Abstract
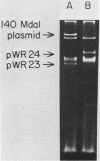
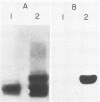
The requirement for both plasmid and chromosomal genes in the biosynthesis of Shigella dysenteriae 1 lipopolysaccharide O antigen was demonstrated in Escherichia coli-Shigella hybrids. A 6-megadalton S. dysenteriae 1 plasmid, designated pWR23, was phenotypically tagged with the Tn3 ampicillin-resistance transposon. The tagged plasmid, designated pWR24, was transferred by transformation or conjugal mobilization to a rough E. coli K-12 recipient. Although the resultant hybrids were agglutinated in S. dysenteriae 1 antiserum, they did not remove all of the anti-Shiga agglutinins in absorption experiments. Modified lipid A core structure was detected in these hybrids, but Shiga O antigen was not expressed. When the his+ locus of the S. dysenteriae 1 chromosome was transferred by transduction to E. coli K-12 containing pWR24, complete Shiga O antigen was expressed. Lipopolysaccharide extracted from these hybrids was indistinguishable chemically, electrophoretically, and serologically from native S. dysenteriae 1 lipopolysaccharide.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clewell D. B., Helinski D. R. Effect of growth conditions on the formation of the relaxation complex of supercoiled ColE1 deoxyribonucleic acid and protein in Escherichia coli. J Bacteriol. 1972 Jun;110(3):1135–1146. doi: 10.1128/jb.110.3.1135-1146.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitriev B. A., Knirel Y. A., Kochetkov N. K. Somatic antigens of shigella. Structural investigation on the O-specific polysaccharide chain of Shigella dysenteriae type 1 lipopolysaccharide. Eur J Biochem. 1976 Jul 15;66(3):559–566. doi: 10.1111/j.1432-1033.1976.tb10582.x. [DOI] [PubMed] [Google Scholar]
- Formal S. B., Gemski P., Baron L. S., Labrec E. H. Genetic Transfer of Shigella flexneri Antigens to Escherichia coli K-12. Infect Immun. 1970 Mar;1(3):279–287. doi: 10.1128/iai.1.3.279-287.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formal S. B., Hale T. L., Kapfer C., Cogan J. P., Snoy P. J., Chung R., Wingfield M. E., Elisberg B. L., Baron L. S. Oral vaccination of monkeys with an invasive Escherichia coli K-12 hybrid expressing Shigella flexneri 2a somatic antigen. Infect Immun. 1984 Nov;46(2):465–469. doi: 10.1128/iai.46.2.465-469.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamian A., Romanowska E. The core structure of Shigella sonnei lipopolysaccharide and the linkage between O-specific polysaccharide and the core region. Eur J Biochem. 1982 Dec;129(1):105–109. doi: 10.1111/j.1432-1033.1982.tb07027.x. [DOI] [PubMed] [Google Scholar]
- Goldman R. C., Leive L. Heterogeneity of antigenic-side-chain length in lipopolysaccharide from Escherichia coli 0111 and Salmonella typhimurium LT2. Eur J Biochem. 1980;107(1):145–153. doi: 10.1111/j.1432-1033.1980.tb04635.x. [DOI] [PubMed] [Google Scholar]
- Hale T. L., Sansonetti P. J., Schad P. A., Austin S., Formal S. B. Characterization of virulence plasmids and plasmid-associated outer membrane proteins in Shigella flexneri, Shigella sonnei, and Escherichia coli. Infect Immun. 1983 Apr;40(1):340–350. doi: 10.1128/iai.40.1.340-350.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecko D. J., Holcombe J., Formal S. B. Molecular characterization of plasmids from virulent and spontaneously occurring avirulent colonial variants of Shigella flexneri. Infect Immun. 1979 May;24(2):580–582. doi: 10.1128/iai.24.2.580-582.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecko D. J., Washington O., Formal S. B. Genetic and physical evidence for plasmid control of Shigella sonnei form I cell surface antigen. Infect Immun. 1980 Jul;29(1):207–214. doi: 10.1128/iai.29.1.207-214.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mäkelä P. H. Genetic determination of the O antigens of Salmonella groups B (4,5,12) and C1(6,7). J Bacteriol. 1966 Mar;91(3):1115–1125. doi: 10.1128/jb.91.3.1115-1125.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Nikaido K., Rapin A. M. Biosynthesis of thymidine diphosphate L-rhamnose in Escherichia coli K-12. Biochim Biophys Acta. 1965 Dec 16;111(2):548–551. doi: 10.1016/0304-4165(65)90068-1. [DOI] [PubMed] [Google Scholar]
- Peppler M. S. Two physically and serologically distinct lipopolysaccharide profiles in strains of Bordetella pertussis and their phenotype variants. Infect Immun. 1984 Jan;43(1):224–232. doi: 10.1128/iai.43.1.224-232.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner J. L. Formation, induction, and curing of bacteriophage P1 lysogens. Virology. 1972 Jun;48(3):679–689. doi: 10.1016/0042-6822(72)90152-3. [DOI] [PubMed] [Google Scholar]
- Sansonetti P. J., Hale T. L., Dammin G. J., Kapfer C., Collins H. H., Jr, Formal S. B. Alterations in the pathogenicity of Escherichia coli K-12 after transfer of plasmid and chromosomal genes from Shigella flexneri. Infect Immun. 1983 Mar;39(3):1392–1402. doi: 10.1128/iai.39.3.1392-1402.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., Kopecko D. J., Formal S. B. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect Immun. 1982 Mar;35(3):852–860. doi: 10.1128/iai.35.3.852-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., Kopecko D. J., Formal S. B. Shigella sonnei plasmids: evidence that a large plasmid is necessary for virulence. Infect Immun. 1981 Oct;34(1):75–83. doi: 10.1128/iai.34.1.75-83.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P., David M., Toucas M. Corrélation entre la perte d'ADN plasmidique et le passage de la phase I virulente à la phase II avirulente chez Shigella sonnei. C R Seances Acad Sci D. 1980 Mar 31;290(13):879–882. [PubMed] [Google Scholar]
- Seid R. C., Jr, Schneider H., Bondarew S., Boykins R. A. Quantitation of L-glycero-D-manno-heptose and 3-deoxy-D-manno-octulosonic acid in rough core lipopolysaccharides by partition chromatography. Anal Biochem. 1982 Aug;124(2):320–326. doi: 10.1016/0003-2697(82)90046-x. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Timmis K. N. A small plasmid in Shigella dysenteriae 1 specifies one or more functions essential for O antigen production and bacterial virulence. Infect Immun. 1984 Jan;43(1):391–396. doi: 10.1128/iai.43.1.391-396.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]