Abstract
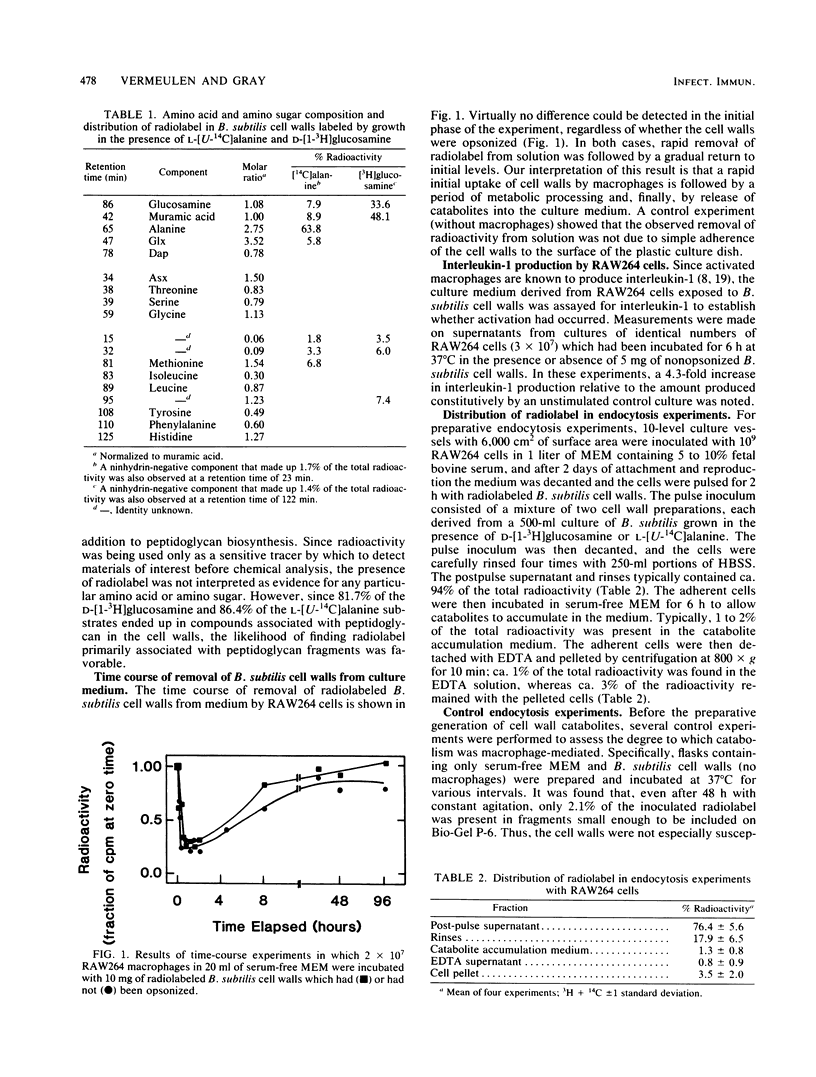
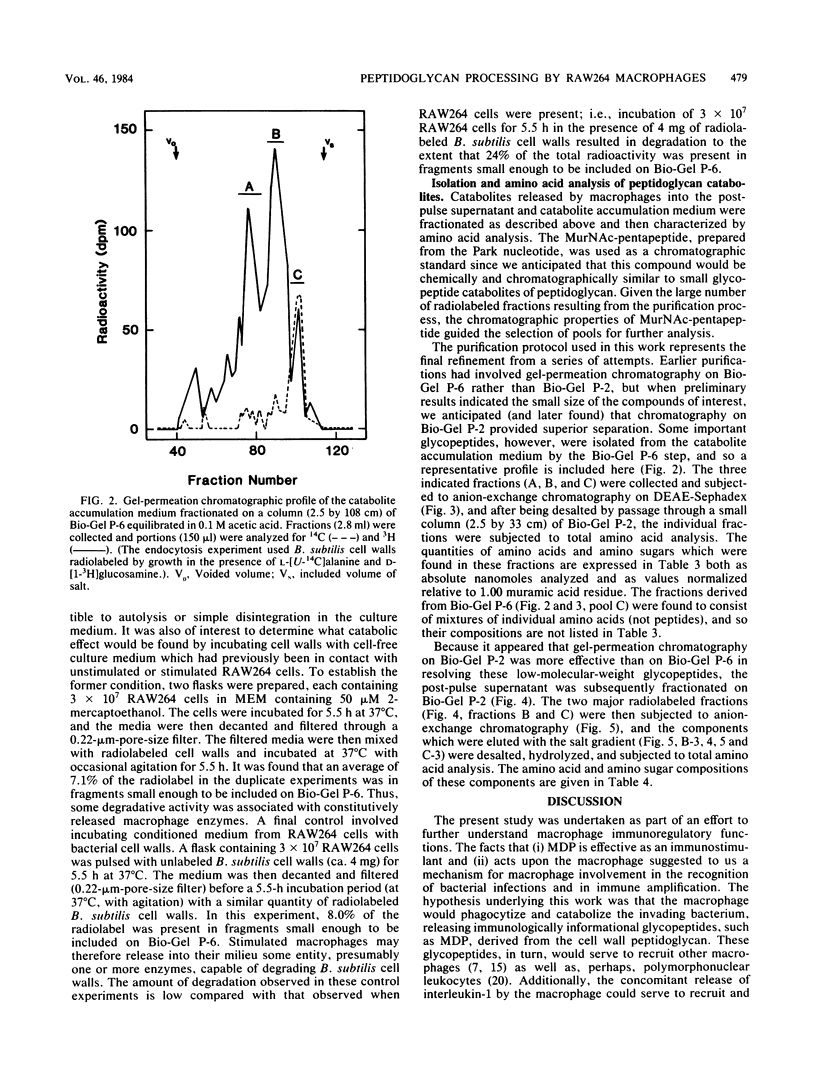
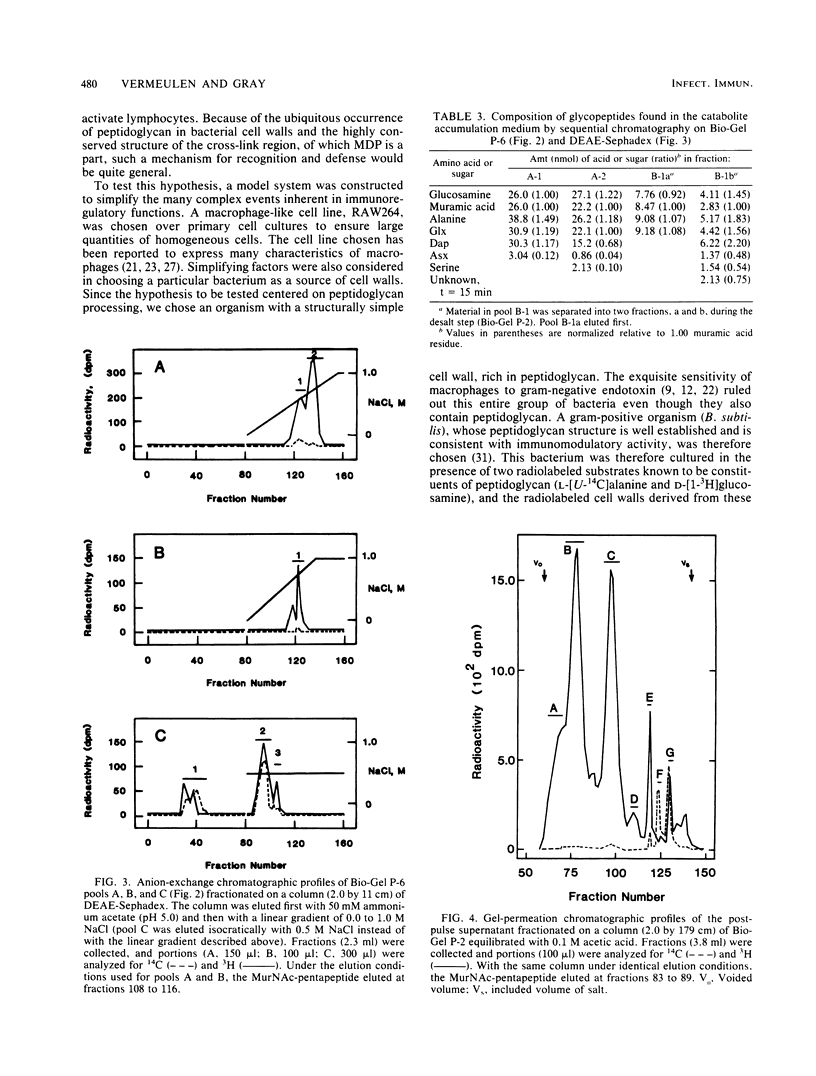
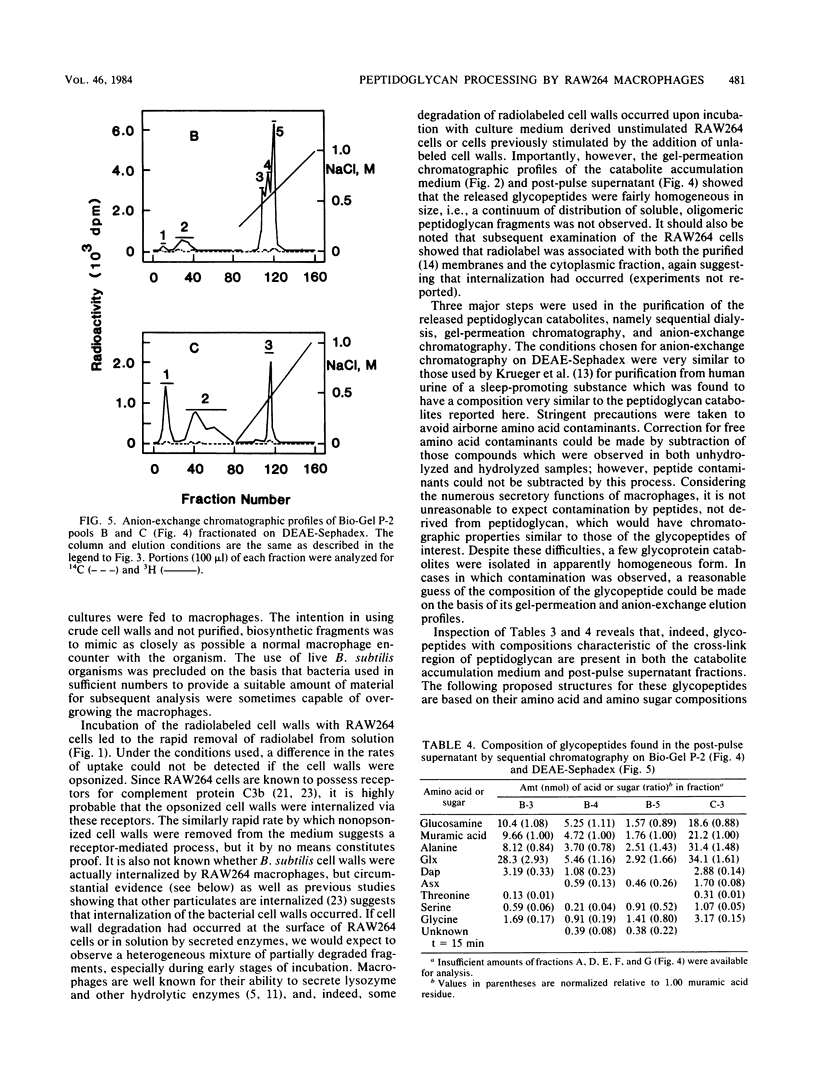
It has previously been established that muramyl dipeptide (N-acetylmuramyl-L-alanyl-D-isoglutamine) is an effective immunostimulant whose primary target cell type is the macrophage. Muramyl dipeptide is known to be structurally identical to a portion of the monomer unit of peptidoglycan, a nearly ubiquitous component of bacterial cell walls. To establish whether muramyl dipeptide or glycopeptides structurally related to it are formed as a result of macrophage processing of peptidoglycan, Bacillus subtilis cell walls radiolabeled in the muramic acid, glucosamine, and alanine residues of the constituent peptidoglycan were incubated in the presence of the cultured macrophage-like cell line RAW264, and the glycopeptides which released into the medium were fractionated and analyzed. Although muramyl dipeptide was not found in the culture medium, at least three glycopeptides structurally related to it were found, namely GlcNAc-MurNAc-Ala-isoGln-Dap-Ala, GlcNAc-MurNAc-Ala-isoGln-Dap, and GlcNAc-MurNAc-Ala-isoGln.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam A., Ciorbaru R., Ellouz F., Petit J. F., Lederer E. Adjuvant activity of monomeric bacterial cell wall peptidoglycans. Biochem Biophys Res Commun. 1974 Feb 4;56(3):561–567. doi: 10.1016/0006-291x(74)90640-8. [DOI] [PubMed] [Google Scholar]
- Bracha R., Chang M., Fiedler F., Glaser L. Biosynthesis of teichoic acids. Methods Enzymol. 1978;50:387–340. doi: 10.1016/0076-6879(78)50046-3. [DOI] [PubMed] [Google Scholar]
- COHN Z. A. The fate of bacteria within phagocytic cells. I. The degradation of isotopically labeled bacteria by polymorphonuclear leucocytes and macrophages. J Exp Med. 1963 Jan 1;117:27–42. doi: 10.1084/jem.117.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A. The fate of bacteria within phagocytic cells. II. The modification of intracellular degradation. J Exp Med. 1963 Jan 1;117:43–53. doi: 10.1084/jem.117.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellouz F., Adam A., Ciorbaru R., Lederer E. Minimal structural requirements for adjuvant activity of bacterial peptidoglycan derivatives. Biochem Biophys Res Commun. 1974 Aug 19;59(4):1317–1325. doi: 10.1016/0006-291x(74)90458-6. [DOI] [PubMed] [Google Scholar]
- Fevrier M., Birrien J. L., Leclerc C., Chedid L., Liacopoulos P. The macrophage, target cell of the synthetic adjuvant muramyl dipeptide. Eur J Immunol. 1978 Aug;8(8):558–562. doi: 10.1002/eji.1830080804. [DOI] [PubMed] [Google Scholar]
- Gillis S. Interleukin biochemistry and biology: summary and introduction. Fed Proc. 1983 Jun;42(9):2635–2638. [PubMed] [Google Scholar]
- Juy D., Chedid L. Comparison between macrophage activation and enhancement of nonspecific resistance to tumors by mycobacterial immunoadjuvants. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4105–4109. doi: 10.1073/pnas.72.10.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnovsky M. L., Lazdins J. K. Biochemical criteria for activated macrophages. J Immunol. 1978 Sep;121(3):809–813. [PubMed] [Google Scholar]
- Krueger J. M., Pappenheimer J. R., Karnovsky M. L. The composition of sleep-promoting factor isolated from human urine. J Biol Chem. 1982 Feb 25;257(4):1664–1669. [PubMed] [Google Scholar]
- Lederer E. Synthetic immunostimulants derived from the bacterial cell wall. J Med Chem. 1980 Aug;23(8):819–825. doi: 10.1021/jm00182a001. [DOI] [PubMed] [Google Scholar]
- Mizel S. B., Oppenheim J. J., Rosenstreich D. L. Characterization of lymphocyte-activating factor (LAF) produced by the macrophage cell line, P388D1. I. Enhancement of LAF production by activated T lymphocytes. J Immunol. 1978 May;120(5):1497–1503. [PubMed] [Google Scholar]
- Oppenheim J. J., Stadler B. M., Siraganian R. P., Mage M., Mathieson B. Lymphokines: their role in lymphocyte responses. Properties of interleukin 1. Fed Proc. 1982 Feb;41(2):257–262. [PubMed] [Google Scholar]
- Osada Y., Otani T., Sato M., Une T., Matsumoto K., Ogawa H. Polymorphonuclear leukocyte activation by a synthetic muramyl dipeptide analog. Infect Immun. 1982 Dec;38(3):848–854. doi: 10.1128/iai.38.3.848-854.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph P., Nakoinz I. Antibody-dependent killing of erythrocyte and tumor targets by macrophage-related cell lines: enhancement by PPD and LPS. J Immunol. 1977 Sep;119(3):950–954. [PubMed] [Google Scholar]
- Raschke W. C., Baird S., Ralph P., Nakoinz I. Functional macrophage cell lines transformed by Abelson leukemia virus. Cell. 1978 Sep;15(1):261–267. doi: 10.1016/0092-8674(78)90101-0. [DOI] [PubMed] [Google Scholar]
- Schwab J. H., Ohanian S. H. Degradation of streptococcal cell wall antigens in vivo. J Bacteriol. 1967 Nov;94(5):1346–1352. doi: 10.1128/jb.94.5.1346-1352.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab J. H., Smialowicz R. Interaction of bacterial cell wall polymers and rat macrophages. Z Immunitatsforsch Exp Klin Immunol. 1975 Jul;149(2-4):283–288. [PubMed] [Google Scholar]
- Taniyama T., Holden H. T. Direct augmentation of cytolytic activity of tumor-derived macrophages and macrophage cell lines by muramyl dipeptide. Cell Immunol. 1979 Dec;48(2):369–374. doi: 10.1016/0008-8749(79)90131-x. [DOI] [PubMed] [Google Scholar]
- Tsujimoto M., Kinoshita F., Okunaga T., Kotani S., Kusumoto S., Yamamoto K., Shiba T. Higher immunoadjuvant activities of N-acetyl-beta-D-glucosaminyl-(1-4)-N-acetylmuramyl-L-alanyl-D-isoglutamine in comparison with N-acetylmuramyl-L-alanyl-D-isoglutamine. Microbiol Immunol. 1979;23(9):933–936. doi: 10.1111/j.1348-0421.1979.tb02828.x. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Cerottini J. C., Bedford M. Persistence of antigen on the surface of macrophages. Nature. 1969 Jun 21;222(5199):1193–1195. doi: 10.1038/2221193a0. [DOI] [PubMed] [Google Scholar]
- Wachsmuth E. D. Local cellular reactions following intracutaneous injections of various adjuvants in rats and guinea-pigs. Adv Exp Med Biol. 1979;114:451–457. doi: 10.1007/978-1-4615-9101-6_74. [DOI] [PubMed] [Google Scholar]
- Warth A. D., Strominger J. L. Structure of the peptidoglycan from vegetative cell walls of Bacillus subtilis. Biochemistry. 1971 Nov 23;10(24):4349–4358. doi: 10.1021/bi00800a001. [DOI] [PubMed] [Google Scholar]
- Waxman D. J., Yu W., Strominger J. L. Linear, uncross-linked peptidoglycan secreted by penicillin-treated Bacillus subtilis. Isolation and characterization as a substrate for penicillin-sensitive D-alanine carboxypeptidases. J Biol Chem. 1980 Dec 10;255(23):11577–11587. [PubMed] [Google Scholar]


