Abstract
MDM2 is a key negative regulator of tumor suppressor p53. A single nucleotide polymorphism in the MDM2 promoter, SNP309, enhances transcriptional activation of MDM2 and has been associated with early onset of several types of cancer. In this study, we attempted to determine if the MDM2 SNP309 polymorphism plays a role in the aggressive phenotype seen in African American (AA) prostate cancer by examining the association between MDM2 SNP309 and MDM2 protein levels in prostate cancer (PCa) patients of different racial backgrounds. Prospectively enrolled PCa patients (AA=51, CA=50) were evaluated for MDM2 SNP309 and MDM2 protein expression. MDM2 overexpression, defined as >10% of tumor cells in three tissue cores, was assessed using immunohistochemistry on tissue microarray. MDM2 protein expression was significantly greater in CA than AA patients (78% versus 45% respectively, p=0.0007). Germline DNA was analyzed by PCR-RFLP then confirmed by DNA sequencing. MDM2 SNP309 genotype frequencies did not differ significantly between AA and CA PCa patients (AA: TT 68.6%, TG 25.5%, GG 5.9%; CA: TT 62.0%, TG 20.0%, GG 18.0%; p=0.16), suggesting that the MDM2 SNP309 allele does not play a significant role in the observed overexpression.
Keywords: MDM2, prostate cancer, single nucleotide polymorphism, SNP309
Introduction
In response to stress, cells activate a complex pathway involving tumor suppressor p53 that is responsible for cell cycle arrest, DNA repair, and apoptosis as protection from the deleterious effects of mutation [1]. A key negative regulator of p53, MDM2 targets p53 for proteasomal degradation via an E3 ubiquitin ligase [2-4]. Overexpression of MDM2 has been associated with lack of response to chemoradiotherapy in laryngeal cancer and has been shown to induce androgen independence in prostate cancer cell lines [5, 6]. We previously reported that MDM2 overexpression was significantly associated with advanced stage prostate cancer (PCa) [7], a finding later reproduced by other investigators [8, 9]. Recent studies have also shown that inhibiting MDM2 expression enhances the effects of radiation and chemotherapy on PCa cells [10-12]. Thus, it is conceivable that altered regulation of MDM2 may play a role, at least in part, in the aggressive nature of PCa in African American (AA) patients.
Recently, the G allele of a single nucleotide polymorphism (SNP) at position 309 in the P2 promoter of MDM2 (rs2279744; T/G) was shown to increase the binding affinity of the transcriptional activator Sp1, resulting in higher levels of MDM2 protein expression [13]. Additionally, steroid hormone receptors including androgen (AR) and estrogen receptors (ER) have been shown to form complexes with Sp1 and act as co-regulators [14, 15]. Studies in ER-positive tumors such as breast and ovarian cancer have shown associations between younger age at onset and the MDM2 SNP309 G allele [16, 17]. In the ovarian cancer study, the age of onset in women with both the SNP 309 G allele and high expression of ER was 8 years earlier than those without the SNP 309 G allele. Similarly, in a cohort of women with breast cancer, patients with the G/G SNP 309 genotype had an age of onset 7 years earlier than patients with the T/T genotype. Moreover, premenopausal women (with active estrogen signaling) who have an MDM2 SNP309 G allele display early-onset soft-tissue sarcoma, diffuse large B-cell lymphoma, colorectal cancer, and non-small cell lung cancer [18-22].
In this study, we tested the postulate that a higher frequency of the SNP309 G allele at the MDM2 promoter in AA patients may contribute to the aggressive phenotype and the young age of onset associated with their tumors. This is the first study to examine the implication of the MDM2 SNP 309 as it applies to racial differences in the clinicopathologic presentation of prostate cancer. Additionally, this is the first report to examine both the SNP 309 genotype and the corresponding MDM2 protein expression in a group of prospectively enrolled prostate cancer patients.
Materials and Methods
Patient Population
The study cohort consisted of 101 PCa patients prospectively enrolled at the Manhattan Veterans Affairs Medical Center (VAMC). Patients self-identified as African American (n=51) or Caucasian (n=50) during the hospital registration process, and this identification was confirmed by a review of the clinical notes before data analysis. Clinicopathologic, demographic, and survival data was recorded prospectively for all patients. The VAMC Institutional Review Board approved the study. Biochemical recurrence (BCR) was defined as a PSA of 0.2 or greater with a confirmatory rise [23].
Genotype Analysis
Germline DNA was isolated from 101 normal prostate tissue specimens collected at the time of patient surgery (Qiagen, DNA Mini Kit, Maryland, USA). Paraffin embedded blocks of normal prostate tissues removed at the time of radical prostatectomy were chosen after review of the corresponding H&E by an attending pathologist (PL). We did not use normal prostate tissue adjacent to prostate cancer to avoid any possible confounding effect of tumor cells. The MDM2 SNP309 genotype was determined by PCR amplification followed by restriction fragment length polymorphism (RFLP) and confirmed by DNA sequencing.
The MDM2 promoter region was amplified by PCR using the primer pair F (5′-CGGGAGTTCAGGGTAAAGGT-3′) and R (5′-AGCAAGTCGGTGCTTACCTG-3′) to amplify a 352 base pair (bp) product. This assay was performed using at least 20 ng of genomic DNA, 10 uM of each primer, 1 U of Amplitaq Gold DNA polymerase (Applied Biosystems, Foster City, CA), 10 mM dNTP, and 25 mM MgCl2. After an enzyme activation step at 94°C for 10 min, PCR was conducted for 45 cycles at 94°C for 1 min, 56°C for 1 min, 72°C for 2 min, and was concluded with a final extension step of 72°C for 10 min.
For RFLP analysis, 7 uL of the 352 bp PCR product was digested in a 20 uL reaction with 2 U of MspA1I (New England Biolabs, Ipswich, MA), 1× BSA, and 1× NEBuffer 4 (50 mM potassium acetate, 20 mM Tris-acetate, 10 mM magnesium acetate, 1 mM DTT, pH 7.9) for 1 h at 37°C.
Digestion products were resolved on 2% agarose gels and stained with ethidium bromide. SNP309 introduces a new MspA1I restriction enzyme site. The wild type product (TT) is digested into three bands (233, 88, 31 bp), the SNP309 homozygous product (GG) is digested into four bands (187, 88, 46, 31 bp), and the heterozygous product (TG) yields a combination of the five bands (233, 187, 88, 46, 31 bp).
For confirmation of genotypes, DNA sequencing was performed at the DNA Sequencing Facility of the Skirball Institute at New York University Medical Center. The sequencing reaction contained 1 uL of purified PCR products (Qiagen, QIAquick PCR Purification Kit, Maryland, USA), 1 uL of forward primer, and 14 uL of water, and was conducted on an ABI 3730XL sequencing instrument that utilizes ABI BigDye Terminators cycle sequencing.
Immunohistochemistry
Immunohistochemistry (IHC) was performed with monoclonal anti-MDM2 antibody (2A10, Calbiochem, San Diego, CA; 2μg/mL) using an avidin-biotin immunoperoxidase method. Antigen retrieval of tissue microarray (TMA) sections were performed by boiling in 0.01 M solution of citric acid, pH 6.0 for 15 min followed by incubation with the primary antibody overnight at 4°C. Biotinylated horse antimouse IgG antibody was applied for 1 h (Vector Laboratories, Burlingame, CA; 1:500 dilution), followed by avidin-biotin peroxidase complexes for 30 min (Vector Laboratories; 1:25 dilution). Diaminobenzidine was used as the final chromogen, and hematoxylin was used as the nuclear counterstain. Nuclear immunoreactivity was determined on a continuous scale by a pathologist with values that ranged from undetectable levels (0%) to homogeneous staining (100%). Protein overexpression was defined as >10% cells positive for staining when averaged across three cores [24].
Statistical Analysis
Descriptive statistics were calculated for baseline demographic and clinicopathologic characteristics. Associations among MDM2 SNP309 genotype, MDM2 protein overexpression (>10% positivity averaged across three cores), and clinicopathologic/ histopathologic features of PCa were evaluated by the Wilcoxon rank-sum test, the Kruskal-Wallis test, the chi-square test, or Fisher’s exact test, as appropriate. All p-values were two-sided with statistical significance evaluated at the 0.05 alpha level. All analyses were performed in SAS Version 9.1 (SAS Institute Inc., Cary, North Carolina).
Results
MDM2 Protein Overexpression is More Common in Caucasian Patients
MDM2 protein expression in 101 prostatectomy tumor specimens was assessed using immunohistochemistry on TMA with 51 AA and 50 CA cancer cases. Tissue specimens from each patient consisted of three core samples, each 0.6 um thick. Fifty tumor cells from each core were analyzed for MDM2 protein expression. Figure 1 shows an example of MDM2 overexpression as demonstrated by immunohistochemical staining. Table 1 summarizes the baseline characteristics of the 101 patients studied. MDM2 protein overexpression was significantly greater in CA patients (78%) than AA patients (45%) (p=0.0007) by Chi-square test.
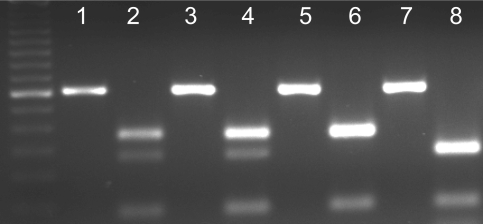
Figure 1.
Representative RFLP genotyping of MDM2 SNP309. Each pair of lanes (e.g., 1+2, 3+4, etc) represents the analysis from a single patient. A 50 bp ladder (first lane on the left) was used to distinguish band size. The 352 bp PCR product is digested with MspA1I. MDM2 SNP309 introduces a new restriction enzyme site, allowing for discrimination between genotypes. Odd lanes were loaded with undigested PCR products and even lanes with the products digested with MspA1I. One upper band represents the TT genotype (lane 6), two bands represent the TG genotype (lanes 2 and 4), and one lower band represents the GG genotype (lane 8).
Table 1.
Summary of baseline patient characteristics
| Patient characteristics | Total number (%) | AA number (%) | CA number (%) |
|---|---|---|---|
| Number of patients | 101(100) | 51(50.5) | 50(49.5) |
| Pathologic stage | |||
| pTl/T2 | 59(58.4) | 29(56.9) | 30(60.0) |
| pT3/T4 | 42(41.6) | 22(43.1) | 20(40.0) |
| PSA | |||
| <10 | 63(66.3)* | 28(56.0) | 35(77.8) |
| >10 | 32(33.7) | 22(44.0) | 10(22.2) |
| Gleason grade | |||
| <7 | 53(52.5) | 24(47.1) | 29(58.0) |
| >7 | 48(47.5) | 27(52.9) | 21(42.0) |
| Recurrence | 29(32.2) | 18(40.0) | 11(24.4) |
some percentages based on denominators less than 101, 51 and 50 due to missing data
Genotype Frequencies of MDM2 SNP309 is Not Associated with Racial Disparity between AA and CA Patients
We successfully amplified DNA by PCR for genotype analysis of MDM2 SNP309 in 101/101 (100%) cases of PCa patients. An example of the genotyping results is shown in Figure 2. The genotype frequencies in AA patients were TT 68.6%, TG 25.5%, and GG 5.9% (allele frequency: T 81.4%, G 18.6%), while those in CA patients were TT 62.0%, TG 20.0%, and GG 18.0% (allele frequency: T 72%, G 28%). These frequencies were not significantly different (p=0.16, chi-square test).
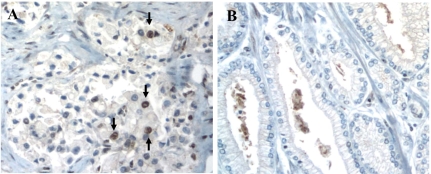
Figure 2.
Immunohistochemical analysis of MDM2 protein expression in PCa. Protein overexpression was defined as >10% cells positive for staining averaged across 3 cores on TMA. Images showing representative cases with positive (A, indicated by arrows) and negative (B) staining for MDM2 protein.
No Correlation between MDM2 SNP309 Allele and Age at Diagnosis, Level of MDM2 Expression or Histopathologic Features
We examined age at diagnosis of PCa in AA and CA patients with respect to MDM2 SNP309 genotype. No statistically significant association was found between the MDM2 SNP309 G allele and early-onset PCa (<60) among the entire cohort (p=0.35). When stratified by race, there was a trend among AA patients with early-onset PCa and the TT MDM2 SNP309 allele (p=0.07). Table 2 presents genotype frequencies by race and age at diagnosis. Histopathologic features of PCa, including tumor stage, Gleason grade, PSA, recurrence, and survival were categorized by MDM2 SNP309 genotype and MDM2 protein overexpression. No significant associations were observed between genotypes and protein expression or between protein expression and histopathologic features of PCa (Table 3).
Table 2.
Genotype frequencies by race and age at diagnosis
| TT No. (%) | TG No. (%) | GG No. (%) | Total No. (%) | P value* | |
|---|---|---|---|---|---|
| All patients | 66(65.4) | 23(22.8) | 12(11.9) | 101(100) | |
| AA | 35(68.6) | 13(25.5) | 3(5.9) | 51(100) | |
| CA | 31(62.0) | 10(20.0) | 9(18.0) | 50(100) | 0.16 |
| AA | |||||
| <60 yr | 13(92.9) | 1(7.1) | 0(0) | 14(100) | |
| >60 yr | 22(59.5) | 12(32.4) | 3(8.1) | 37(100) | 0.07 |
| CA | |||||
| <60 yr | 9(60.0) | 4(26.7) | 2(13.3) | 15(100) | |
| >60 yr | 22(62.9) | 6(17.1) | 7(20.0) | 35(100) | 0.69 |
Chi-square test or Fisher’s exact test as appropriate
Table 3.
Patient characteristics categorized by MDM2 protein expression
| AA | CA | |||||||
|---|---|---|---|---|---|---|---|---|
| Patient characteristics | Total AA No. (%) | ≤10% No. (%) | >10% No. (%) | P value* | Total CA No. (%) | ≤10% No. (%) | >10% No. (%) | P value |
| No. of patients | 51(100) | 28(54.9) | 23(45.1) | - | 50(100) | 11(22. 0) | 39(78.0) | - |
| SNP300 genotype | ||||||||
| TT | 35(100) | 22(62.9) | 13(37.1) | 0.09 | 31(100) | 7(22.6) | 24(77.4) | 0.9 |
| TG or GG | 16(100) | 6(37.5) | 10(62.5) | 19(100) | 4(21.1) | 15(78.9) | ||
| Age at diagnosis | ||||||||
| <60 yr | 14(100) | 6(42.9) | 8(57.1) | 0.29 | 15(100) | 3(20) | 12(80.0) | 0.82 |
| >60 yr | 37(100) | 22(59.5) | 15(40.5) | 35(100) | 8(22.9) | 27(77.1) | ||
| Pathologic stage | ||||||||
| pTl/T2 | 29(100) | 16(55.2) | 13(44.8) | 0.96 | 30(100) | 7(23.3) | 23(76.7) | 0.78 |
| pT3/T4 | 22(100) | 12(54.5) | 10(45.5) | 20(100) | 4(20.0) | 16(80.0) | ||
| PSA | ||||||||
| ≤10 | 28(100)** | 14(50.0) | 14(50.0) | 0.52 | 35(100)** | 6(17.1) | 23(76.7) | 0.78 |
| >10 | 22(100) | 13(59.1) | 9(40.9) | 10(100) | 4(20.0) | 16(80.0) | ||
| Gleason grade | ||||||||
| <7 | 24(100) | 11(45.8) | 13(54.2) | 0.22 | 29(100) | 6(20.7) | 23(79.3) | 0.79 |
| ≥7 | 27(100) | 17(63.0) | 10(37.0) | 21(100) | 5(23.8) | 16(76.2) | ||
| Recurrence | 18(100) | 10(55.6) | 8(44.4) | 0.63 | 11(100) | 2(18.2) | 9(81.8) | 0.86 |
Chi-square or Fisher’s exact test as appropriate
One AA and 5 CA patients missing from this analysis
Discussion
Recent work by Bond et al demonstrated that not only can a specific SNP 309 allele increase MDM2 expression but that it may do so in a gender-specific and hormone-dependent manner, making SNP 309 particularly appealing in hormonally regulated cancers such as prostate [17]. Our data showed a higher expression of MDM2 protein in the CA tumors compared to the AA tumors. We hypothesized that the MDM2 SNP309 polymorphism (G allele) might contribute to the aggressive tumor phenotype and younger age at onset observed in AA patients with PCa. We performed genotyping analysis of germline DNA from PCa patients which revealed no significant difference in allele frequency between AA and CA patients. Our findings suggest different mechanisms in the regulation of MDM2 expression in PCa patients of different racial backgrounds. The data does not, however, support a role of the MDM2 SNP 309G allele as a significant regulator of MDM2 overexpression in PCa.
In contrast to the Bond study, our data is consistent with the observation from a recent case-control study by Stoehr et al from Germany which suggests that the MDM2 SNP309 does not have the same diagnostic or prognostic potential in prostate cancer as has been demonstrated in other malignancies [25]. The Stoehr study consisted of 145 PCa patients and 124 male controls. SNP genotyping of normal prostate tissue from the cases and peripheral blood from the controls showed no significant difference in MDM2 SNP 309 allele frequency between the two groups. There was, however, a trend toward a higher frequency of the T/T genotype in the cancer patients. In concordance with our study, there was no association between the MDM2 SNP 309 variant and cancer stage or early onset disease. Our study, however, further demonstrated that there is no difference in MDM2 SNP 309 allele frequency between racial groups within a cohort of prostate cancer patients.
Another recent study by Kibel and colleagues [26], however, is in discordance with our conclusion. They attempted to correlate SNP variants of nine different cell cycle genes including MDM2 with an aggressive prostate cancer phenotype. Their study revealed a significant association between the MDM2 SNP 309 TT/GT genotype (vs. GG) and patients with androgen-independent disease (AID). They demonstrated that of 71 patients who developed AID, 93% had the TT/GT genotype which equated to a statistically significant odds ratio of 2.28. Additionally, when stratified by age at diagnosis, the same high-risk MDM2 genotypes were significantly associated with earlier onset of disease. Interestingly, it was the T allele, not the G allele as had been described previously, that was associated with aggressive phenotype. Their data suggests that the MDM2 SNP309 allele may be more useful as a predictor of aggressive disease among a cohort of patients already diagnosed than as a predictor of PCa risk in the population overall. However, the study was retrospective, and thus was unable to determine if the high-risk genotypes were associated with aggressive disease at onset or with the progression to aggressive disease from an initially indolent disease. In our prospective study of 101 patients, there were 29 recurrences, 90% of which had the GT/TT genotype. Of the 18 AA patients who recurred, 100% had the GT/TT phenotype and 78% had the TT genotype, suggesting that the MDM2 SNP309 may in fact play a role in the aggressive nature of prostate cancer in AAs. Clearly, the number of recurrences in our study was not large enough to detect true associations, but its concordance with the Kibel study may be enough to warrant further study of the T allele and its role in predicting poor prognosis. Unlike our study, the Kibel study did not study MDM2 expression in their patient cohort, so they were unable to speculate about whether the MDM2 SNP309 contributes to advanced disease due to up-regulation of MDM2 or due to a different pathway. Our data showing no association between the SNP variant and the level of MDM2 expression suggests that the latter scenario is more likely.
Both the Stoehr and Kibel studies compare the frequency of MDM2 SNP309 alleles between prostate cancer patients and healthy controls. In the Kibel study, they explicitly stated that all patients are of Caucasian decent, and it is not unreasonable to expect that most patients in the German study are also Caucasians. Our study is the first to examine differences in the MDM2 SNP309genotype between AA and CA patients with PCa. A previous case-control study by Pine et al compared the frequencies of SNP309 genotypes between AA and CA patients with lung cancer [27]. They were not able to demonstrate that the MDM2 SNP309 was associated with lung cancer in either racial group. However, their data provides a control population of 255 AAs to which we can compare the genotype frequencies of our AA patients. This comparison reveals that our AA population has a higher frequency of the G allele compared to the AA controls in the Pine study (18.6% vs 11%). This finding might be interpreted in two ways. It may be an indication that the G allele confers an increased risk of PCa in the AA population, or it may indicate that the degree of CA admixture in our cohort of AA patients is higher than that in other studies.
Our results somewhat unexpectedly revealed that MDM2 was overexpressed in prostate cancer in the CA population. While MDM2 and AA ethnicity have both been associated with poor prognosis, the relationship between the two variables in our study was neither causative nor correlative. One possible reason for the seemingly paradoxical results is that prior studies examining the utility of MDM2 as a prognostic marker included predominantly if not exclusively CA patients (or simply did not stratify by race) thus providing no opportunity for such a disparity to be revealed. Also, MDM2 is a negative regulator of p53, which has been shown to be rarely mutated in primary PCa. In a recent study of 2514 post-prostatectomy tumors, only 2.5% were found to have p53 overexpression by immunohistochemistry [28]. In contrast, p53 is more frequently inactivated in other types of cancers including breast and ovarian [16, 29].
In conclusion, our data reveals that MDM2 expression in PCa differs between AA and CA patients. The data does not, however, support a role for the MDM2 SNP309 in the development of the aggressive nature of PCa in AA patients, including younger age at onset.
References
- 1.Pietsch EC, Humbey O, Murphy ME. Polymorphisms in the p53 pathway. Oncogene. 2006;25:1602–1611. doi: 10.1038/sj.onc.1209367. [DOI] [PubMed] [Google Scholar]
- 2.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 3.Kubbutat MH, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature. 1997;387:299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- 4.Bond GL, Hu W, Levine AJ. MDM2 is a central node in the p53 pathway: 12 years and counting. Curr Cancer Drug Targets. 2005;5:3–8. doi: 10.2174/1568009053332627. [DOI] [PubMed] [Google Scholar]
- 5.Osman I, Sherman E, Singh B, Venkatraman E, Zelefsky M, Bosl G, Scher H, Shah J, Shaha A, Kraus D, Cordon-Cardo C, Pfister DG. Alteration of p53 pathway in squamous cell carcinoma of the head and neck: impact on treatment outcome in patients treated with larynx preservation intent. J Clin Oncol. 2002;20:2980–2987. doi: 10.1200/JCO.2002.06.161. [DOI] [PubMed] [Google Scholar]
- 6.Agus DB, Cordon-Cardo C, Fox W, Drobnjak M, Koff A, Golde DW, Scher HI. Prostate cancer cell cycle regulators: response to androgen withdrawal and development of androgen independence. J Natl Cancer Inst. 1999;21:1869–1876. doi: 10.1093/jnci/91.21.1869. [DOI] [PubMed] [Google Scholar]
- 7.Osman I, Drobnjak M, Fazzari M, Ferrara J, Scher HI, Cordon-Cardo C. Inactivation of the p53 pathway in prostate cancer: impact on tumor progression. Clin Cancer Res. 1999;5:2082–2088. [PubMed] [Google Scholar]
- 8.Leite KR, Franco MF, Srougi M, Nesrallah A, Bevilacqua RG, Darini E, Carvalho CM, Meirelles MI, Santana I, Camara-Lopes LH. Abnormal expression of MDM2 in prostate carcinoma. Mod Pathol. 2001;5:428–436. doi: 10.1038/modpathol.3880330. [DOI] [PubMed] [Google Scholar]
- 9.Khor LY, Desilvio M, Al-Saleem T, Hammond ME, Grignon DJ, Sause W, Pilepich M, Okunieff P, Sandler H, Pollack A, Radiation Therapy Oncology Group MDM2 as a predictor of prostate carcinoma outcome: an analysis of Radiation Therapy Oncology Group Protocol 8610. Cancer. 2005;104:962–967. doi: 10.1002/cncr.21261. [DOI] [PubMed] [Google Scholar]
- 10.Bianco R, Caputo R, Damiano V, De Placido S, Ficorella C, Agrawal S, Bianco AR, Ciardiello F, Tortora G. Combined targeting of epidermal growth factor receptor and MDM2 by gefitinib and antisense MDM2 cooperatively inhibit hormone-independent prostate cancer. Clin Cancer Res. 2004;10:4858–4864. doi: 10.1158/1078-0432.CCR-03-0497. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Yu D, Agrawal S, Zhang Experimental therapy of human prostate cancer by inhibiting MDM2 expression with novel mixed-backbone antisense oligonucleotides: in vitro and in vivo activities and mechanisms. Prostate. 2003;54:194–205. doi: 10.1002/pros.10187. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z, Li M, Wang H, Agrawal S, Zhang R. Antisense therapy targeting MDM2 oncogene in prostate cancer: Effects on proliferation, apoptosis, multiple gene expression, and chemotherapy. Proc Natl Acad Sci USA. 2003;100:11636–11641. doi: 10.1073/pnas.1934692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bond GL, Hu W, Bond EE, Robins H, Lutzker SG, Arva NC, Bargonetti J, Bartel F, Taubert H, Wuerl P, Onel K, Yip L, Hwang SJ, Strong LC, Lozano G, Levine AJ. A single nucleotide polymorphism in the MDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans. Cell. 2004;119:591–602. doi: 10.1016/j.cell.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 14.Yuan H, Gong A, Young CY. Involvement of transcription factor Sp1 in quercetin-mediated inhibitory effect on the androgen receptor in human prostate cancer cells. Carcinogenesis. 2005;26:793–801. doi: 10.1093/carcin/bgi021. [DOI] [PubMed] [Google Scholar]
- 15.Saville B, Wormke M, Wang F, Nguyen T, Enmark E, Kuiper G, Gustafsson JA, Safe S. Ligand-, cell-, and estrogen receptor subtype (alpha/beta)-dependent activation at GC-rich (Sp1) promoter elements. J Biol Chem. 2000;275:5379–5387. doi: 10.1074/jbc.275.8.5379. [DOI] [PubMed] [Google Scholar]
- 16.Bartel F, Jung J, Bohnke A, Gradhand E, Zeng K, Thomssen C, Hauptmann S. Both germ line and somatic genetics of the p53 pathway affect ovarian cancer incidence and survival. Clin Cancer Res. 2008;14:89–96. doi: 10.1158/1078-0432.CCR-07-1192. [DOI] [PubMed] [Google Scholar]
- 17.Bond GL, Hirshfield KM, Kirchhoff T, Alexe G, Dond EE, Robbins H, Bartel F, Taubert H, Wuerl P, Hait W, Toppmeyer D, Offit K, Levine AJ. MDM2 SNP309 accelerates tumor formation in a gender-specific and hormone-dependent manner. Cancer Res. 2006;66:5104–5110. doi: 10.1158/0008-5472.CAN-06-0180. [DOI] [PubMed] [Google Scholar]
- 18.Bond GL, Menin C, Bertorelle R, Alhopuro P, Aaltonen LA, Levine AJ. MDM SNP309 accelerates colorectal tumour formation in women. J Med Genet. 2006;43:950–952. doi: 10.1136/jmg.2006.043539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lind H, Zienolddiny S, Ekstrom PO, Skaug V, Haugen A. Association of a functional polymorphism in the promoter of the MDM2 gene with risk of nonsmall cell lung cancer. Int J Cancer. 2006;119:718–721. doi: 10.1002/ijc.21872. [DOI] [PubMed] [Google Scholar]
- 20.Menin C, Scaini MC, De Salvo GL, Biscuola M, Quaggio M, Esposito G, Belluco C, Montagna M, Agata S, D’Andrea E, Nitti D, Amadori A, Bertorelle R. Association between MDM2-SNP309 and age at colorectal cancer diagnosis according to p53 mutation status. J Natl Cancer Inst. 2006;98:285–288. doi: 10.1093/jnci/djj054. [DOI] [PubMed] [Google Scholar]
- 21.Alhopuro P, Ylisaukko-Oja SK, Koskinen WJ, Bono P, Arola J, Jarvinen HJ, Mecklin JP, Atula T, Kontio R, Makitie AA, Suominen S, Leivo I, Vahteristo P, Aaltonen LM, Aaltonen LA. The MDM2 promoter polymorphism SNP309T–>G and the risk of uterine leiomyosarcoma, colorectal cancer, and squamous cell carcinoma of the head and neck. J Med Genet. 2005;42:694–698. doi: 10.1136/jmg.2005.031260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yarden RI, Friedman E, Metsuyanim S, Olender T, Ben-Asher E, Papa MZ. MDM2 SNP309 accelerates breast and ovarian carcinogenesis in BRCA1 and BRCA2 carriers of Jewish-Ashkenazi descent. Breast Cancer Res Treat. 2008;111:497–504. doi: 10.1007/s10549-007-9797-z. [DOI] [PubMed] [Google Scholar]
- 23.Cookson M, Aus G, Burnett AL, Canby-Hagino Ed, D’Amico AV, Dmochowski RR, Eton DT, Forman JD, Goldenberg SL, Hernandez J, Higano CS, Kraus SR, Moul JW, Tangen C, Thrasher JB, Thompson I. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: The American Urological Association prostate guidelines for localized prostate cancer update panel report and recommendations for a standard in the reporting of surgical outcomes. J Urol. 2007;177:540–545. doi: 10.1016/j.juro.2006.10.097. [DOI] [PubMed] [Google Scholar]
- 24.Hammock L, Lewis M, Phillips C, Cohen C. Strong HER-2/neu protein overexpression by immunohistochemistry often does not predict oncogene amplification by fluorescence in situ hybridization. Hum Pathol. 2003;34:1043–1047. doi: 10.1053/s0046-8177(03)00409-x. [DOI] [PubMed] [Google Scholar]
- 25.Stoehr R, Hitzenbichler F, Kneitz B, Hammerschmied CG, Burger M, Tannapfel A, Hartmann A. MDM2-SNP309 polymorphism in prostate cancer: no evidence for association with increased risk or histopathological tumor characteristics. Br J Cancer. 2008;99:78–82. doi: 10.1038/sj.bjc.6604441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kibel A, Jin C, Klim A, Luly J, A Roehl K, Wu WS, Suarez BK. Association between polymorphisms in cell cycle genes and advanced prostate carcinoma. Prostate. 2008;68:1179–1186. doi: 10.1002/pros.20784. [DOI] [PubMed] [Google Scholar]
- 27.Pine S, Mechanic L, Bowman E, Welsh JA, Chanock SC, Shields PG, Harris CC. MDM2 SNP309 and SNP354 are not associated with lung cancer risk. Cancer Epidemiol Biomarkers Prev. 2006;15:1559–1561. doi: 10.1158/1055-9965.EPI-06-0217. [DOI] [PubMed] [Google Scholar]
- 28.Schlomm T, Iwers L, Kirstein P, Jessen B, Kollermann J, Minner S, Passow-Drolet A, Mirlacher M, Milde-Langosch K, Graefen M, Haese A Steuber T, Simon R, Huland H, Sauter G, Erbersdobler A. Clinical significance of p53 alterations in surgically treated prostate cancers. Mod Pathol. 2008 doi: 10.1038/modpathol.2008.104. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Langerød A, Zhao H, Borgan Ø, Nesland JM, Bukholm IR, Ikdahl T, Karesen R, Borresen-Dale AL, Jeffrey SS. TP53 mutation status and gene expression profiles are powerful prognostic markers of breast cancer. Breast Cancer Res. 2007;9:R30. doi: 10.1186/bcr1675. [DOI] [PMC free article] [PubMed] [Google Scholar]