Abstract
Dipyridamole (DP) is a phosphodiesterase inhibitor that increases the intracellular levels of cyclic adenosine monophosphate (cAMP) and cyclic guanine monophosphate (cGMP) by preventing their conversion to AMP and GMP, respectively. By increasing cAMP and cGMP levels in platelets, DP reversibly inhibits platelet aggregation and platelet-mediated thrombotic disease. In addition, DP may potentiate some of the vascular protective effects of endothelium-derived nitric oxide (NO), which increases cGMP by stimulating soluble guanylyl cyclase. Endothelium-derived NO is an important regulator of vascular tone, blood flow, and tissue perfusion. Indeed, endothelial NO synthase-deficient (eNOS−/−) mice exhibit elevated systemic blood pressure and have larger myocardial and cerebral infarct size after ischemic injury. Other NO/cGMP-dependent effects that may be potentiated by DP include inhibition of vascular smooth muscle proliferation and prevention of endothelial-leukocyte interaction. In addition, DP increases local concentrations of adenosine and prostacyclin, which could affect vascular tone and inflammation. Finally, DP has antioxidant properties, which could stabilize platelet and vascular membranes as well as prevent the oxidation of low-density lipoprotein. These platelet and nonplatelet actions of DP may contribute to some of its therapeutic benefits in vascular disease.
Keywords: platelets, endothelium, vascular, dipyridamole, oxidation, inflammation, perfusion
This article is part of a multi-part CME-certified activity titled Translational Therapeutics at the Platelet Vascular Interface. In order to achieve all of the activity’s learning objectives, please read all of the components of the activity listed in the Table of Contents and follow the “Instructions for Participation and Obtaining CME Credit” outlined prior to the Introduction.
Antiplatelet therapy such as aspirin (ASA) has been the cornerstone for the treatment of cardiovascular disease, particularly ischemic strokes. However, the relatively small magnitude of benefits derived from aspirin monotherapy, ie, 14% to 20% relative risk (RR) reduction compared with placebo, has spurred the search for more effective antiplatelet agents or regimens.1-3 Surprisingly, the Management of Atherothrombosis With Clopidogrel in High-Risk Patients (MATCH) and Clopidogrel for High Atherothrombosis Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) studies indicate that the addition of another antiplatelet, clopidogrel, to ASA does not confer additional protection for secondary strokes compared with ASA or clopidogrel alone.4,5 Bleeding rates, however, were increased with this combination. In contrast, addition of dipyridamole (DP) to ASA in the European Stroke Prevention Study (ESPS)-2 and the European/Australian Stroke Prevention in Reversible Ischemia Trial (ESPRIT) reduced the RR of stroke by about 20% compared with ASA alone, without incurring excess bleeding.6,7 Surprisingly, the risk of bleeding was less with DP plus ASA compared with ASA alone. These findings suggest that DP may exert vascular protective effects beyond platelet inhibition.
Adenosine and Platelet Inhibition
DP was initially found to increase extracellular levels of adenosine by inhibiting adenosine uptake by red blood cells, thereby leading to inhibition of platelet aggregation (Figure 1).8 Adenosine is released from vascular wall cells and platelets into the extracellular space as a breakdown product of adenosine triphosphate (ATP). Released adenine nucleotides are rapidly converted to adenosine by nucleases. In circulating blood, free adenosine is rapidly removed from plasma by a specific adenosine carrier into red blood cells. At clinically relevant doses, DP inhibits adenosine uptake by red blood cells by >90% and increases plasma adenosine levels by 60%.9,10 Adenosine, acting through adenosine receptors, stimulates adenylyl cyclase in platelets and increases intracellular levels of cyclic adenosine monophosphate (cAMP), which is a potent inhibitor of platelet activation.11 It should be noted that DP can also increase intracellular levels of cAMP in platelets by preventing the breakdown of cAMP via inhibition of phosphodiesterase (PDE).12,13 Indeed, DP has been shown to inhibit platelet aggregation in whole blood in vitro and potentiate the antiaggregatory effect of adenosine in vitro.14,15
Figure 1.

Mechanism of Action of DP. DP increases local concentrations of adenosine, which stimulates adenylyl cyclase in platelets leading to increased intracellular cAMP levels. In addition, by inhibiting PDE, DP prevents the breakdown of cAMP. Increased intracellular levels of cAMP keep platelets from being activated. Furthermore, by inhibiting PDE in the vascular wall, DP increases PGI2 production and vascular smooth muscle cGMP levels, leading to vasodilation.
Vasodilation and Perfusion
By inhibiting cyclic guanine monophosphate (cGMP) PDE, DP enhances cGMP-dependent downstream vasodilatory effects in smooth muscle (Figure 1).16 DP can also stimulate prostacyclin (PGI2) production by increasing intracellular levels of cAMP.17 PGI2 is not only a potent inhibitor of platelet aggregation, but also a vasodilator. PGI2 is generated by a cyclooxygenase-dependent pathway in a variety of cells, including endothelial cells.18 Finally, DP can potentiate vasodilation by increasing local adenosine levels.8 Thus, DP can exert direct and indirect vasodilatory effects on vascular smooth muscle.
Because of its vasodilatory properties, DP is often used in conjunction with electrocardiographic or imaging studies to detect underlying coronary ischemia.19,20 The basis of these studies is to augment the difference in myocardial perfusion, ie, coronary steal, via non–rate-limiting atherosclerotic coronary arteries compared with that of fixed rate-limiting lesions. The DP myocardial imaging studies are performed with IV infusion of DP, which results in 4 to 5 times higher acute blood levels of DP than what can be achieved with oral dose. A smaller increase in myocardial perfusion is observed with sustained-release oral DP, showing improved hyperemic myocardial blood flow and left ventricular systolic function in patients with ischemic cardiomyopathy.21
Antioxidative Effects
The molecular structure of DP allows it to accept electrons, thus functioning as a free radical scavenger and antioxidant. Using lipid oxidation assays based on the generation of peroxy radicals by azo compounds, DP was found to scavenge both hydrophilic and hydrophobic radicals.22 Compared with ascorbic acid, α-tocopherol, and probucol, DP was more efficient in inhibiting chemically or cellularly induced low-density lipoprotein (LDL) oxidation as monitored by diene formation, evolution of hydroperoxides and thiobarbituric acid reactive substances, apoprotein modification, and by the fluorescence of cis-parinaric acid.23
The antioxidative effects of DP could also occur at the cellular level. At clinically relevant concentrations, DP protects erythrocyte membranes from oxidation and spares the antioxidant power of erythrocytes.24 Furthermore, DP suppresses oxygen free radical formation in platelets and endothelial cells and improves cellular redox status.25 These antixodiative effects of DP may extend the half-life and increase the bioavailability of endothelium-derived nitric oxide (NO), which is vascular protective.
Antiinflammatory Effects
Besides indirect antiinflammatory effects of DP via adenosine and PGI2, DP may also exert direct antiinflammatory effects through inhibition of platelet-monocyte interaction. For example, activated platelets adhere to and stimulate monocytes, causing monocytes to secrete monocyte chemotactic protein-1 (MCP-1) and matrix metalloproteinase-9 (MMP-9).26 Treatment of activated platelets with DP, but not ASA, prevented monocyte secrection of MCP-1, MCP-9, and tissue factor.26,27 DP has also been shown to inhibit the adhesion of neutrophils to the vascular endothelium in ischemic stroke patients via a specific downregulation of Mac-1.28 Furthermore, DP inhibits lymphocyte recruitment, activation, and secretion of proinflammatory mediators.29,30 Thus, the potential antiinflammatory effects of DP may contribute to some of its clinical benefits.
Potentiation of NO-Mediated Pathways
By increasing intracellular levels of cGMP, DP could augment many of the downstream signaling pathways of NO (Figure 2). Loss of endothelial-derived NO activity leading to reduction in intracellular cGMP levels contributes to impaired vascular responses,31 enhanced platelet aggregation,32 and vascular smooth muscle proliferation.33 Inhibition of endothelial NO production by the endothelial NO synthase (eNOS) inhibitor, Nω-monomethyl-l-arginine (l-NMA), causes vasoconstriction34 and vascular inflammation by promoting endothelial-leukocyte adhesion.35 Indeed, lower vascular cGMP levels in mutant mice lacking eNOS are associated with systemic and pulmonary hypertension,36,37 greater propensity for intimal smooth muscle proliferation in response to vascular cuff injury,38 and larger stroke sizes in response to cerebral ischemia.39 Thus, by inhibiting cGMP PDE, DP may potentiate the downstream effects of NO. Indeed, DP has been shown to potentiate NO/cGMP vasodilatory and platelet antiaggregatory effects,40 enhance ischemia-induced angiogenesis,41 increase myocardial perfusion in heart failure and stable coronary artery disease,21,42 and ameliorate the severity of ischemic strokes via NO- and adenosine-mediated effects.43
Figure 2.
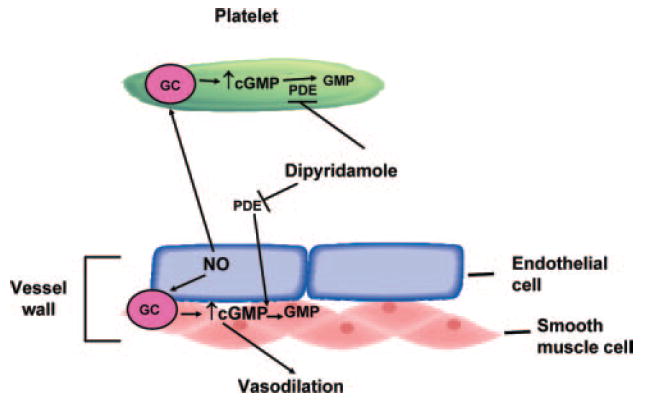
Potentiation of NO/cGMP-mediated pathway by DP. Endothelium-derived NO stimulates guanylyl cyclase (GC) in platelets and vascular smooth muscle cells, leading to increase in intracellular cGMP. DP enhances cGMP levels by inhibiting PDE and preventing the breakdown of cGMP to GMP. Increased intracellular levels of cGMP in platelets and vascular smooth muscle cell (VSMC) inhibits platelet activation and enhances vasodilation, respectively.
Translational Benefits of DP in Secondary Stroke Protection in Antiplatelet Clinical Trials
The mechanism by which DP, especially the extended-release formulation, could reduce the risks for secondary strokes without incurring excess bleeding may be attributable to some of its effects beyond platelet inhibition on the vascular wall (Table). As previously mentioned, DP could augment many of the downstream signaling pathways of NO (Figure 2). Indeed, lower vascular cGMP levels in mutant mice lacking eNOS are associated with larger stroke sizes in response to cerebral ischemia.39 These findings suggest that DP may protect against stroke, in part, through a platelet-independent mechanism. Similar mechanisms may occur with other cardiovascular agents such as statins (Stroke Prevention by Aggressive Reduction in Cholesterol Levels [SPARCL] trial), angiotensin-converting enzyme inhibitors (Heart Outcomes Prevention Evaluation [HOPE] trial), and angiotensin II receptor blockers (Losartan Intervention for Endpoint Reduction [LIFE] Study), which protect the vascular wall and confer stroke protection without any direct effects on platelet aggregation. Indeed, the combination of DP plus lower doses of statins synergizes to protect against ischemia-reperfusion injury44 and ischemic stroke (Hyung-Huan Kim and James K. Liao, 2008).
Table.
Vascular Effects of DP
| Endothelial cell | ↑ cGMP and potentiate downstream actions of endothelium-derived NO |
| ↑ PGI2 production | |
| ↓ Thrombus formation | |
| ↓ Oxidative stress | |
| ↓ Inflammation | |
| ↑ Angiogenesis | |
| Smooth muscle cell | ↓ Migration and proliferation |
| ↓ Reactive oxygen species | |
| ↑ Vasorelaxation | |
| Platelet | ↓ Platelet reactivity via increase in local adenosine levels |
| ↓ Platelet aggregation via increase in intracellular cAMP and cGMP | |
| ↓ Soluble CD40L secretion | |
| ↑ Stabilization of platelet membranes | |
| Monocyte/macrophage | ↓ Platelet-monocyte interaction |
| ↓ MMP-9 expression and secretion | |
| ↓ MCP-1 secretion | |
| ↓ Tissue factor expression and activity | |
| ↓ Interleukin-8 secretion | |
| Vascular inflammation | ↓ High-sensitivity C-reactive protein level |
| ↓ Leukocyte-endothelial cell adhesion | |
| ↑ CD40/CD40L expression | |
| Other effects | ↓ LDL oxidation |
| ↑ Plasma adenosine levels | |
| ↑ Perfusion |
Acknowledgments
Sources of Funding This work was supported by grants from the National Institutes of Health (NS10828) and ZEMA Corporation.
Footnotes
Disclosures Dr Kim received unrestricted research support from ZEMA Corporation. Dr Liao is on the speakers’ bureaus and is a consultant for Pfizer Inc, Merck & Co Inc, AstraZeneca, and Boehringer Ingelheim. He has also received unrestricted research support from Boehringer Ingelheim.
The online version of this article, along with updated information and services, is located on the World Wide Web at:
Subscriptions: Information about subscribing to Arteriosclerosis, Thrombosis, and Vascular Biology is online at
Permissions: Permissions & Rights Desk, Lippincott Williams & Wilkins, a division of Wolters Kluwer Health, 351 West Camden Street, Baltimore, MD 21202-2436. Phone: 410-528-4050. Fax: 410-528-8550. E-mail:
journalpermissions@lww.com
Reprints: Information about reprints can be found online at
References
- 1.Swedish Aspirin Low-Dose Trial (SALT) of 75 mg aspirin as secondary prophylaxis after cerebrovascular ischaemic events. The SALT Collaborative Group. Lancet. 1991;338:1345–1349. [PubMed] [Google Scholar]
- 2.A comparison of two doses of aspirin (30 mg vs. 283 mg a day) in patients after a transient ischemic attack or minor ischemic stroke. The Dutch TIA Trial Study Group. N Engl J Med. 1991;325:1261–1266. doi: 10.1056/NEJM199110313251801. [DOI] [PubMed] [Google Scholar]
- 3.Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, Cacoub P, Cohen EA, Creager MA, Easton JD, Flather MD, Haffner SM, Hamm CW, Hankey GJ, Johnston SC, Mak KH, Mas JL, Montalescot G, Pearson TA, Steg PG, Steinhubl SR, Weber MA, Brennan DM, Fabry-Ribaudo L, Booth J, Topol EJ for the CHARISMA Investigators. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706–1717. doi: 10.1056/NEJMoa060989. [DOI] [PubMed] [Google Scholar]
- 5.Diener HC, Bogousslavsky J, Brass LM, Cimminiello C, Csiba L, Kaste M, Leys D, Matias-Guiu J, Rupprecht HJ for the MATCH Investigators. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331–337. doi: 10.1016/S0140-6736(04)16721-4. [DOI] [PubMed] [Google Scholar]
- 6.Diener HC, Cunha L, Forbes C, Sivenius J, Smets P, Lowenthal A. European Stroke Prevention Study. 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996;143:1–13. doi: 10.1016/s0022-510x(96)00308-5. [DOI] [PubMed] [Google Scholar]
- 7.ESPRIT Study Group. Halkes PH, van Gijn J, Kappelle LJ, Koudstaal PJ, Algra A. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet. 2006;367:1665–1673. doi: 10.1016/S0140-6736(06)68734-5. [DOI] [PubMed] [Google Scholar]
- 8.Best LC, McGuire MB, Jones PB, Holland TK, Martin TJ, Preston FE, Segal DS, Russell RG. Mode of action of dipyridamole on human platelets. Thromb Res. 1979;16:367–379. doi: 10.1016/0049-3848(79)90084-7. [DOI] [PubMed] [Google Scholar]
- 9.Dresse A, Chevolet C, Delapierre D, Masset H, Weisenberger H, Bozler G, Heinzel G. Pharmacokinetics of oral dipyridamole (Persantine) and its effect on platelet adenosine uptake in man. Eur J Clin Pharmacol. 1982;23:229–234. doi: 10.1007/BF00547559. [DOI] [PubMed] [Google Scholar]
- 10.German DC, Kredich NM, Bjornsson TD. Oral dipyridamole increases plasma adenosine levels in human beings. Clin Pharmacol Ther. 1989;45:80–84. doi: 10.1038/clpt.1989.12. [DOI] [PubMed] [Google Scholar]
- 11.Born GV, Cross MJ. Effect of adenosine diphosphate on the concentration of platelets in circulating blood. Nature. 1963;197:974–976. doi: 10.1038/197974a0. [DOI] [PubMed] [Google Scholar]
- 12.Mills DC, Smith JB. The influence on platelet aggregation of drugs that affect the accumulation of adenosine 3′:5′-cyclic monophosphate in platelets. Biochem J. 1971;121:185–196. doi: 10.1042/bj1210185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith JB, Mills DC. Inhibition of adenosine 3′,5′-cyclic monophosphate phosphodiesterase. Biochem J. 1970;120:20P. doi: 10.1042/bj1200020pa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gresele P, Arnout J, Deckmyn H, Vermylen J. Mechanism of the antiplatelet action of dipyridamole in whole blood: modulation of adenosine concentration and activity. Thromb Haemost. 1986;55:12–18. [PubMed] [Google Scholar]
- 15.Gresele P, Zoja C, Deckmyn H, Arnout J, Vermylen J, Verstraete M. Dipyridamole inhibits platelet aggregation in whole blood. Thromb Haemost. 1983;50:852–856. [PubMed] [Google Scholar]
- 16.Schoeffter P, Lugnier C, Demesy-Waeldele F, Stoclet JC. Role of cyclic AMP- and cyclic GMP-phosphodiesterases in the control of cyclic nucleotide levels and smooth muscle tone in rat isolated aorta. A study with selective inhibitors. Biochem Pharmacol. 1987;36:3965–3972. doi: 10.1016/0006-2952(87)90465-5. [DOI] [PubMed] [Google Scholar]
- 17.Blass KE, Block HU, Förster W, Pönicke K. Dipyridamole: a potent stimulator of prostacyclin (PGI2) biosynthesis. Br J Pharmacol. 1980;68:71–73. doi: 10.1111/j.1476-5381.1980.tb10700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehta J, Mehta P. Dipyridamole and aspirin in relation to platelet aggregation and vessel wall prostaglandin generation. J Cardiovasc Pharmacol. 1982;4:688–693. doi: 10.1097/00005344-198207000-00022. [DOI] [PubMed] [Google Scholar]
- 19.Baron JF, Mundler O, Bertrand M, Vicaut E, Barré E, Godet G, Samama CM, Coriat P, Kieffer E, Viars P. Dipyridamole-thallium scintigraphy and gated radionuclide angiography to assess cardiac risk before abdominal aortic surgery. N Engl J Med. 1994;330:663–669. doi: 10.1056/NEJM199403103301002. [DOI] [PubMed] [Google Scholar]
- 20.Leppo JA, O’Brien J, Rothendler JA, Getchell JD, Lee VW. Dipyridamole-thallium-201 scintigraphy in the prediction of future cardiac events after acute myocardial infarction. N Engl J Med. 1984;310:1014–1018. doi: 10.1056/NEJM198404193101603. [DOI] [PubMed] [Google Scholar]
- 21.Akhtar M, Ordovas K, Martin A, Higgins CB, Michaels AD. Effect of chronic sustained-release dipyridamole on myocardial blood flow and left ventricular function in patients with ischemic cardiomyopathy. Congest Heart Fail. 2007;13:130–135. doi: 10.1111/j.1527-5299.2007.06047.x. [DOI] [PubMed] [Google Scholar]
- 22.Iuliano L, Pedersen JZ, Rotilio G, Ferro D, Violi F. A potent chain-breaking antioxidant activity of the cardiovascular drug dipyridamole. Free Radic Biol Med. 1995;18:239–247. doi: 10.1016/0891-5849(94)e0123-z. [DOI] [PubMed] [Google Scholar]
- 23.Iuliano L, Colavita AR, Camastra C, Bello V, Quintarelli C, Alessandroni M, Piovella F, Violi F. Protection of low density lipoprotein oxidation at chemical and cellular level by the antioxidant drug dipyridamole. Br J Pharmacol. 1996;119:1438–1446. doi: 10.1111/j.1476-5381.1996.tb16056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kusmic C, Picano E, Busceti CL, Petersen C, Barsacchi R. The antioxidant drug dipyridamole spares the vitamin E and thiols in red blood cells after oxidative stress. Cardiovasc Res. 2000;47:510–514. doi: 10.1016/s0008-6363(00)00058-4. [DOI] [PubMed] [Google Scholar]
- 25.Chakrabarti S, Vitseva O, Iyu D, Varghese S, Freedman JE. The effect of dipyridamole on vascular cell-derived reactive oxygen species. J Pharmacol Exp Ther. 2005;315:494–500. doi: 10.1124/jpet.105.089987. [DOI] [PubMed] [Google Scholar]
- 26.Weyrich AS, Denis MM, Kuhlmann-Eyre JR, Spencer ED, Dixon DA, Marathe GK, McIntyre TM, Zimmerman GA, Prescott SM. Dipyridamole selectively inhibits inflammatory gene expression in platelet-monocyte aggregates. Circulation. 2005;111:633–642. doi: 10.1161/01.CIR.0000154607.90506.45. [DOI] [PubMed] [Google Scholar]
- 27.Brozna JP, Horan M, Carson SD. Dipyridamole inhibits O2- release and expression of tissue factor activity by peripheral blood monocytes stimulated with lipopolysaccharide. Thromb Res. 1990;60:141–156. doi: 10.1016/0049-3848(90)90293-l. [DOI] [PubMed] [Google Scholar]
- 28.Hallevi H, Hazan-Halevy I, Paran E. Modification of neutrophil adhesion to human endothelial cell line in acute ischemic stroke by dipyridamole and candesartan. Eur J Neurol. 2007;14:1002–1007. doi: 10.1111/j.1468-1331.2007.01847.x. [DOI] [PubMed] [Google Scholar]
- 29.Coeugniet E, Bendtzen K, Bendixen G. Leucocyte migration inhibitory activity of concanavalin-A-stimulated human lymphocytes. Modification by dipyridamole, lysine-acetylsalicylate and heparin. Acta Med Scand. 1976;199:99–104. doi: 10.1111/j.0954-6820.1976.tb06698.x. [DOI] [PubMed] [Google Scholar]
- 30.Dong H, Osmanova V, Epstein PM, Brocke S. Phosphodiesterase 8 (PDE8) regulates chemotaxis of activated lymphocytes. Biochem Biophys Res Commun. 2006;345:713–719. doi: 10.1016/j.bbrc.2006.04.143. [DOI] [PubMed] [Google Scholar]
- 31.Liao JK, Bettmann MA, Sandor T, Tucker JI, Coleman SM, Creager MA. Differential impairment of vasodilator responsiveness of peripheral resistance and conduit vessels in humans with atherosclerosis. Circ Res. 1991;68:1027–1034. doi: 10.1161/01.res.68.4.1027. [DOI] [PubMed] [Google Scholar]
- 32.Radomski MW, Rees DD, Dutra A, Moncada S. S-nitroso-glutathione inhibits platelet activation in vitro and in vivo. Br J Pharmacol. 1992;107:745–749. doi: 10.1111/j.1476-5381.1992.tb14517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garg UC, Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest. 1989;83:1774–1777. doi: 10.1172/JCI114081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurose I, Kubes P, Wolf R, Anderson DC, Paulson J, Miyasaka M, Granger DN. Inhibition of nitric oxide production. Mechanisms of vascular albumin leakage. Circ Res. 1993;73:164–171. doi: 10.1161/01.res.73.1.164. [DOI] [PubMed] [Google Scholar]
- 35.De Caterina R, Libby P, Peng HB, Thannickal VJ, Rajavashisth TB, Gimbrone MA, Jr, Shin WS, Liao JK. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest. 1995;96:60–68. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 37.Steudel W, Ichinose F, Huang PL, Hurford WE, Jones RC, Bevan JA, Fishman MC, Zapol WM. Pulmonary vasoconstriction and hypertension in mice with targeted disruption of the endothelial nitric oxide synthase (NOS 3) gene. Circ Res. 1997;81:34–41. doi: 10.1161/01.res.81.1.34. [DOI] [PubMed] [Google Scholar]
- 38.Moroi M, Zhang L, Yasuda T, Virmani R, Gold HK, Fishman MC, Huang PL. Interaction of genetic deficiency of endothelial nitric oxide, gender, and pregnancy in vascular response to injury in mice. J Clin Invest. 1998;101:1225–1232. doi: 10.1172/JCI1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang Z, Huang PL, Ma J, Meng W, Ayata C, Fishman MC, Moskowitz MA. Enlarged infarcts in endothelial nitric oxide synthase knockout mice are attenuated by nitro-L-arginine. J Cereb Blood Flow Metab. 1996;16:981–987. doi: 10.1097/00004647-199609000-00023. [DOI] [PubMed] [Google Scholar]
- 40.Aktas B, Utz A, Hoenig-Liedl P, Walter U, Geiger J. Dipyridamole enhances NO/cGMP-mediated vasodilator-stimulated phosphoprotein phosphorylation and signaling in human platelets: in vitro and in vivo/ex vivo studies. Stroke. 2003;34:764–769. doi: 10.1161/01.STR.0000056527.34434.59. [DOI] [PubMed] [Google Scholar]
- 41.Kawasaki K, Smith RS, Jr, Hsieh CM, Sun J, Chao J, Liao JK. Activation of the phosphatidylinositol 3-kinase/protein kinase Akt pathway mediates nitric oxide-induced endothelial cell migration and angiogenesis. Mol Cell Biol. 2003;23:5726–5737. doi: 10.1128/MCB.23.16.5726-5737.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jagathesan R, Rosen SD, Foale RA, Camici PG, Picano E. Effects of long-term oral dipyridamole treatment on coronary microcirculatory function in patients with chronic stable angina: a substudy of the persantine in stable angina (PISA) study. J Cardiovasc Pharmacol. 2006;48:110–116. doi: 10.1097/01.fjc.0000245404.20922.9f. [DOI] [PubMed] [Google Scholar]
- 43.Gamboa A, Abraham R, Diedrich A, Shibao C, Paranjape SY, Farley G, Biaggioni I. Role of adenosine and nitric oxide on the mechanisms of action of dipyridamole. Stroke. 2005;36:2170–2175. doi: 10.1161/01.STR.0000179044.37760.9d. [DOI] [PubMed] [Google Scholar]
- 44.Ye Y, Lin Y, Perez-Polo R, Huang MH, Hughes MG, McAdoo DJ, Manickavasagam S, Uretsky BF, Birnbaum Y. Enhanced cardioprotection against ischemia-reperfusion injury with a dipyridamole and low-dose atorvastatin combination. Am J Physiol Heart Circ Physiol. 2007;293:H813–H818. doi: 10.1152/ajpheart.00210.2007. [DOI] [PubMed] [Google Scholar]


