Abstract
Autism spectrum disorders (ASD) are characterized by impairment in social interactions, communication deficits, and restricted repetitive interests and behaviors. There is evidence of both immune dysregulation and autoimmune phenomena in autism. We examined the regulatory cytokine transforming growth factor beta-1 (TGFβ1) because of its role in controlling immune responses. Plasma levels of active TGFβ1 were evaluated in 75 children with ASD compared with 96 controls. Children with ASD had significantly lower plasma TGFβ1 levels compared with typically developing controls (p=0.0017) and compared with children with developmental disabilities other than ASD (p=0.0037) but not siblings, after adjusting for age and gender. In addition, there were significant correlations between psychological measures and TGFβ1 levels, such that lower TGFβ1 levels were associated with lower adaptive behaviors and worse behavioral symptoms. The data suggest that immune responses in autism may be inappropriately regulated due to reductions in TGFβ1. Such immune dysregulation may predispose to the development of possible autoimmune responses and/or adverse neuroimmune interactions during critical windows in development.
INTRODUCTION
Autism spectrum disorders (ASD) are complex neurodevelopmental disorders that are distinguished by qualitative impairments in social interaction, deficits in verbal and non-verbal communication, and restricted repetitive and stereotyped patterns of behavior and interests [Filipek et al., 2000; Lord et al., 2000]. There is growing evidence that an abnormal immune response may exert a negative influence on neurodevelopment, potentially contributing to the etiology of some cases of ASD. Alterations in appropriate regulation of the immune response may result in chronic inflammation, autoimmunity, or an inappropriate response to immune challenge in children with ASD [reviewed in Ashwood et al., 2006]. Furthermore, abnormally regulated immune responses could potentially cause inflammation of the CNS or brain leading to altered neurodevelopment.
Systemic immunologic aberrations in autism have often been associated with immune dysregulation, in particular, the generation of antibodies reactive against brain and CNS proteins [Ashwood et al., 2004; Cabanalit et al., 2007; Connolly et al., 1999; Connolly et al., 2006; Kozlovskaia et al., 2000; Singh et al., 1993; Singh et al., 1997a; Singh et al., 1997b; Singh et al., 2002; Singh et al., 2004; Todd et al., 1988; Wills et al., 2007]. Indeed, autoantibodies to critical neuronal components have been reported in as many as 25–70% of individuals with autism [Connolly et al., 1999; Connolly et al., 2006; Singh et al., 2002; Singh et al., 2004; Todd et al., 1988]. However, it must be noted that it is not currently known whether these antibodies are a cause of autism or generated as a result of inflammation in the brain and CNS. In addition, many genetic studies have indicated a link between autism and genes that have immune functions, including complement C4 alleles, MHC haplotypes B44-SC30-DR4, human leukocyte antigen (HLA)-DRB1, and DR13 [Ferrante et al., 2003; Torres et al., 2001; Warren et al., 1991; Warren et al., 1996]. Moreover, several studies have shown peripheral immune abnormalities in patients with autism including abnormal or skewed T helper cell cytokine profiles, decreased lymphocyte numbers, decreased T cell mitogen response, and an imbalance of serum immunoglobulin levels [reviewed in Ashwood et al., 2006a]. Of particular note, Vargas et al., recently described increased neuroinflammation in brain and CNS specimens obtained from subjects with ASD [Vargas et al., 2005]. In addition, gene expression profiles in the temporal cortex of autistic subjects show increased transcript levels of many immune system-related genes when compared with matched controls [Garbett et al., 2008]. Taken together these data are suggesting of the presence of ongoing neuroinflammatory processes in the brain and CSF, as well as indicative of widespread changes in the peripheral immune response, of at least a significant proportion of children with ASD. We propose that the disturbances in immune function that have been reported in autism stem from deficits and defects in the regulatory immune system controlling the overall immune response.
Regulatory responses by the immune system are essential for the maintenance of tolerance to self-antigen and to innocuous non-harmful substances such as food nutrients. The regulatory immune response is also critical in the down-regulation of the inflammatory immune response following infection, thus limiting potential tissue damage. Immunosuppressive cytokines such as transforming growth factor beta1 (TGFβ1) are critical for immune homeostasis, and are important in the induction of unresponsiveness in activated T cells [den Haan et al., 2007; Marra et al., 2004; Sonoda et al., 2001]. Published findings support widespread changes in the immune systems of at least a significant proportion of children with autism, yet the exact nature of this immune dysfunction is not yet fully characterized. Reports of increased expression of TH1 cytokines [Croonenberghs et al., 2002; Molloy et al., 2006; Singh, 1996; Vargas et al., 2005], acute inflammatory cytokines [Croonenberghs et al., 2002; Singh, 1996; Vargas et al., 2005; Zimmerman et al., 2005], and TH2 cytokines [Molloy et al., 2006; Gupta et al., 1998] in autism do not implicate a specific inflammatory profile but instead suggest that a loss of regulation in the immune response may have occurred.
One of the most important immune regulators that can effectively control diverse aspects of the immune response is TGFβ1. In the autism literature, there are several studies that demonstrate an alteration or dysregulation of immune responses in autism compared with matched controls [Ashwood et al., 2004; Ashwood and Wakefield, 2006b; Cohly and Panja, 2005; Jyonouchi et al., 2005]. In addition, altered TGFβ1 levels have been observed in brain specimens of subjects with autism [Vargas et al. 2005]. Although one recent study revealed decreased plasma TGFβ1 in adults with autism [Okada et al., 2007] thus far we are unaware of any studies addressing the levels of TGFβ1 in children with autism, close to the time of their diagnosis, and compared with age matched control children. To better define the immune status of children with ASD, we utilized a large population based study of well-characterized children with confirmed ASD and age-matched controls, with and without developmental disabilities, to examine whether circulating active TGFβ1 differed between groups. If a defect in the immune regulatory function were confirmed, such a finding would contribute to the understanding of role of the immune system in autism, and could potentially offer a new diagnostic or treatment target.
MATERIALS AND METHODS
Subjects
Participants in the study were recruited from the case-control population based CHARGE (Childhood Autism Risk from Genetics and Environment) study conducted at the UC Davis M.I.N.D. Institute [Hertz-Picciotto et al., 2006]. One hundred and forty-three (143) children were investigated in this study and were the first subjects enrolled in the CHARGE study. Participants met one of the following 4 criteria: 1) diagnosed with an autism spectrum disorder (ASD); 2) diagnosed with developmental disability but not ASD; or 3) age-matched typically developing general population controls. The descriptive statistics of the study population are summarized in Table 1. This study was approved by the UC Davis institutional review board and complied with all requirements regarding human subjects. Informed consent was obtained from the parent of each participating child. Upon enrollment, all diagnoses were confirmed using Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) (American Psychiatric Association, 1994) criteria by qualified clinicians at the M.I.N.D. Institute. In addition, all participants with ASD met research criteria for ASD on both the Autism Diagnostic Interview-Revised (ADI-R) [Lord et al., 1997] and the Autism Diagnostic Observation Schedule (ADOS) [Lord et al., 2000] which were administered at the M.I.N.D. Institute by trained clinicians. The ADI-R is a comprehensive clinical interview administered to parents or caregivers of children suspected of having autism. The ADI-R assesses function in areas of language and communication, reciprocal social interaction, and repetitive, restricted, stereotyped behaviors or interests. Results from the interview are interpreted using a diagnostic algorithm that correlates with the DSM-IV and ICD-10 definitions of autism. The ADOS is an observational assessment that provides a standardized set of conditions to observe behavior in the areas of communication, play, and other areas relevant to autism. Based on DSM-IV, ADI-R and ADOS scores, within the ASD group 67 children met the cut-off for autistic disorder with a further 8 meeting the cut-off for ASD. The majority of the ASD group (81%) completed ADOS Module 1, the administration of which was partly determined based on the child’s language development level. The ADOS Module 2 was used in assessment of the remaining ASD cases (19%).
Table 1.
Descriptive group statistics of the study population
| Autism Spectrum Disorders
|
General Population | Developmental Disabilities | |||
|---|---|---|---|---|---|
| Total | Regression | Early Onset | |||
| N= | 75 | 42 | 33 | 36 | 32 |
| % Males | 91 | 88 | 94 | 67 | 88 |
| Median Age | 3.4 | 3.6 | 3.3 | 3.6 | 3.9 |
| Interquartile Range | 3.0–4.2 | 3.1–4.3 | 2.7–3.9 | 2.8–4.5 | 3.1–4.5 |
Children from the developmental disability and general population groups were screened for autism traits by the Social Communication Questionnaire, using a cut-off score of 15 or more to indicate a need for further autism evaluation. The ADI-R and ADOS were administered to those children in the developmental disability and general population group who scored above the screening threshold (SCQ score ≥15) to determine autism status. Children who scored above the cut-off for the SCQ and met criteria for ASD on the ADOS and ADI-R were included in the ASD group. The status of siblings was based on parental report.. In children from the ASD, general population and developmental disability groups, adaptive function was assessed by parental interview using the Vineland Adaptive Behavior Scales (VABS) [Sparrow et al., 1984]. The VABS covers the domains of socialization (interpersonal relationships, play and leisure time, and coping skills); daily living skills (personal, domestic and community skills); motor skills (gross and fine motor); and communication skills (receptive, expressive, and written communication). Cognitive function was assessed directly using the Mullen Scales of Early Learning (MSEL) [Mullen, 1995], which measures expressive and receptive language, fine motor, and nonverbal cognitive skills. The Aberrant Behavior Checklist (ABC) [Aman MG, et al., 1985] was used to measure behavior problems, such as irritability, stereotypy, and hyperactivity.
Children confirmed as ASD subjects were divided into subgroups of early onset or regressive autism based on the developmental trajectory of the child. Children were classified as having early onset autism if parents answered “no” to both of two questions from the ADI-R regarding developmental skill loss (language loss (Q11) and social skills loss (Q25)) and regressive autism if parents answered “yes” to either of these questions, indicating loss of language skills and/or social interest and engagement.
For each subject, peripheral blood was collected in acid-citrate-dextrose Vacutainers (BD Biosciences; San Jose, CA), centrifuged at 2300 rpm for 10 min, and plasma collected and stored at −80°C until time of analysis. Activated TGFβ1 concentrations in plasma were measured by a commercial enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN) using manufacturer’s protocol. Laboratory personnel were blinded to the subjects diagnosis. ELISA measurements were performed in duplicate for each patient sample. To measure the active form of TGFβ1, plasma samples were acidified according to the manufacturer’s recommendation. All solutions and activation reagents required were prepared following the manufacturer’s instructions. Activation and neutralization of plasma followed procedures listed. Samples were run in duplicate and in accordance with the instructions of the kit protocol. Sample concentrations were derived from the standard curve generated according to the kit protocol. All samples concentrations fell within the standard curve. Plasma aliquots had no more than 1 freeze/thaw cycle.
The TGFβ1 levels were not normally distributed, and hence, a natural logarithm transformation was applied to the dataset to meet necessary regression assumptions. Analysis of the data was performed using an analysis of covariance (ANCOVA) to compare across the diagnostic groups, with adjustment for age and gender. Evaluations of the relationship between the levels of TGFβ1 and psychological measures among children with autism were determined by multiple linear regression methods. P values were corrected for multiple comparisons using Tukey-Kramer Adjustment. Results were considered statistically significant after adjustment for multiple comparisons if p<0.05 (*). All analyses were performed using the Statistical Analysis System, version 9.1 (SAS Institute Inc, Cary, NC).
RESULTS
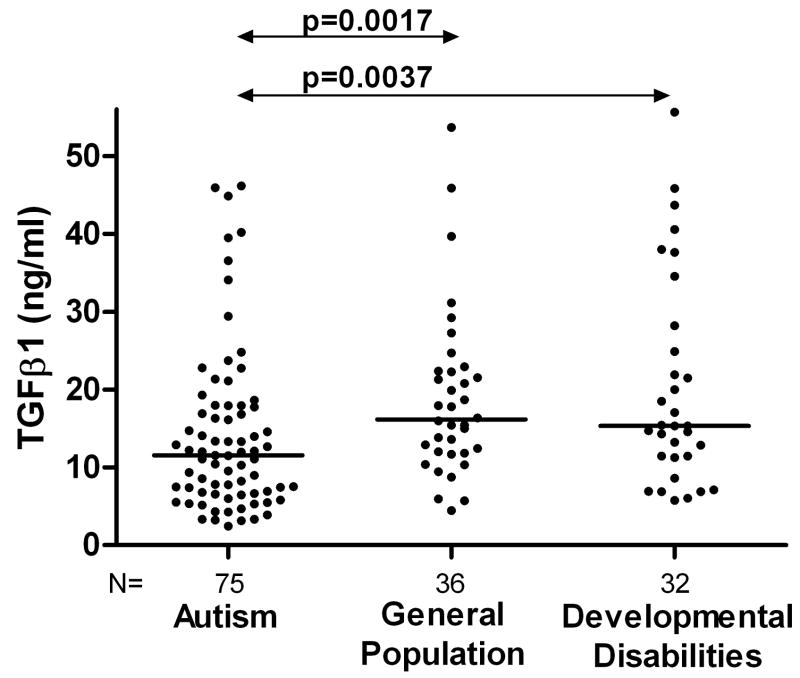
Plasma TGFβ1 levels were significantly lower in children with ASD compared with typically developing general population controls (median = 11.54, interquartile range (6.73–17.84) ng/ml versus 16.16 (11.97–22.32) ng/ml, p=0.0017) or compared with children having other developmental disabilities (15.34 (11.41–25.71) ng/ml, p=0.0037). Individual results are represented in Figure 1. Indeed, the combined group of non-ASD controls (i.e. children with developmental disabilities or typical controls, 15.43 (11.63–22.52) ng/ml) were significantly different when compared with ASD subjects (p<0.0001).
Figure 1.
Circulating TGFβ1 levels (ng/ml) in autism. TGFβ1 levels were significantly decreased in children with autism compared with typically developing general population controls (p=0.0017) and children with other developmental disabilities (p=0.0037. The bar represents the median TGFβ1 levels in each group.
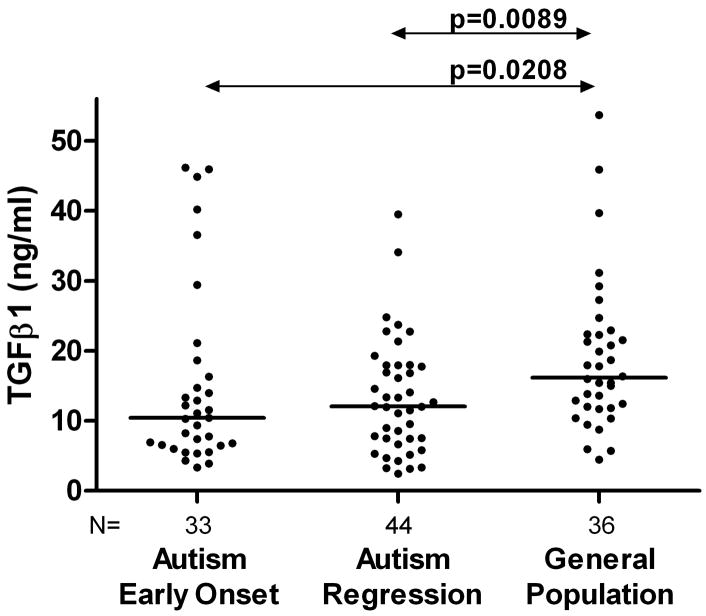
The children with ASD were further subdivided into those who exhibited regression and those with early onset ASD. There were no significant differences in psychological measures based on the ABC, ADI-R, MSEL and VABS scores between ASD children who had regressed and those that had not (data not shown). No differences were observed in the levels of TGFβ1 between the early onset ASD (10.43(6.54–16.29) ng/ml) and regressive ASD (12.07(7.43–17.89) ng/ml, p=0.9, Figure 2). Both children with early onset autism and children with autism who had regression had significantly reduced plasma TGFβ1 levels when compared with typically developing general population controls (p<0.02, Figure 2) or with children who had developmental disabilities (p<0.03).
Figure 2.
TGFβ1 levels (ng/ml) in children with distinct autism phenotypes. There were no significant differences in the plasma TGFβ1 levels noted between the subjects with early onset autism compared with those with regressive autism (p>0.5). Plasma TGFβ1 levels were significantly reduced in children with regressive autism when compared with the typically developing general population controls (p<0.0089). Similarly, Plasma TGFβ1 levels were significantly reduced in children with early onset autism when compared with the typically developing general population controls (p<0.0208).
To determine if there was a relationship between TGFβ1 levels and specific behavioral domains we used multiple linear regression analysis models with adjustment for potential age and gender confounders to compare across groups. Using the ABC assessment we observed several inverse correlations between TGFβ1 and psychological measures of irritability, lethargy. stereotypy and hyperactivity (Table 2, p<0.02). Overall, low TGFβ1 levels were associated with higher (worse) total ABC scores (R2=−0.098, p=0.011, Table 2). These relationships suggest as TGFβ1 decreases, stereotypy, irritability, hyperactivity and other behavioral symptoms measured by the ABC worsen. Similar trends were observed using the VABS assessment i.e. that as TGFβ1 levels decreased, adaptive behavior levels worsened (in the case of the VABS measure, in which lower scores indicate worse performance, this was a positive correlation). Specifically, the relationship between the VABS social interaction score and TGFβ1 levels was statistically significant (R2=0.043, p=0.018). TGFβ1 levels were also significantly associated with composite VABS scores (R2=0.036, p=0.027) across groups. If the ASD group was analyzed alone there was a significant correlation between total ABC scores and plasma TGFβ1 levels (R2=−0.393, p=0.038) but not with any measures assessed by ADI-R, ADOS, MSEL or VABS scores. If general population typically developing children and/or, children with developmental disabilities were considered alone there were no marked correlations observed between plasma TGFβ1 levels and clinical variables using ABC, MSEL and VABS measures. Furthermore, there were no significant correlations between the levels of TGFβ1 in the plasma and clinical assessment scores based on the ABC, ADI-R, MSEL and VABS in children with either early onset or regressive autism. However, there was a significant correlation between the measures of social interaction on the VABS assessment and plasma TGFβ1 levels in children who had early onset ASD (R2=0.125, p=0.029) with the composite VABS score also significantly associated with TGFβ1 levels (R2=0.099, p=0.047). In addition, in children with early onset ASD, there were trends to associations between TGFβ1 and total ABC scores (R2=−0.536, p=0.106), scores of communication (R2=−0.265, p=0.077) and social interaction (R2=−0.126, p=0.094) as measured by ADOS and for non-verbal communication (R2=−0.128, p=0.109) as assessed by ADI-R, again suggesting that as TGFβ1 levels decrease behavior becomes more atypical. In children with ASD that had regressed there was a significant association of decreasing TGFβ1 levels and increasing irritability as measured by the ABC (R2=−0.315, p=0.029).
Table 2.
Multiple linear regression analysis of TGFβ1 and association with behavioral outcome measures as assessed by the Aberrant Behavioral Checklist. Age of subject and gender of subject were considered as confounders. Significant correlations were observed between TGFβ1 levels and the severity of impairments in behavior in children enrolled in this study
| Range | R2 value | p-value | |
|---|---|---|---|
| Aberrant Behavioral Checklist | |||
| Subscale I: Irritability | (0–35) | −0.108 | 0.016 |
| Subscale II: Lethargy | (0–43) | −0.099 | 0.021 |
| Subscale III: Stereotypy | (0–20) | −0.128 | 0.004 |
| Subscale IV: Hyperactivity | (0–47) | −0.123 | 0.007 |
| Subscale V: Inappropriate Speech | (0–10) | −0.092 | 0.108 |
| Total | (0–109) | −0.098 | 0.011 |
DISCUSSION
The current study describes a significant decrease in plasma TGFβ1 levels in children with ASD when compared with age-matched typically developing controls and age-matched children with developmental disabilities in cognitive or adaptive function but who do not have ASD. Low TGFβ1 levels may lead to an inappropriate control of the immune response in these children. While Okada et al., recently published a similar finding of reduced TGFβ1 in a small cohort of 19 adults with autism compared with 21 healthy male controls [Okada et al., 2007], ours is the first study to show such reduced TGFβ1 levels in a large, well-defined cohort of children with autism. Taken together, these findings suggest that TGFβ1 may play a role in the pathophysiology of ASD in childhood that continues into adulthood, although further work is needed to confirm these reports.
Furthermore, data from our study showed that decreasing TGFβ1 levels in plasma correlated with worse behavioral scores on the ABC and lower adaptive levels on the VABS measures. These findings were more apparent when comparing across the study groups, most likely due to the larger spread in the behavioral measures, ranging from normalized typical values for the general population controls to more impaired values in the ASD children. However, it is notable that there were also associations between TGFβ1 levels and increasing behavioral difficulties in the ASD group alone, both in children with early onset ASD and those who had regressed, but not for the typically developing or developmental disabilities control groups. This is the first time that cytokine measures, such as TGFβ1, have been associated with worsening of behavioral measures in ASD. While the interpretation of these results is complicated, it remains possible that low TGFβ1 may result in immune dysregulation that could contribute to the symptoms and behaviors that are associated with ASD. Although these data need to be confirmed, they may suggest that peripheral immune markers may reflect biological factors that could influence behaviors in children with ASD. Given the key role of TGFβ1 in the immune response and during neurogenesis, decreased levels of TGFβ1 may contribute to the pathophysiology and influence behavioral manifestations of ASD. Given that a major role of TGFβ1 is to control inflammation, the negative correlations observed for TGFβ1 and behaviors may suggest that there is increased inflammation and/or ongoing inflammatory processes in subjects that exhibit higher (worse) behavioral scores. Additional studies are needed to explore these potential links further. In particular, the use of TGFβ1 as a serological marker in children who have recently been diagnosed with ASD, as well as its use as a biological marker to monitor the potential efficacies of therapies that target behavioral outcomes, warrants further investigation.
The transforming growth factors (TGFβ1, 2 and 3) play crucial and important roles in cell growth and differentiation, organ development, migration, matrix formation, apoptosis, as well as critical roles in the regulation of immune cells and cellular homeostasis [Letterio and Roberts, 1998; Aoki et al., 2005]. The role of TGFβ1 with respect to the immune response is complex. Early during an immune response, TGFβ1 has the ability to enhance inflammation. Conversely, TGFβ1 has a number of profound down-regulatory effects on T and B cell development and function, as well as the ability to modulate the differentiation and activation status of NK cells, dendritic cells, monocytes/macrophages, granulocytes and mast cells [Li et al., 2006a]. The immune regulatory effects of TGFβ1 are most evident in TGFβ1 deficient mice that develop severe multifocal inflammatory autoimmune diseases after only two weeks of life [Shull et al., 1992]. Similarly, blocking TGFβ signaling by deletion of TGFβ receptor type II (TGFR2), leads to a disruption of T cell development and results in multi-organ autoimmune inflammation [Li et al., 2006b; Marie et al., 2006]. As such, TGFβ has often been considered as one of the crucial regulators within the immune system and a key mediator in the development of autoimmune and systemic inflammation.
The location and developmental stage of the target cell or tissue, as well as the presence of other cytokines and growth factors within the local milieu may, in large part, determine the action of TGFβ1. In the brain, there is evidence that glial and neuronal cells produce TGFβ1 and that it plays a role in regulating CNS development [reviewed in Gomes et al., 2005]. Due to the distribution of TGFβ1 and the receptors for TGFβ1 within the developing nervous system, this cytokine may have important roles in the development of the brain and in neurodevelopmental disorders. For example, widespread and important roles of TGFβ1 in the CNS have been reported including involvement in neuroprotection against glutamate cytotoxicity, control of astrocyte differentiation and morphology, inhibition of astrocyte and microglia proliferation, cell migration in the cerebral cortex, control of neuronal death and microgliosis, wound healing and immunosuppression, induction of blood-brain barrier characteristics in endothelial cells, and the survival of neurons [reviewed in Gomes et al., 2005]. Interestingly, it has been recently shown that proliferation of neural stem and progenitor cell cultures and neurogenesis can be inhibited, over a long period, by TGFβ1 [Wachs et al., 2006]. These data suggest that TGFβ1 may have long lasting effects on the control of neural stem and progenitor cell proliferation during neurogenesis of the CNS. Macrocephaly and mini-columnar pathology reported in some cases of autism may arise from a direct imbalance of neurogenesis [Courchesne et al., 2003; Courchesne et al., 2001]. Whether abnormal cytokine levels such as TGFβ or inappropriate TGFβ signaling contribute significantly to this imbalance deserves further investigation.
In summary, this study demonstrates that there is a significant reduction in TGFβ1 levels in the plasma of young children who have ASD compared with typically developing children and with non-ASD developmentally delayed controls who were frequency-matched on age. Such immune dysregulation may predispose to the development of autoimmunity and/or adverse neuroimmune interactions that could occur during critical windows in development. As a decrease in TGFβ1 levels could reflect a role for cytokine imbalance affecting early neurodevelopment and the generation of clinical symptoms in ASD, these findings provide a framework for further longitudinal studies to investigate the changes in TGFβ1 levels over the natural history of the disorder. In particular, it is currently unknown if altered TGFβ1 levels are active determinants in the development of an immune mediated neuropathology in ASD, or accompanying phenomena secondary to the onset of the disease itself. As such, further work needs to be carried out in order to determine whether the presence of low TGFβ1 occurs prior to the diagnosis or development of ASD symptoms. Furthermore, in children with ASD there were correlations between circulating TGFβ1 levels and measurements of behaviors. This finding suggests that ongoing inflammatory responses may be linked to disturbances in behavior and require confirmation in a larger study. Future studies designed to evaluate TGFβ1 levels during critical periods of neurodevelopment of ASD would be essential towards addressing the potential role of TGFβ1 as a biological component in ASD.
Acknowledgments
This study was funded by the NIEHS Children’s Center grant (2 P01 ES011269), US EPA STAR program grant (R833292 and R829388), NIEHS CHARGE study (R01ES015359) Cure Autism Now, the Ted Lindsay Foundation, Visceral, Peter Emch Foundation and a generous gift from the Johnson Family. We would like to thank the staff of both the UC Davis M.I.N.D Institute and the CHARGE study for their technical support. The commitment of the families who took part in these studies, at both the M.I.N.D Institute and as part of the CHARGE study, is also gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aman MG, Singh NN, Stewart AW, Field CJ. The aberrant behavior checklist: a behavior rating scale for the assessment of treatment effects. Am J Ment Defic. 1985;89(5):485–491. [PubMed] [Google Scholar]
- Aoki CA, Borchers AT, Li M, Flavell RA, Bowlus CL, Ansari AA, Gershwin ME. Transforming growth factor beta (TGF-beta) and autoimmunity. Autoimmun Rev. 2005;4(7):450–459. doi: 10.1016/j.autrev.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Ashwood P, Anthony A, Torrente F, Wakefield AJ. Spontaneous mucosal lymphocyte cytokine profiles in children with autism and gastrointestinal symptoms: mucosal immune activation and reduced counter regulatory interleukin-10. J Clin Immunol. 2004;24(6):664–673. doi: 10.1007/s10875-004-6241-6. [DOI] [PubMed] [Google Scholar]
- Ashwood P, Wills S, Van de Water J. The immune response in autism: a new frontier for autism research. J Leukoc Biol. 2006a;80(1):1–15. doi: 10.1189/jlb.1205707. [DOI] [PubMed] [Google Scholar]
- Ashwood P, Wakefield AJ. Immune activation of peripheral blood and mucosal CD3+ lymphocyte cytokine profiles in children with autism and gastrointestinal symptoms. J Neuroimmunol. 2006b;173(1–2):126–134. doi: 10.1016/j.jneuroim.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Cabanlit M, Wills S, Goines P, Ashwood P, Van de Water J. Brain-specific autoantibodies in the plasma of subjects with Autistic Spectrum Disorder. Ann NY Acad Sci. 2007;1107:92–103. doi: 10.1196/annals.1381.010. [DOI] [PubMed] [Google Scholar]
- Cohly HH, Panja A. Immunological findings in autism. Int Rev Neurobiol. 2005;71:317–341. doi: 10.1016/s0074-7742(05)71013-8. [DOI] [PubMed] [Google Scholar]
- Connolly AM, Chez M, Streif EM, Keeling RM, Golumbek PT, Kwon JM, Riviello JJ, Robinson RG, Neuman RJ, Deuel RM. Brain-derived neurotrophic factor and autoantibodies to neural antigens in sera of children with autistic spectrum disorders, Landau-Kleffner syndrome, and epilepsy. Biol Psychiatry. 2006;59(4):354–363. doi: 10.1016/j.biopsych.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Connolly AM, Chez MG, Pestronk A, Arnold ST, Mehta S, Deuel RK. Serum autoantibodies to brain in Landau-Kleffner variant, autism, and other neurologic disorders. J Pediatr. 1999;134(5):607–613. doi: 10.1016/s0022-3476(99)70248-9. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. JAMA. 2003;290:337–344. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, Chisum HJ, Moses P, Pierce K, Lord C, Lincoln AJ, Pizzo S, Schreibman L, Haas RH, Akshoomoff NA, Courchesne RY. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- Croonenberghs J, Bosmans E, Deboutte D, Kenis G, Maes M. Activation of the inflammatory response system in autism. Neuropsychobiology. 2002;45(1):1–6. doi: 10.1159/000048665. [DOI] [PubMed] [Google Scholar]
- den Haan JM, Kraal G, Bevan MJ. Cutting edge: Lipopolysaccharide induces IL-10-producing regulatory CD4+ T cells that suppress the CD8+ T cell response. J Immunol. 2007;178(9):5429–5433. doi: 10.4049/jimmunol.178.9.5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante P, Saresella M, Guerini FR, Marzorati M, Musetti MC, Cazzullo AG. Significant association of HLA A2-DR11 with CD4 naive decrease in autistic children. Biomed Pharmacother. 2003;57(8):372–374. doi: 10.1016/s0753-3322(03)00099-4. [DOI] [PubMed] [Google Scholar]
- Filipek PA, Accardo PJ, Ashwal S, Baranek GT, Cook EH, Jr, Dawson G, Gordon B, Gravel JS, Johnson CP, Kallen RJ, Levy SE, Minshew NJ, Ozonoff S, Prizant BM, Rapin I, Rogers SJ, Stone WL, Teplin SW, Tuchman RF, Volkmar FR. Practice parameter: screening and diagnosis of autism: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Child Neurology Society. Neurology. 2000;55(4):468–479. doi: 10.1212/wnl.55.4.468. [DOI] [PubMed] [Google Scholar]
- Garbett K, Ebert PJ, Mitchell A, Lintas C, Manzi B, Mirnics K, Persico AM. Immune transcriptome alterations in the temporal cortex of subjects with autism. Neurobiology of Disease. 2008;30:303–311. doi: 10.1016/j.nbd.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes FC, Sousa Vde O, Romao L. Emerging roles for TGF-beta1 in nervous system development. Int J Dev Neurosci. 2005;23(5):413–424. doi: 10.1016/j.ijdevneu.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Gupta S, Aggarwal S, Rashanravan B, Lee T. Th1- and Th2-like cytokines in CD4+ and CD8+ T cells in autism. J Neuroimmunol. 1998;85(1):106–109. doi: 10.1016/s0165-5728(98)00021-6. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Croen LA, Hansen R, Jones CR, Van de Water J, Pessah IN. The CHARGE study: an epidemiologic investigation of genetic and environmental factors contributing to autism. Env Health Persp. 2006;114(7):1119–1125. doi: 10.1289/ehp.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyonouchi H, Geng L, Ruby A, Zimmerman-Bier B. Dysregulated innate immune responses in young children with autism spectrum disorders: their relationship to gastrointestinal symptoms and dietary intervention. Neuropsychobiology. 2005;51(2):77–85. doi: 10.1159/000084164. [DOI] [PubMed] [Google Scholar]
- Kozlovskaia GV, Kliushnik TP, Goriunova AV, Turkova IL, Kalinina MA, Sergienko NS. Nerve growth factor auto-antibodies in children with various forms of mental dysontogenesis and in schizophrenia high risk group. Zh Nevrol Psikhiatr Im S S Korsakova. 2000;100(3):50–52. [PubMed] [Google Scholar]
- Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–161. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006a;25(3):455–471. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006b;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- Lord C, Pickles A, McLennan J, Rutter M, Bregman J, Folstein S, Fombonne E, Leboyer M, Minshew N. Diagnosing autism: Analyses of data from the autism diagnostic interview. J Autism Devel Disord. 1997;27(5):501–517. doi: 10.1023/a:1025873925661. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The Autism Diagnostic Observation Schedule-Generic: A standard measure of social and communication deficits associated with the spectrum of autism. J Autism Devel Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Marie JC, Liggitt D, Rudensky AY. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity. 2006;25(3):441–454. doi: 10.1016/j.immuni.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Marra LE, Zhang ZX, Joe B, Campbell J, Levy GA, Penninger J, Zhang L. IL-10 induces regulatory T cell apoptosis by up-regulation of the membrane form of TNF-alpha. J Immunol. 2004;172(2):1028–1035. doi: 10.4049/jimmunol.172.2.1028. [DOI] [PubMed] [Google Scholar]
- Molloy CA, Morrow AL, Meinzen-Derr J, Schleifer K, Dienger K, Manning-Courtney P, Altaye M, Wills-Karp M. Elevated cytokine levels in children with autism spectrum disorder. J Neuroimmunol. 2006;172(1–2):198–205. doi: 10.1016/j.jneuroim.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Mullen EM. Mullen scales of early learning. Circle Pines, MN: American Guidance Services, Inc; 1995. [Google Scholar]
- Okada K, Hashimoto K, Iwata Y, Nakamura K, Tsujii M, Tsuchiya KJ, Sekine Y, Suda S, Suzuki K, Sugihara G, Matsuzaki H, Sugiyama T, Kawai M, Minabe Y, Takei N, Mori N. Decreased serum levels of transforming growth factor-beta1 in patients with autism. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(1):187–190. doi: 10.1016/j.pnpbp.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, Annunziata N, Doetschman T. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359(6397):693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VK, Warren RP, Odell JD, Warren WL, Cole P. Antibodies to myelin basic protein in children with autistic behavior. Brain Behav Immun. 1993;7(1):97–103. doi: 10.1006/brbi.1993.1010. [DOI] [PubMed] [Google Scholar]
- Singh VK. Plasma increase of interleukin-12 and interferon-gamma. Pathological significance in autism. J Neuroimmunol. 1996;66(1–2):143–145. doi: 10.1016/0165-5728(96)00014-8. [DOI] [PubMed] [Google Scholar]
- Singh VK, Singh EA, Warren RP. Hyperserotoninemia and serotonin receptor antibodies in children with autism but not mental retardation. Biol Psychiatry. 1997a;41(6):753–755. doi: 10.1016/S0006-3223(96)00522-7. [DOI] [PubMed] [Google Scholar]
- Singh VK, Warren R, Averett R, Ghaziuddin M. Circulating autoantibodies to neuronal and glial filament proteins in autism. Pediatr Neurol. 1997b;17(1):88–90. doi: 10.1016/s0887-8994(97)00045-3. [DOI] [PubMed] [Google Scholar]
- Singh VK, Lin SX, Newell E, Nelson C. Abnormal measles-mumps-rubella antibodies and CNS autoimmunity in children with autism. J Biomed Sci. 2002;9(4):359–364. doi: 10.1007/BF02256592. [DOI] [PubMed] [Google Scholar]
- Singh VK, Rivas WH. Prevalence of serum antibodies to caudate nucleus in autistic children. Neurosci Lett. 2004;355(1–2):53–56. doi: 10.1016/j.neulet.2003.10.026. [DOI] [PubMed] [Google Scholar]
- Sonoda KH, Faunce DE, Taniguchi M, Exley M, Balk S, Stein-Streilein J. NK T cell-derived IL-10 is essential for the differentiation of antigen-specific T regulatory cells in systemic tolerance. J Immunol. 2001;166(1):42–50. doi: 10.4049/jimmunol.166.1.42. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Balla DA, Cicchetti DV. Vineland adaptive behavior scales interview edition expanded form manual. Circle Pines, MN: American Guidance Services, Inc; 1984. [Google Scholar]
- Todd RD, Hickok JM, Anderson GM, Cohen DJ. Antibrain antibodies in infantile autism. Biol Psychiatry. 1988;23(6):644–7. doi: 10.1016/0006-3223(88)90012-1. [DOI] [PubMed] [Google Scholar]
- Torres AR, Maciulis A, Odell D. The association of MHC genes with autism. Front Biosci. 2001;6:D936–943. doi: 10.2741/torres. [DOI] [PubMed] [Google Scholar]
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57(1):67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- Wachs FP, Winner B, Couillard-Despres S, Schiller T, Aigner R, Winkler J, Bogdahn U, Aigner L. Transforming growth factor-beta1 is a negative modulator of adult neurogenesis. J Neuropathol Exp Neurol. 2006;65(4):358–370. doi: 10.1097/01.jnen.0000218444.53405.f0. [DOI] [PubMed] [Google Scholar]
- Warren RP, Singh VK, Cole P, Odell JD, Pingree CB, Warren WL, White E. Increased frequency of the null allele at the complement C4b locus in autism. Clin Exp Immunol. 1991;83(3):438–440. doi: 10.1111/j.1365-2249.1991.tb05657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren RP, Singh VK, Averett RE, Odell JD, Maciulis A, Burger RA, Daniels WW, Warren WL. Immunogenetic studies in autism and related disorders. Mol Chem Neuropathol. 1996;28(1–3):77–81. doi: 10.1007/BF02815207. [DOI] [PubMed] [Google Scholar]
- Wills S, Cabanlit M, Bennett J, Ashwood P, Amaral D, Van de Water J. Autoantibodies in autism spectrum disorder. Ann NY Acad Sci. 2007;1107:79–91. doi: 10.1196/annals.1381.009. [DOI] [PubMed] [Google Scholar]
- Zimmerman AW, Jyonouchi H, Comi AM, Connors SL, Milstien S, Varsou A, Heyes MP. Cerebrospinal fluid and serum markers of inflammation in autism. Pediatr Neurol. 2005;33(3):195–201. doi: 10.1016/j.pediatrneurol.2005.03.014. [DOI] [PubMed] [Google Scholar]