-HindIII fragment from pN5.2 cloned into pUC19 |
This study |
| pR5.2 | Miniplasmid obtained from rescue of pSJ5.2 from a R64::pSJ5.2 cointegrate |
This study |
| pUC3.5 | 3, 475 bp BamH1-HindIII fragment from pR5.2 cloned into pUC19 |
This study |
| pN1.0 | Miniplasmid obtained from the rescue of pUC1.0 from a N3::pUC1.0 cointegrate |
This study |
| pR1.0 | Miniplasmid obtained from the rescue of pUC1.0 from a R64::pUC1.0 cointegrate |
This study |
Cloning of the 2.4 kb BamH1-BamH1, 1.8 kb BamH1-HindIII and 1.0 kb BamH1-HindIII fragments of pSJ5.2 into pUC19 was done in order to analyze the minimal region required for pSJ5.2 transfer (data not shown). Among the clones generated (pUC2.4, pUC1.8 and pUC1.0, respectively), the pUC1.0 construct was selected for study of its cointegrative properties in the presence of N3 and R64 plasmids.
Plasmid pUC3.2 was constructed by cloning the 2.4 kb BamH1-BamH1 fragment of a pSJ5.2 IS-derivative transmitted with the help of the tet(M) conjugative plasmid into pUC18. This fragment contains the IS1 insertion.
2.2 Rescue cloning of DNA surrounding the recombination junctions
Isolation of recombination junctions between pSJ5.2 and the self-transmissible plasmids was made by rescue of pSJ5.2 from the R64::pSJ5.2, N3::pSJ5.2, R64::pUC1.0 and N3::pUC1.0 cointegrates. E. coli transconjugants containing the cointegrates were isolated from LB plates containing both ampicillin and tetracycline and subjected to plasmid DNA extraction by alkaline lysis (Sambrook et al., 1989). Residual bacterial genomic DNA and single-stranded fragments were removed from DNA lysates by incubation with an ATP-dependent DNase (Plasmid-Safe®; Epicentre). The R64::pSJ5.2 and N3::pSJ5.2 DNA lysates were digested with AlwN1 restriction enzyme, which digests the conjugative plasmid but does not cut the integrated pSJ5.2. The BglII restriction enzyme was used for the digestion of N3::pUC1.0 DNA, since it does not cut either the 1.0 kb insert nor the pUC19 vector. Likewise, the EcoRV and Bsm1 restriction enzymes were used to cut the R64::pUC1.0 cointegrate. The DNA fragments were self-ligated and transformed into E. coli DH10B cells (Invitrogen), with subsequent selection for resistance to ampicillin. The resultant ampicillin-resistant (ampR) colonies were then screened for plasmids larger than the size of pSJ5.2 or pUC1.0. The miniplasmids were then mapped by double digestion with BamH1-HindIII and the restriction patterns compared with those of wild type pSJ5.2 and pUC1.0. The fragments obtained upon digestion of R64::pUC1.0 and N3::pUC1.0 miniplasmids were compared with those obtained with the parental pUC1.0.
2.3 Plasmid constructs derived from the rescue of pSJ5.2 from cointegrates
Plasmid pN5.2 resulted from the rescue of pSJ5.2 from a N3::pSJ5.2 cointegrate (Table 1). The pN5.2 construct was double digested with BamH1-HindIII and the resultant restriction fragment containing a “nonconserved” 3.0 kb BamH1-HindIII fragment which replaced the wild type 1.8 kb BamH1-HindIII fragment was subcloned into pUC19, producing pUC3.0. Likewise, plasmid pR5.2 was obtained by rescue of pSJ5.2 from a R64::pSJ5.2 cointegrate. Double digestion with BamH1-HindIII produced a non-conserved 3.5 kb restriction fragment instead of the 1.8 kb fragment. Cloning of the 3.5 kb fragment into pUC19 produced pUC3.5.
Plasmids pN1.0 and pR1.0 resulted from the rescue of clone pUC1.0 from the N3::pUC1.0 and R64::pUC1.0 cointegrates; respectively.
2.4 Polymerase Chain Reaction, non-radioactive probes and Southern Blot experiments
The oligonucleotides used for the generation of non-radioactive probes, pUC-derived clones and the recombination junctions within pN5.2 and pR5.2 are listed in Table 2. Synthesis of oligonucleotides was carried out at the RCMI-Molecular Genetics Core Facility, at Medical Sciences Campus, University of Puerto Rico.
Table 2.
Oligonucleotides used in the study.
| Oligonucleotides | Description / (Coordinates, bp) |
Nucleotide sequence (5′ – 3′) |
Source |
|---|---|---|---|
| Primers for PCR amplification of IS1 | |||
| IS1F119 | InsB gene from IS1 (421)* | AGCTCCACCGATTTTGAGAA | This study |
| IS1R119 | InsB gene from IS1, reverse (520)* | AAGCTGCACGTAATCAGCAA | |
| Sequencing Primers for pUC19-derived clones | |||
| M13F | Universal Primers | GTTTTCCCAGTCACGAC | Invitrogen |
| M13R | CAGGAAACAGCTATGAC | ||
| Walking Primers for Sequencing IS1 in pUC3.6 | |||
| M13FI | Primer based on M13 right border sequence |
AGCTCCACCGATTTTGAGAA | This study |
| M13FII | Primer based on M13 FI right border sequence |
TCTCTGCTCTCACTGCCGTA | This study |
| TEM-P 1R | Primer based on TEM-1 beta lactamase flanking sequence and used to close the gap between beta-lactamase and IS1 |
CGCCGCATACACTATTCTCA | This study |
| Primers for sequencing junctions within pN5.2 and pR5.2 | |||
| REPA | Anneal adjacent to BamH1 site from pSJ5.2 repA gene (1497) |
TATTGCGTGAATTGCCCTTT | This study |
| REPB | Anneal adjacent to HindIII site from pSJ5.2 repB gene (3296, reverse) |
TGCGGATTCTGGCTTTAACT | This study |
Coordinates 421 to 520 are relative to the IS1 mapped within the pSJ5.2 insertion derivatives
To elucidate the source of the IS1 isolated in previous triparental crosses (Scharbaai-Vazquez, 2006), a non-radioactive probe for IS1 was generated. Primers IS1F119 and IS1R119 were specific for the InsB gene of IS1 (Table 2 and Fig. 2b). A 117 bp PCR product was generated with the primers annealed at coordinates 421-520 respective to a pSJ5.2::IS1 derivative used as template. The probe was used in Southern Blot hybridizations involving EcoR1-digested chromosomal DNA from N. gonorrhoeae and E. coli.
Figure 2.
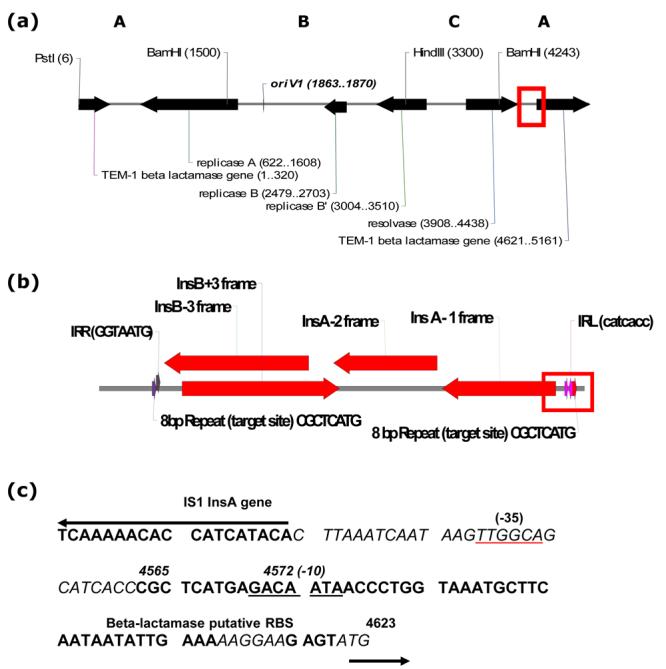
IS1 integration within the 2.4 kb BamH1-BamH1 site (A region) of pSJ5.2 after transfer by tet(M) conjugative plasmid. (a) Pst1-linearized map of pSJ5.2 showing the site of IS1 insertion (open red square). Refer text for details. (b) Detail of the two overlapped IS1 copies found inserted in the pSJ5.2 derivative. Open Reading Frames and direction of transcription of the InsB and InsA genes are shown in red solid arrows. Red open square denote the formation of an IS1-beta lactamase hybrid promoter. (c) The DNA sequence of the IS1-beta-lactamase hybrid promoter in detail (Accession Number EF527806). Only the promoter region of the InsA gene from one IS1 and part of the pSJ5.2 beta-lactamase gene promoter are shown. Coordinates are in bp and relative to pSJ5.2. InsA gene and the 28 bp inverted repeat are shown in red and red italics, respectively. The beta-lactamase promoter region is shown in black. The 8 bp IS1 insertion site from pSJ5.2 is shown in blue. Black arrows denote direction of both InsA and beta-lactamase gene transcription. The -35 and -10 regions of the promoter are shown underlined. RBS (Ribosome Binding Site) in italics.
Insert DNA fragments in pUC3.6, pUC3.0 and pUC3.5 were sequenced using the M13 universal primers (Invitrogen, CA). Additional walking primers were designed to assemble the sequence of IS1 in clone pUC3.6 (Table 2).
Sequencing primers REPA and REPB were designed to anneal adjacent to the BamH1 site at the repA gene (coordinate 1497) and to the HindIII site from the repB gene (coordinate 3296) of pSJ5.2 in pN5.2 and pR5.2.
The integration of pUC1.0 into the genomes of N3 and R64 plasmids during previous mating assays was confirmed with a non-radioactive probe consisting of the 1.0 kb BamH1-HindIII fragment of pSJ5.2.
All probes were randomly labeled using the Genius nonradioactive labeling and detection kit (Roche Diagnostic Corp., Indianapolis, IN). Southern analysis of both total and restricted DNA of all donor and transconjugant bacteria was done according to the manufacturer.
2.5 Determination and analysis of nucleotide sequences
DNA sequencing reactions were performed with an ABI 310 automated DNA sequencer (Applied Biosystems, Foster City, CA) at the RCMI-Molecular Genetics Core Facility, at Medical Sciences Campus, University of Puerto Rico and SeqWright, Inc. (Houston, TX).
The nucleotide and the predicted protein sequences were analyzed with Visual Cloning, version 3.0 (Redasoft Corp., Ontario, Canada) and available tools at the National Center of Biotechnology Information website (http://www.ncbi.nlm.nih.gov). Assembly of IS1 DNA sequences was done with GeneStudio Professional Software (GeneStudio, Inc., Suwanee, GA).
2.6 Nucleotide sequence accession numbers
Nucleotide sequences were deposited at the NCBI GenBank database under accession nos. EF527803, EF527804, EF523805, EF523806, EF527807 and EF523808.
3. Results
3.1 The 1.8 kb BamH1-HindIII fragment of pSJ5.2 contains a site for non-homologous recombination which mediates cointegration with self-transmissible plasmids N3 and R64
Previously published physical maps of the N3::pSJ5.2 and R64::pSJ5.2 cointegrate DNA of transconjugants with BamH1 and HindIII revealed that a region within the 1.8 kb BamH1-HindIII fragment of pSJ5.2 is deleted during fusion with the R64 and N3 plasmids (Scharbaai-Vázquez et al., 2007; Region B in Fig. 1). Other than this deletion, analysis showed no differences in the restriction bands, allowing us to easily delimit the recombination ends upon selection of representative transconjugants containing the N3::pSJ5.2 and R64::pSJ5.2 plasmid cointegrates. Plasmid DNA from pSJ5.2 was rescued from the stable N3::pSJ5.2 and R64::pSJ5.2 plasmid cointegrates and the resultant pSJ5.2 miniplasmids containing fragments of the conjugative plasmid (pN5.2 and pR5.2, respectively) were then mapped by double digestion with BamH1 and HindIII.
Figure 1.
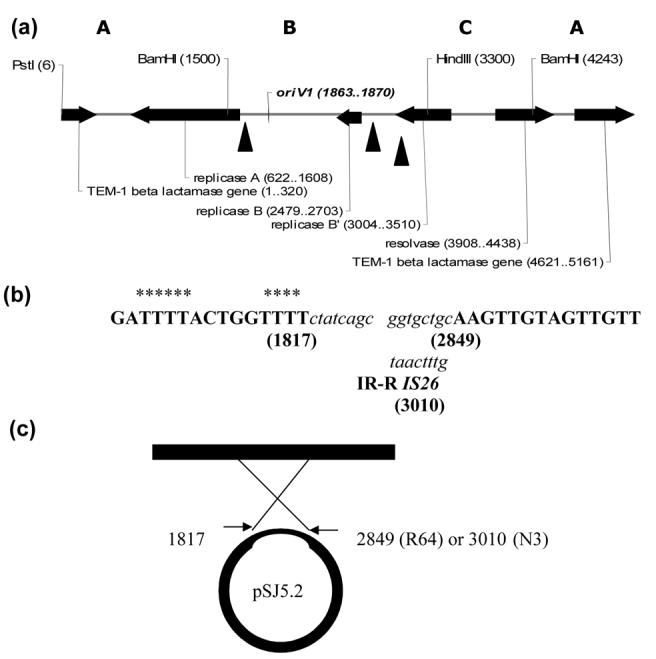
Hot spots for cointegration of N3 and R64 self-transmissible plasmids within the 1.8 kb BamH1-HindIII site of pSJ5.2 (B region). (a) The Pst1-linearized map of pSJ5.2 showing the enzymes used in the study. Letters A, B and C refer to the 2.4 kb, 1.8 kb and 1.0 kb fragments generated by double digestion with BamH1 and HindIII. Genes, origin of replication oriV1 and restriction enzyme coordinates are in parentheses. Hot-spots for cointegration are marked by three solid black triangles. (b) DNA sequence of the three recombinational crossover points at coordinates 1817, 2849 and 3010 of pSJ5.2; they were deposited in the GenBank under Accession Numbers EF527803, EF527804 and EF527805; respectively. The partial sequence from pSJ5.2 is shown in bold capital letters; partial sequences from N3 and R64 are shown in lower case italics letters. Parentheses denote the DNA coordinate of recombination. Asterisks represents AT-rich region at the 5′-end of junction. Refer text for details. (c) Schematic representation of the structure and proposed mechanism of the N3-pSJ5.2 and R64-pSJ5.2 cointegrates at the 1.8 kb BamH1-HindIII of pSJ5.2. The solid black rectangle represent DNA insert from N3 and R64; the thick loop represents pSJ5.2 with deleted DNA indicated by the thin region of the circle at the site of recombination.
In order to test the hypothesis of a recombination occurring within the 1.8 kb BamH1-HindIII fragment of pSJ5.2, the nucleotide sequences of the 5′– and 3′- junctions for pN5.2 and pR5.2 were determined and deposited in the GenBank database under Accession Numbers EF 527803, EF527804 and EF527805. Sequencing primers were designed to anneal close to the 5′-end and 3′-end recombinations, respectively. The results are summarized in Figures 1a and 1b. The analysis revealed the following: (i) the junctions at the 5′-end start at coordinate 1817 respective to pSJ5.2 and are the same for the N3::pSJ5.2 and R64::pSJ5.2 junctions tested — Accession Number EF527804, (ii) an AT-rich domain of pSJ5.2 was noted in each of the 5′-end junctions analyzed, (iii) the 5′-end junction is part of the Replicase, repA gene promoter and it is very close to the origin of replication 1, oriV1 described by Pagotto and Dillon (2001) which lies at coordinate 1867 in the parental 7.4 kb “Asian” R-plasmid, (iv) the junction at the 3′-end of the R64::pSJ5.2 cointegrate - Accession Number EF527803 - was mapped at coordinate 2849 from pSJ5.2, (v) an IS26 transposon was found at the 3′-end junction of the N3::pSJ5.2 cointegrate at coordinate 3010 — Accession Number EF527805; only the terminal inverted repeat is shown in the figure, (vi) no overlap nucleotides between pSJ5.2 and the respective conjugative plasmid sequences at the 5′ and 3′ — end junctions were found. These results indicate that recombination between pSJ5.2 and conjugative plasmid N3 was mediated by a non-homologous recombination at the 5′-end combined with a transposition of IS26 at the 3′-end. Recombination between pSJ5.2 and conjugative plasmid R64 was the result of two non-homologous recombinations occurring at both 5′-end and 3′-end borders of the fusion.
The 3.0 kb and 3.5 kb fragments generated from the digestion of pN5.2 and pR5.2 with BamH1 and HindIII, respectively were analyzed by sequencing. BLAST analysis of 3,010 bp sequence cloned into pUC19 (pUC3.0; Table1) confirmed the presence of IS26 at the N3 right border and at coordinates 2191 to 3010 respective to the fragment (data not shown). Sequence analysis of 3,475 bp fragment cloned into pUC19 (pUC3.5; Table 1) revealed 100% identity to R64 with no transposable element flanking either border of the junction (data not shown).
3.2 The Insertion element 1 (IS1) promoted cointegration of pSJ5.2 with the 41kb tet(M) self-transmissible plasmid by recruiting two IS1 elements from E. coli
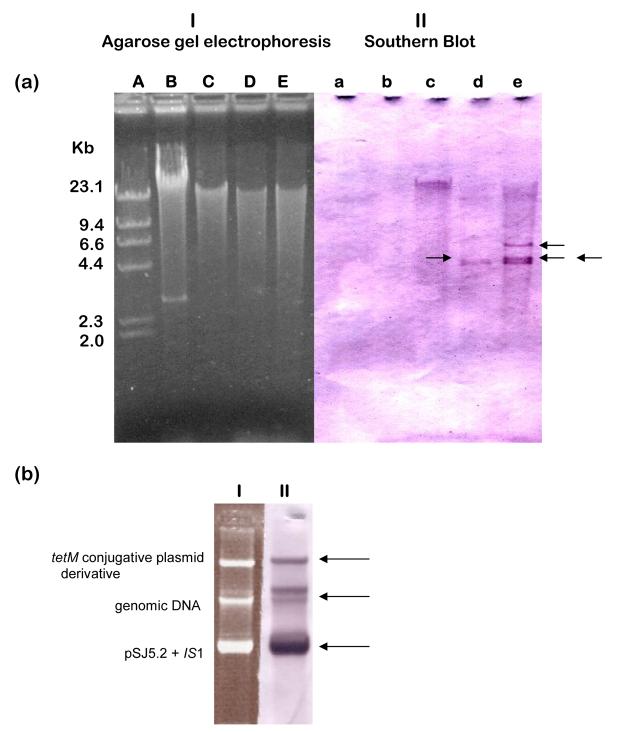
Mobilization of pSJ5.2 by the gonococcal 41 kb tet(M) self-transmissible plasmid using an intergeneric triparental cross resulted in E. coli transconjugants containing both the self-transmissible plasmid and pSJ5.2 derivative with an insertion sequence located within the 2.4 kb BamH1-BamH1 fragment (Scharbaai-Vazquez, et. al., 2007; Region A in Fig. 2a). The new BamH1-BamH1 fragment of about 3.6 kb was cloned in pUC19 (pUC3.6; Table 1) for sequence analysis. Sequence analysis revealed the presence of two overlapped copies of IS1 inserted in the 2.4 kb BamH1-BamH1 fragment of pSJ5.2 (Figure 2b). Moreover, the InsA gene from one of the IS1 copies was found inserted at position 4565 relative to pSJ5.2 and disrupting the promoter of the beta-lactamase gene creating an IS1::beta-lactamase hybrid promoter (Fig. 2c; Accession Number EF527806). The presence of two overlapped copies of IS1 inserted in the 2.4 kb BamH1-BamH1 fragment of the pSJ5.2 transconjugants produced with the tet(M) plasmid suggests an unusual transposition event. To gain insight into the role of IS1 in pSJ5.2 cointegrative mobilization by tet(M), the genomic DNA from the bacterial strains used in the triparental cross were evaluated for the presence of IS1 by Southern blot hybridization with an IS1-derived probe (Experimental Procedures). The results demonstrate that both the intermediate and recipient strains of E. coli, but not N. gonorrhoeae contained sequences that could have been the source of the two IS1 elements (Figure 3a; Panel II).
Figure 3.
Copy number and distribution of the Insertion Sequence IS1 in the Neisseria gonorrhoeae and Escherichia coli strains used in the study. (a) Agarose gel electrophoresis of EcoR1-digested genomic DNA’s (Panel I) and Southern Blot hybridization with an IS1-derived non-radioactive probe (Roche, IN; Panel II). Lane Aa, Lambda + Hind III control (NEB Labs); Lane Bb, N. gonorrhoeae 15315 (showing the undigested 4.2 kb cryptic plasmid); Lane Cc, E. coli DH5α; Lane E. coli DH10B; Lane Ee, E. coli HMS174. Black arrows indicate the IS1 hybridization signals. (b) Undigested DNA from a representative E. coli HMS174 transconjugant showing the conjugative teM and pSJ5.2 both resolved (Panel I) and with at least one IS1 hybridization signal each (black arrows in Panel II).
3.3 The 1.0 kb BamH1-HindIII fragment of pSJ5.2 contains a DNA site for cointegrative transfer by homologous recombination with R64 and N3 self-transmissible plasmids
The mobilization properties of the 1.0 kb BamH1-HindIII fragment from pSJ5.2 (Region C in Fig. 1) cloned into a nonmobilizable pUC19 vector prompted us to investigate the site and mechanism responsible for this type of transfer. Fifteen pUC1.0 ampR transconjugants produced in the presence of conjugative plasmids R64 or N3 were randomly selected for analysis. Plasmid DNA content of the representative transconjugants analyzed by agarose gel electrophoresis is shown in Fig. 4. Analysis of transconjugants did not reveal the presence of a plasmid of 3.7 kb, which is the size of pUC1.0 (Fig. 4; lanes C to E and lanes G to M in panel I). Southern Blot hybridization using a non-radioactive 1.0 kb BamH1-BamH1 fragment of pSJ5.2 as probe revealed the integration of pUC1.0 into both R64 and N3 conjugative plasmids (Fig 4; Panel II; lanes c to e and g to m). Double digestion of the integrated pUC1.0 DNA with BamH1 and HindIII revealed that this 1.0 kb fragment is not conserved, indicating that integration occurred within that fragment (Fig 5). A weak hybridization signal was also detected using the 1.0 kb BamH1-HindIII probe in the undigested R64 and N3 DNA from the donor E. coli strains (Figure 4, lanes Bb and Ff; top of gel), which may indicate some homology between DNA regions in the 1.0 kb fragment and the N3 and R64 self-transmissible plasmids. This, in turn, suggests that integration occurred between homologous sequences in the 1.0 kb fragment and the self-transmissible plasmids. To test this hypothesis, the pUC1.0-derived plasmid was rescued from the N3::pUC1.0 and R64::pUC1.0 cointegrates. The resultant miniplasmids (pN1.0 and pR1.0, respectively; Table 1) were used as DNA template for the sequencing of the N3::pUC1.0 and R64::pUC1.0 junctions using the M13 universal primers. The results are shown in Figure 6. Analysis of the R64::pUC1.0 revealed overlap of DNA sequences between the pSJ5.2 resolvase and the Tn5393 transposase from R64 (Figure 6A; diagram I; Accession Number EF527807). Likewise, the Tn3 resolvase from the pSJ5.2 plasmid was found to overlap with the Tn2 resolvase from the IS26 in conjugative plasmid N3 (Fig. 6A; diagram II; Accession Number EF527808). Only the right arm of the inverted repeat from IS26 (IRR) was found, which indicates that IS26 is partially deleted at the resolvase region. The results suggest that the N3::pUC1.0 and R64::pUC1.0 cointegrates were formed by homologous recombination between the truncated resolvases found on both plasmids.
Figure 4.
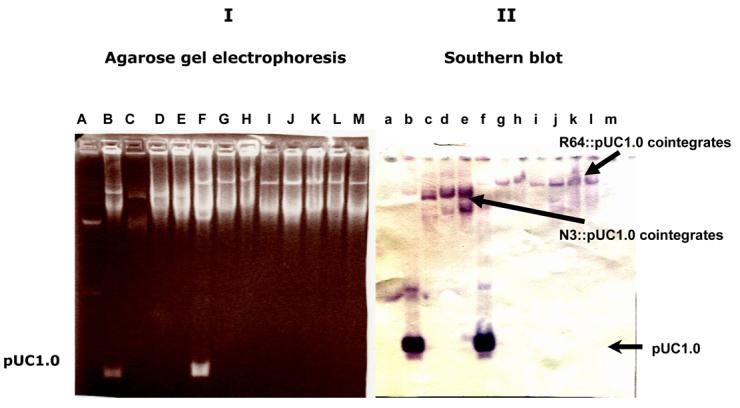
Formation of N3::pUC1.0 and R64::pUC1.0 cointegrates during mobilization of pUC1.0 with self-transmissible plasmids N3 and R64. (I) Agarose gel electrophoresis of undigested DNA. (II) Southern Blot hybridization with a 1.0 kb BamH1-HindIII probe from pSJ5.2. Lanes Aa - BacTracker supercoiled DNA ladder (Epicentre, Madison WI); Lanes Bb - Donor cell with pUC1.0 and N3 plasmids located at the bottom at top of gel, respectively; Lanes Cc to Ee — representative N3/pUC1.0 transconjugants; Lanes Ff — Donor cell with pUC1.0 and R64 plasmids located at bottom and top of gel, respectively; Lanes Gg to Mm — Representative R64/pUC1.0 transconjugants.
Figure 5.
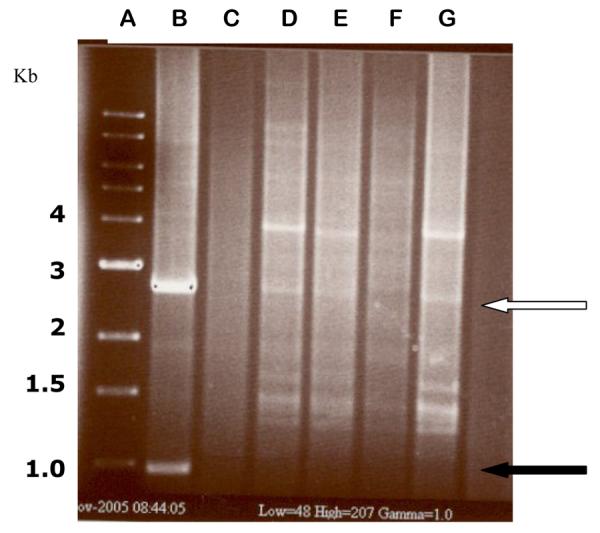
BamH1-HindIII restriction analysis of representative ampicillin-resistant E. coli transconjugant colonies containing N3::pUC1.0 and R64::pUC1.0 plasmid cointegrates. The DNA’s were analyzed with a rapid boiling DNA extraction and restriction screening method (Epicentre; Madison, WI). Lane A - 1 kb control ladder; Lane B - pUC1.0 control with the 2.9 kb band from pUC19 (white arrow) and the 1.0 kb fragment derived from pSJ5.2 (solid black arrow); Lane C - digested R64::pSJ5.2 DNA; D and E - R64::pUC1.0 transconjugants; Lanes F and G -N3::pUC1.0 transconjugants (the 2.9 kb band from pUC19 is barely visible in lane F). The 1.0 kb BamH1-HindIII fragment is not visible in the cointegrates analyzed from lanes D to G, confirming that integration occurred within that fragment.
Figure 6.
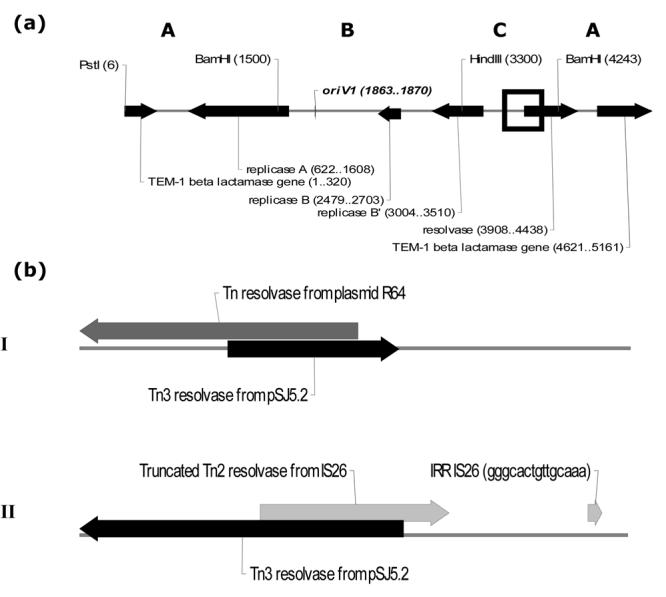
Resolvase-mediated homologous recombination between the conjugative plasmids N3 and R64 and the 1.0 kb BamH1-HindIII fragment of pSJ5.2 (C region). (a) Pst1- linear map of pSJ5.2 showing the site of recombination (open black square). (b) Description of the recombination intermediates recovered in the R64::pUC1.0 (I) and N3::pUC1.0 (II) plasmid cointegrates. The sequences were deposited in the GenBank database under Accession Numbers EF527807 and EF527808, respectively. The Tn3 resolvase gene of pSJ5.2 is shown in solid black arrow. The Tn5393 resolvase from R64 and the IS26-derived resolvase from N3 are shown in dark and light gray arrows, respectively. (IRR) = Right Inverted Repeat from IS26. Note that the scale is different in the two panels.
4. Discussion
Previous reports of the gonococcal beta-lactamase plasmids mobilization have shown that different conjugative plasmids recognized different DNA sites involved in mobilization (McNichol et al., 1983; Tenover et al., 1985; Dillon et al., 1991; Piffaretti et al., 1988; Gauthier, 1990). Here we show that the mechanisms for conjugal mobilization of the 5.2 kb beta lactamase plasmid pSJ5.2 involve all three BamH1-HindIII restriction fragments and depend on cointegration with conjugative plasmids.
Cointegration can occur by: (a) recombination between homologous regions on the two plasmids; also called rec-dependent, and (b) non-homologous or rec-independent mechanisms which include: site-specific recombination mediated by integrases, resolvases, invertases; transposition of an Insertion Sequence (IS) element residing on either plasmid; and illegitimate recombination (Reimmann and Haas, 1993). Conjugative transfer of a small R-plasmid by cointegration with a larger self-transmissible plasmid rarely involves more than one mechanism of recombination. In Gram-negative bacteria, Gram-positive bacteria and rhizobia, cointegration has been reported to occur through one type of RecA-dependent recombination among homologous sequences or through one specific cointegration involving transposon, insertion sequences or specific att sites (Merlin, et al. 2000; Miller and Cohen, 1980; Hille et al., 1983 Leclaporn et al., 1996; Vaudequin-Dransart et al., 1998; Trotter et al., 2004; Brom et al., 2004). To our knowledge, this is the first report of a naturally-occurring small resistance plasmid that employs four different mechanisms for cointegrate formation at three different sites to ensure its spread. We had found that the type of recombination leading to the cointegration of pSJ5.2 with different conjugative plasmids occurred at different frequencies, which reflects the varied efficiencies of each event (Scharbaai-Vázquez, 2006).
In order to deduce the recombination mechanisms that occurred in the three BamH1-HindIII fragments of pSJ5.2, we analyzed representative N3::pSJ5.2, N3::pUC1.0, R64::pSJ5.2, and R64::pUC1.0 stable cointegrates and the pSJ5.2::IS1 insertion derivatives. The use of a recA-deficient E. coli host for mating assays reduced the likelihood of mobilization via possible homologous recombination with the self-transmissible plasmids, and facilitated the trapping of stable cointegrate intermediates for the study of the plasmid-plasmid junctions. Analysis of pSJ5.2 mobilization showed that the plasmid utilizes two non-homologous recombination mechanisms in the 1.8 kb BamH1-HindIII fragment and a replicative transposition of IS1 in the 2.4 kb BamH1-BamH1 fragment. A fourth cointegrative mechanism consisted of a resolvase-mediated homologous recombination in the 1.0 kb BamH1-HindIII fragment of pSJ5.2. This suggests that each fragment contains at least one hot-spot site for mobilization by cointegration with self-transmissible plasmids.
A hot-spot for non-homologous recombination with self-transmissible plasmids N3 and R64 was found in the 1.8 kb BamH1-HindIII fragment of pSJ5.2. Moreover, nucleotide sequence analysis of the N3::pSJ5.2 and R64::pSJ5.2 junctions (pN5.2 and pR5.2; respectively) revealed two different types of non-homologous recombination (Fig. 1b). Interestingly, nucleotide sequences at the junctions showed that both R64 and N3 recombined at the same coordinate (1817) within the 1.8 kb BamH1-HindIII fragment from pSJ5.2. We refer to coordinate 1817 as the 5′-end of the junction. The fact that two unrelated conjugative plasmids can recombine at the same spot in the pSJ5.2 lead us to search for distinctive structural characteristics in the site surrounding the 1817 coordinates. Computer analysis of the ORF flanking coordinate 1817 from pSJ5.2 (repA; GenBank Accession No. DQ355980) using the BLAST homology search (Altschul et al., 1997) was performed. The repA gene showed significant homologies with the conserved domain of the type I topoisomerases which is known to have single-strand nicking and joining activity during plasmid replication (Horowitz and Deonier, 1985; Ikeda, H., 1986; Michel and Ehrlich, 1986; Shuman, 1989; Marsin et al., 2000; Zhu and Schiestl,1996 and 2004). This activity could mistakenly join DNA strands from R64 or N3 promoting the illegitimate intermolecular recombination event with pSJ5.2. This activity is consistent with previous in vitro nicking assays in which a non-oriT related nic site was located in the 1.8 kb BamH1-HindIII fragment from the 7.4 kb gonococcal beta-lactamase “Asian” plasmid p22209 (McNichol et al., 1983).
In contrast with the 5′-end junction, analysis of the 3′-end recombination junctions of N3 and R64 within the 1.8 kb BamH1-HindIII fragment of pSJ5.2 revealed structural differences which suggest differences in the mechanism of cointegrate formation. The IS26 found at the 3′-end of the pSJ5.2-N3 junction is 820 bp in length, and is known to form cointegrates by generation of directly repeated copies of IS26 present at each cointegrate junction by a replicative transposition mechanism (Mollet et al., 1983; Brown et al., 1984; Chandler and Mahillon, 2002; Kim et al., 2002). It is known that IS-mediated cointegration can mediate the transfer of the entire DNA sequence of a nonconjugative plasmid by a conjugative one (Bennett et al., 1988).The presence of one copy of IS26 could be consistent with the formation of N3::pSJ5.2 cointegrates by one-ended transposition, which is another type of illegitimate recombination mechanism described by Reimmann and Haas (1993). However, deletion of the sequence between the two recombination sites is not a consequence of such mechanism (Reimmann and Haas, 1993). Moreover, no deletion of DNA associated with the transposition of IS26 has been reported previously (Mollet et al., 1983; Brown et al., 1984).The IS26-mediated one-ended transposition occurring at the 3′-end of the fusion, combined with the non-homologous recombination event found at the 5′-end in the N3:: pSJ5.2 cointegrates suggest that two recombination mechanisms occurred simultaneously at the two ends (Fig. 1b).
Although there were differences in the mechanisms for pSJ5.2 cointegration with N3 and R64 in the 1.8 kb BamH1-HindIII fragment, the net results were the same: crossover between coordinates 1817 and 2849 or 3010 led to the deletion of ≈1,032 bp or 1193 bp from pSJ5.2 during the intermolecular R64::pSJ5.2 and N3::pSJ5.2 non-homologous recombination event, respectively (Figure 1c). Based on these observations, we conclude that the 1.8 kb BamH1-HindIII fragment of pSJ5.2 contains hot-spots for intermolecular recombination which promote the formation of pSJ5.2 fusions with conjugative plasmids N3 and R64 during conjugal transfer.
Mobilization of pSJ5.2 by the 41 kb tet(M) self-transmissible plasmid in E. coli involved IS1-mediated cointegration. It is known that the Insertion Sequence 1 play an active role in the formation of plasmid cointegrates and resolution by a replicative site-specific recombination mechanism (Crisona et al., 1980; Masepohl and Puehler, 1983; Elhai et al., 1994; Mahillon and Chandler, 1998; Martinez and De la Cruz, 1998; Shiga et al., 2001; Grindley, N., 2002). The presence of two IS1 copies within the 2.4 kb BamH1-BamH1 fragment in the pSJ5.2 insertion derivatives isolated from the E. coli transconjugants is compatible with the presence of at least one copy of IS1 in both the 41 kb tet(M) conjugative and pSJ5.2 plasmids. However, we did not find IS1 Southern hybridization signals in the N. gonorrohoeae genomic and plasmids DNAs. Therefore, the genomic DNA from the E. coli strains used in the previous triparental matings (Scharbaai-Vázquez, et al. 2007) were the source of the IS1. To our knowledge, this type of IS recruitment to form plasmid cointegrates has not been reported. In addition, the results demonstrate that IS1 is likely to play an important natural role in mobilizing plasmids across the species and generic boundaries between Neisseria and non-related Gram negative bacteria.
The IS1 insertion element possesses outwardly directed −35 promoter hexamers located in the terminal inverted repeats that creates a hybrid promoter when transposing at the correct distance from a resident −10 hexamer (Iida et al., 1987; Olliver et al., 2005). The effect of the new hybrid promoter on the TEM-beta lactamase gene of pSJ5.2 remains to be elucidated. Other transposable elements show similar mechanisms of altering expression of adjacent genes (Barker and Matsumura, 2004; Siu et al., 2003; Olliver et al., 2005).
Our evaluation of recombination events occurring at the 1.0 kb BamH1-HindIII fragment of pSJ5.2 indicates that the region contains a hot spot for pSJ5.2 cointegration with self-transmissible plasmids N3 and R64. Previous BLAST computer analysis of the 1.0 kb BamH1-HindIII fragment from pSJ5.2 (GenBank Accession No. DQ355980) revealed that it contains part of the ORF which corresponds to coordinates 3908-4438 of the Tn3 resolvase, TnpR gene (Scharbaai-Vázquez, et al., 2007; Fig. 6). The TnpR gene is a site-specific recombinase involved in the process of cointegrates resolution and together with the transposase, TnpA and beta-lactamase, bla genes constitute the Tn3 compound transposon. In the gonococcal R-plasmids, however, the whole TnpA and approximately 84% of the TnpR genes are truncated by a natural deletion (Candelas, 2000). Nevertheless, the remnant of the TnpR gene in pSJ5.2 can recombine with other conjugative plasmids containing Tn3 transposons such as N3 and R64, provided that their resolvase DNA regions have significant homologies with it. In the case of the R64::pUC1.0 junction, crossover between the two plasmids comprises a 155 bp homologous DNA region within the resolvase genes (Figure 6b). Similarly, the DNA homology between the resolvases from pSJ5.2 and N3 comprises a 209 bp overlapped region (Figure 6b). BLAST analysis of the pSJ5.2 Tn3 partial resolvase gene also showed between 89 to 100% similarity at the nucleotide level to several resolvases from the Tn1, and Tn2 families of resolvases (data not shown). Therefore, plasmids with resolvases that share extensive homology with the resolvase of pSJ5.2 could form cointegrates. The 1.0 kb fragment of pSJ5.2 may therefore function as a hot-spot site for homologous recombination with resolvase-encoding regions.
Analysis of the four mechanisms of recombination reported here for the conjugal transfer of pSJ5.2 by cointegration (IS1 replicative transposition, illegitimate recombination, non-homologous combined with IS26 transposition and homologous recombination), suggests that N. gonorrhoeae possesses efficient systems for both homologous and non-homologous recombination in a heterologous background. It is of particular interest of what is the role of illegitimate recombination in lateral transfer of genes in the gonococcal genome, due to the fact that N. gonorrhoeae can associate with non-related STD pathogens and commensal organisms from the urogenital flora (Kroll et al. 1998; Reid, 2005).
5. Concluding remarks
The Neisseria gonorrhoeae beta-lactamase encoding (R)-plasmids have evolved into highly successful genetic elements; a characteristic that is shared by its pathogenic host. While gonococcal 7.4 kb and 5.6 kb R-plasmids depend on an oriT / mob system for their conjugal transfer by self-transmissible plasmids, the 5.2 kb R-plasmid has developed an impressive capability of spread and survival using multiple recombinational mechanisms. Multiple sequences that can serve as recombination targets compensate for the lack of a functional oriT for mobilization. This repertoire of recombination events could be a rudimentary, yet efficient mechanism for the mobilization of pSJ5.2 among gonococci. The results of this study might provide a foundation to identify strategies that would abrogate mobilization functions in the resistance plasmids (e.g., antisense RNA or agents that block cis-acting sites involved in recombination). On the other hand, this unique experimental system for mobilization of the naturally occurring β-lactamase plasmid pSJ5.2 can be used to provide the basis for the design of integrative plasmid recombination assays which may help to understand the role of genes involved in DNA recombination in the gonococcus. Moreover, it can be used to design novel recombination gene delivery vectors in Neisseria species and other pathogenic Gram negative bacteria.
Acknowledgements
We thank Dr. Michael J. Leibowitz from University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School for critical reading of this manuscript. We thank Dr. Carlos Sariol and Roberto Medina for their technical assistance. This research work was made possible by the support of: NIH-MBRS-SCORE — S06 GM08224, NIH-MBRS-RISE GM68138 and the Biomedical Sciences Deanship, University of Puerto Rico, Medical Sciences Campus.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. “Gapped BLAST and PSI-BLAST”: A new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker CS, Pruss BM, Matsumura P. Increased motility of Escherichia coli by insertion sequence element integration into the regulatory region of the flhD operon. J. Bacteriol. 2004;186:7529–7537. doi: 10.1128/JB.186.22.7529-7537.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett PM, Grinsted J, Foster TJ. Detection and use of transposons. In: Grinsted J, Bennett PM, editors. Plasmid Technology. Method. Microbiol. Academic Press; London: 1988. pp. 205–231. [Google Scholar]
- Biswas GD, Thompson SA, Sparling F. Gene transfer in Neisseria gonorrhoeae. Clin. Microbiol. Rev. 1989;2:S24–S28. doi: 10.1128/cmr.2.suppl.s24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brom S, Girard L, Garrido CT, los Santos A. García-de, Bustos P, González V, Romero D. Transfer of the symbiotic plasmid of Rhizobium etti CFN42 requires cointegration with p42a, which may be mediated by site-specific recombination. J. Bacteriol. 2004;186:7538–7548. doi: 10.1128/JB.186.22.7538-7548.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AMC, Coupland GM, Willetts NS. Characterization of IS46, an insertion sequence found on two IncN plasmids. J. Bacteriol. 1984;159:472–481. doi: 10.1128/jb.159.2.472-481.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candelas T. Complete sequencing of a 5.2 kb San Juan R-plasmid from Neisseria gonorrhoeae and analysis of a DNA deletion on its mobilization capacity by co-conjugation. Thesis. University of Puerto Rico, Medical Sciences Campus; San Juan, Puerto Rico: 2000. [Google Scholar]
- Chandler M, Mahillon J. In: Mobile DNA II. Craig NL, Craigie R, Gellert M, Lambowitz AM, editors. American Society of Microbiology Press; Washington, DC: 2002. pp. 305–366. [Google Scholar]
- Crisona NJ, Nowak JA, Nagishi H, Clark AJ. Transposon-mediated conjugational transmission of nonconjugative plasmids. J. Bacteriol. 1980;142:701–713. doi: 10.1128/jb.142.2.701-713.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon JR, Yeung K-Y. Beta-lactamase plasmids and chromosomally- mediated antibiotic resistance in pathogenic Neisseria species. Clin. Microbiol. Rev. 1989;2:S125–133. doi: 10.1128/cmr.2.suppl.s125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon JR, Yeung K-Y, Gauthier BR, Hannah KA.Achtman M, Kohl P, Marchal C, Morelli G, Seiler A, Thiesen B.Mobilization of naturally-occurring gonococcal penicillinase-producing plasmids by different conjugative plasmids Neisseriae 1990, Proceedings of the seventh international pathogenic Neisseria conference 1991511–516.Walter de GruyterBerlin, Federal Republic of Germany. [Google Scholar]
- Elhai J, Cai Y, Wolk P. Conduction of pEC22, a plasmid coding for MR EcoT22I, mediated by a resident Tn3-like transposon, Tn5396. J. Bacteriol. 1994;176:5059–5067. doi: 10.1128/jb.176.16.5059-5067.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-López R, Machón C, Longshaw CM, Marin S, Molin S, Zechner EL, Espinosa M, Lanka E, de La Cruz F. Unsaturated faty acids are inhibitors of bacterial conjugation. Microbiology. 2005;151:3517–3526. doi: 10.1099/mic.0.28216-0. [DOI] [PubMed] [Google Scholar]
- Flett F, Humphreys GO, Saunders JR. Intraspecific and intergeneric mobilization of non-conjugative resistance plasmids by a 24.5 Megadalton conjugative plasmid of Neisseria gonorrhoeae. J. Gen. Microbiol. 1981;125:123–129. doi: 10.1099/00221287-125-1-123. [DOI] [PubMed] [Google Scholar]
- Gauthier BR. Development of a novel shuttle system for the transfer of gonococcal genes between Escherichia coli and Neisseria gonorrhoeae. Master Thesis. University of Ottawa; Ontario, Canada: 1990. [Google Scholar]
- Grindley NDF. The movement of Tn3-like elements: Transposition and Cointegrate resolution. In: Craig NL, Craigie R, Gellert M, Lambowitz AM, editors. Mobile DNA II. American Society of Microbiology Press; Washington, DC: 2002. pp. 272–302. [Google Scholar]
- Guinney DG, Ito JI. Transfer of the gonococcal penicillinase plasmid: mobilization in Escherichia coli by IncP plasmids and isolation as a DNA-protein relaxation complex. J. Bacteriol. 1982;150:298–302. doi: 10.1128/jb.150.1.298-302.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagblom P, Korch C, Jonsson A-B, Normark S. Intragenic variation by site-specific recombination in the cryptic plasmid of Neisseria gonorrhoeae. J. of Bacteriol. 1986;167(1):231–237. doi: 10.1128/jb.167.1.231-237.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton HL, Dillard JP. Natural transformation of Neisseria gonorrhoeae: from DNA donation to homologous recombination. Mol. Microbiol. 2006;59(2):376–385. doi: 10.1111/j.1365-2958.2005.04964.x. [DOI] [PubMed] [Google Scholar]
- Hille J, Van Kan J, Klasen I, Schilperoort R. Site-directed mutagenesis in Escherichia coli of a stable R772::Ti cointegrate plasmid from Agrobacterium tumefaciens. J. Bacteriol. 1983;154:693–701. doi: 10.1128/jb.154.2.693-701.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz B, Deonier RC. Formation of traF’ plasmids: Specific recombination at oriT. J. Mol. Biol. 1985;186:267–274. doi: 10.1016/0022-2836(85)90103-2. [DOI] [PubMed] [Google Scholar]
- Iida S, Kulka I, Meyer J, Arber W. Amplification of drug resistance genes flanked by inversely repeated IS1 elements: Involvement of IS1-promoted DNA Rearrangements before amplification. J. Bacteriol. 1987;169:1447–1453. doi: 10.1128/jb.169.4.1447-1453.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda FA, Tsuji A, Kaneko Y, Nishida M, Goto S. Conjugal transfer of beta-lactamase-producing plasmids of Neisseria gonorrhoeae to Neisseria meningitidis. Microbiol. Immunol. 1986;30:737–742. doi: 10.1111/j.1348-0421.1986.tb03000.x. [DOI] [PubMed] [Google Scholar]
- Ikeda H. Bacteriophage T4 DNA topoisomerase mediates illegitimate recombination in vitro. Proc. Natl. Acad. Sci. USA. 1986;83:922–926. doi: 10.1073/pnas.83.4.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SR, Anderson BE, Biddle JW, Perkins GH, DeWitt WE. Characterization of concatameric plasmids of Neisseria gonorrhoeae. Infect. Immune. 1983;40(2):843–846. doi: 10.1128/iai.40.2.843-846.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Shin H-S, Seo S-Y, Cho D-T. Relationship between bla SHV-12 and bla SHV-2a in Korea. J. Antimicrob. Chemother. 2002;49:261–267. doi: 10.1093/jac/49.2.261. [DOI] [PubMed] [Google Scholar]
- Kirvin LA, Thornsberry C. Transfer of beta-lactamase genes of Neisseria gonorrhoeae by conjugation, Antimicrob. Agents Chemother. 1977;11:1004–1006. doi: 10.1128/aac.11.6.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline KA, Sechman EV, Skaar EP, Seifert HS. Recombination, repair and replication in the pathogenic Neisseriae: the 3 R’s of molecular genetics of two human-specific bacterial pathogens. Mol. Microbiol. 2003;50(1):3–13. doi: 10.1046/j.1365-2958.2003.03679.x. [DOI] [PubMed] [Google Scholar]
- Kroll JS, Wilks KE, Farrant JL, Langford PR. Natural genetic exchange between Haemophilus and Neisseria: intergeneric transfer of chromosomal genes between major human pathogens. Proc. Natl. Acad. Sci. USA. 1998;95:12381–12385. doi: 10.1073/pnas.95.21.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclaporn A, Firth N, Paulsen IT, Skurray RA. IS-257 mediated cointegration in the evolution of a family of Staphylococcal thrimethroprim resistance plasmids. J. Bacteriol. 1996;178:6070–6073. doi: 10.1128/jb.178.20.6070-6073.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujón SA, Gougas LM, Ragonese H, Matson SW, Redinbo MR. Disrupting antibiotic resistance propagation by inhibiting the conjugative DNA relaxase. Proc. Natl. Acad. Sci. USA. 2007;104:12282–12287. doi: 10.1073/pnas.0702760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahillon J, Chandler M. Insertion Sequences. Microbiol. Mol. Biol. Rev. 1998;62:725–774. doi: 10.1128/mmbr.62.3.725-774.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez C, Xia M, Borthagaray G, Roberts MC. Conjugal transfer of the 3.05β-lactamase plasmid by the 25.2Mda plasmid in Neisseria gonorrhoeae. Sex. Transm. Dis. 1999;26:157–159. doi: 10.1097/00007435-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Marsin S, Marguet E, Forterre P. Topoisomerase I activity of the hyperthermophilic replication initiator protein Rep75. Nucleic Acid Res. 2000;28:2251–2255. doi: 10.1093/nar/28.11.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez E, De la Cruz F. Transposon Tn21 encodes a recA- independent site-specific integration system. Mol. Gen. Genet. 1998;211:320–325. doi: 10.1007/BF00330610. [DOI] [PubMed] [Google Scholar]
- Masepohl RG, Puehler A. Analysis of IS21-mediated mobilization of plasmid pSCYC184 by R68.45 in Escherichia coli. Plasmid. 1983;10:111–118. doi: 10.1016/0147-619x(83)90063-x. [DOI] [PubMed] [Google Scholar]
- Mc Nicol PJ, Albritton WL, Ronald AR. Characterization of ampicillin resistance plasmids of Haemophilus ducreyi and Neisseria gonorrhoeae with regard to location of origin of transfer and mobilization by conjugative plasmid of Haemophilus ducreyi. J. Bacteriol. 1983;156:437–440. doi: 10.1128/jb.156.1.437-440.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlin C, Mahillon J, Nesvera J, Toussaint A. Gene recruiters and transporters: The modular structure of bacterial mobile elements. In: Thomas CM, editor. The horizontal gene pool. Hardwood Academic Publishers, Amsterdam; The Netherlands: 2000. pp. 363–409. [Google Scholar]
- Michel B, Ehrlich SD. Illegitimate recombination occurs between the replication origin of the plasmid pC194 and a progressing replication fork. EMBO J. 1986;5:3691–3196. doi: 10.1002/j.1460-2075.1986.tb04701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Cohen SN. F-plasmid provides a function that promotes recA-independent site-specific fusions of pSC101 replicon. Nature (London) 1980;285:577–579. doi: 10.1038/285577a0. [DOI] [PubMed] [Google Scholar]
- Mollet B, Iida S, Sheperd J, Arber W. Nucleotide sequence of IS26, a new prokaryotic mobile genetic element. Nucleic Acid. Res. 1983;11:6319–6329. doi: 10.1093/nar/11.18.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olliver A, Vallé M, Chaslus-Dancla E, Cloeckaert A. Overexpression of the multidrug efflux operon acrEF by insertional activation with IS1 or IS10 elements in Salmonella enterica serovar typhimurium DT204 acrB mutants selected with fluoroquinilones. Antimicrob. Agent. Chemother. 2005;49:289–301. doi: 10.1128/AAC.49.1.289-301.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagotto F, Aman AT, Ng LI, Yeung KH, Brett M, Dillon JR. Sequence analysis of the family of penicillinase-producing plasmids of Neisseria gonorrhoeae. Plasmid. 2000;43:24–34. doi: 10.1006/plas.1999.1431. [DOI] [PubMed] [Google Scholar]
- Phillips I. Beta-lactamase-producing penicillin resistant gonococcus. Lancet. 1976;2:656–657. doi: 10.1016/s0140-6736(76)92466-1. [DOI] [PubMed] [Google Scholar]
- Piffareti JC, Arinoi A, Frey J. pUB307 mobilizes resistance plasmids from Escherichia coli into Neisseria gonorrhoeae. Mol. Gen. Genet. 1988;212:215–218. doi: 10.1007/BF00334687. [DOI] [PubMed] [Google Scholar]
- Ray K, Bala M, Kumari S, Narain JP. Antimicrobial resistance of Neisseria gonorrhoeae in selected World Health Organization Southeast Asia Region Countries: An overview. Sex. Transm. Dis. 2005;32:178–184. doi: 10.1097/01.olq.0000154490.40381.15. [DOI] [PubMed] [Google Scholar]
- Reid G. Colonization of the vaginal and urethral mucosa. In: Nataro JP, et al., editors. Colonization of mucosal surfaces. ASM Press; Washington, D.C.: 2005. pp. 431–448. [Google Scholar]
- Reimmann C, Haas D. Mobilization of chromosomes and nonconjugative plasmids by cointegrate mechanisms. In: Clewell DB, editor. Bacterial conjugation. Plenum Press; New York, NY: 1993. pp. 137–188. [Google Scholar]
- Roberts M, Falkow S. Conjugal transfer of R-plasmids in Neisseria gonorrhoeae. Nature. 1977;266:630–631. doi: 10.1038/266630a0. [DOI] [PubMed] [Google Scholar]
- Roberts MC, Knapp JS. Transfer of beta-lactamase plasmids from Neisseria gonorrhoeae to Neisseria meningitidis and commensal Neisseria species by the 25.2-megadalton conjugative plasmid. Antimicrob. Agents Chemother. 1988;32:1430–1432. doi: 10.1128/aac.32.9.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Bonano N, Torres-Bauzá LJ. Molecular analysis of oriT and mobA protein in the 7.4 kb mobilizable beta-lactamase plasmid pSJ7.4 from Neisseria gonorrhoeae. Plasmid. 2004;52:89–101. doi: 10.1016/j.plasmid.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Sambrook JE, Fritsch EF, Maniatis T. 2nd 1-3. Cold Spring Harbor Press, Cold Spring Harbor Laboratory; New York: 1989. Molecular cloning, a laboratory manual. [Google Scholar]
- Scharbaai- Vázquez R. Doctoral Thesis. University of Puerto Rico, Medical Sciences Campus; San Juan, Puerto Rico: 2006. Molecular analysis of the cointegrative transfer mechanisms in the gonococcal 5.2 Kb Beta-lactamase plasmid. [Google Scholar]
- Scharbaai-Vázquez R, Candelas T, Torres-Bauzá LJ. Mobilization of the gonococcal 5.2 kb β-lactamase plasmid pSJ5.2 into Escherichia coli by cointegration with several gram - conjugative plasmids. Plasmid. 2007;57:156–164. doi: 10.1016/j.plasmid.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiga Y, Sekine Y, Kano Y, Ohtsubo E. Involvement of H-NS in transpositional recombination mediated by IS1. J. Bacteriol. 2001;183:2476–84. doi: 10.1128/JB.183.8.2476-2484.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman S. Vaccinia DNA topoisomerase 1 promotes illegitimate recombination in Escherichia coli. Proc. Natl. Acad. Sci. USA. 1989;86:3489–3493. doi: 10.1073/pnas.86.10.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu LK, Lu P-L, Chen J-Y, Lin FM, Chang S-C. High-level expression of AmpC β -lactamase due to insertion of nucleotides between -10 and -35 promoter sequences in Escherichia coli clinical isolates: Cases not responsive to extended-spectrum- cephalosporin treatment. Antimicrob. Agent. Chemother. 2003;47:2138–2144. doi: 10.1128/AAC.47.7.2138-2144.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sox TE, Mohammed W, Blackman E, Biswas G, Sparling F. Conjugative plasmids in Neisseria gonorrhoeae. J. Bacteriol. 1978;134:278–286. doi: 10.1128/jb.134.1.278-286.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenover FC, Stein DC, Young FE, Clark VL. Construction and characterization of chimeric beta- lactamase plasmids of Neisseria gonorrhoeae with altered ability to be mobilized during conjugation. Sex. Transm. Dis. 1985;12:76–82. doi: 10.1097/00007435-198504000-00005. [DOI] [PubMed] [Google Scholar]
- Trotter M, McAuliffe DE, Fitzgerald GF, Hill C, Ross RP, Coffey A. Variable bacteriocin production in the commercial starter Lactococcus lactis DPC 4275 is linked to the formation of the cointegrated plasmid pMRC02. Appl. Env. Microbiol. 2004;70:34–42. doi: 10.1128/AEM.70.1.34-42.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Passel MWJ, Van der Ende A, Bart A. Plasmid diversity in Neisseriae. Infect. Immun. 2006;74:4892–4899. doi: 10.1128/IAI.02087-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudequin-Dransart V, Petit A, Chilton WS, Dessaux Y. The cryptic plasmid of Agrobacterium tumefaciens cointegrates with the Ti plasmid and cooperates for opine degradation. Mol. Plant-Microbe Interact. 1998;11:583–591. [Google Scholar]
- Zhu J, Schiestl RH. Topoisomerase 1 involvement in illegitimate recombination in Saccharomyces cerevisiae. Mol. Cell. Biol. 1996;16:1805–1812. doi: 10.1128/mcb.16.4.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Schiestl RH. Human topoisomerase 1 mediates illegitimate recombination leading to DNA insertion into the ribosomal DNA locus in Saccharomyces cerevisiae. Mol. Gen. Genomics. 2004;271:347–358. doi: 10.1007/s00438-004-0987-7. [DOI] [PubMed] [Google Scholar]