Abstract
Identification of metastasis and occult micrometastases of breast cancer demands sensitive and specific diagnostic markers. In this study, we assessed the utility of a mouse monoclonal antibody to human mammaglobin for one such purpose. Immunohistochemical stains were performed on paraffin-embedded sections from a total of 284 cases, which consisted of primary breast invasive carcinomas (41 cases) with matched metastases to ipsilateral axillary lymph nodes, metastatic breast carcinoma to liver (1 case) and kidney (1 case), non-breast neoplasms (161 cases), and normal human tissues (39 cases). The results showed 31 of the 41 cases of primary breast cancer with axillary lymph node metastases were positive for mammaglobin (76%). In the meantime, we documented expression of mammaglobin in occasional cases of endometrial carcinoma (17%). Our data further validated that mammaglobin is a valuable diagnostic marker for metastatic carcinoma of breast origin, although endometrial carcinoma should be considered as a major differential diagnosis.
Keywords: Mammaglobin, breast cancer, metastasis, immunohistochemistry
Introduction
Breast cancer is known for its morphologic diversity and unpredictable clinical behavior. The currently available immunohistochemical markers used for the diagnosis of metastatic breast carcinoma include estrogen receptor (ER) [1], progesterone receptor (PR) [1] and gross cystic disease fluid protein (GCDFP-15) [2-3]. They are valuable diagnostic tools, but there is a need to further improve the sensitivity and specificity of the existing panel of breast markers. Additionally, the lack of organ specificity of these breast carcinoma markers further demonstrates the need for new markers in the diagnosis of metastatic breast cancer.
Mammaglobin is a 93 amino acid glycoprotein with homology to other secretoglobin-uteroglobin family members. Mammaglobin was originally identified as a breast cancer restricted biomarker by differential screening and subsequent studies have focused on further elucidating its function and expression profile [4-10]. There are also reports exploring mammaglobin as a serum marker for breast cancer [11-12]. Although data has been accumulating regarding the clinical utility of mammaglobin as a biomarker for diagnostic purposes, there has been few reports, however, focusing on its utility in identifying metastatic breast cancer [3, 13].
In this study, we surveyed the expression profile of mammaglobin using a mouse monoclonal anti-human antibody on a series of primary invasive breast carcinomas with matched ipsilateral axillary lymph nodes metastasis, non-breast neoplasms and normal human tissues. We report and discuss our findings regarding the sensitivity and specificity of mammaglobin as a diagnostic marker for breast carcinoma, especially metastatic breast carcinoma.
Materials and Methods
Tissue Sources
Breast Carcinomas with Their Matched Metastases on Whole Sections: All 41 cases diagnosed as invasive breast carcinoma with axillary lymph node metastasis were reviewed and graded according to the Elston-Ellis Scarff-Bloom-Richardson (ESBR) criteria. Additionally, one case of metastatic breast carcinoma to live and one case metastatic to kidney were included.
Non-breast Neoplasms and Normal Tissue on Tissue Microarray: A set of tissue microarrays consisting of 63 cases of endometrial carcinoma, 98 cases of non-breast non-endometrial carcinomas, and 49 cases of normal tissue were used in the study. Three1.0 mm punches of each case from the representative areas of the paraffin blocks were taken to construct the above tissue arrays using a manual tissue microarrayer.
All the blocks were retrieved from surgical pathology files from Asan Medical Center, Seoul, Korea, The Methodist Hospital, Houston, TX and Dako North America Inc, CA. These three institutions routinely use Dako immunostainer and follow the similar tissue processing protocols, and our previous collaborative studies demonstrate a consistent and comparable immunostain quality.
Immunohistochemistry
Formalin-fixed paraffin embedded human tissues and tissue arrays were sectioned at 4µm and mounted on charged slides. The slides were deparaffinized in Histo-Clear (National Diagnostics) and rehydrated in graded alcohols. The slides were pretreated with heat-induced epitope retrieval for 40 minutes at 95–99°C in the Target Retrieval Solution pH 9.0 (Dako) and cooled for 20 minutes at room temperature. Immunohistochemistry was performed using the EnVisionTM+ System/HRP, Dual Link Rabbit/Mouse (Dako). Endogenous peroxidase was quenched by incubating sections in 3% hydrogen peroxide solution for five minutes. Mouse monoclonal anti-mammaglobin clone 304-1A5 (Dako) was used at a 1:100 dilution. A negative control reagent was also used with each tissue sample to confirm the specificity of the immunostaining. The primary antibody, detection and chromagen (diaminobenzidine) incubations were performed on the Dako autostainer. Finally, the slides were counterstained with hematoxylin and permanently mounted.
Immunostain Scoring Criteria
Staining intensity was reported using a four-point scale, signal intensity was graded as 0 (negative), 1+ (weak), 2+ (moderate) and 3+ (strong). The proportion of positively stained tumor cells was also recorded. Cases with signal intensity of 1+ were considered positive if ≥10% of the tumors cells were positively stained. Cases with ≥2+ signal intensity were considered positive if ≥1% of the tumor cells were positively stained.
Results
All 41 breast cancer cases were diagnosed as invasive carcinoma with axillary lymph node metastases and 38 of the 41 (93%) cases demonstrated high histological grade (≥2/3) (Table 1). Different histological grades between the primary tumor and lymph node metastasis were recorded in 15 cases (37%).
Table 1.
Primary breast cancer and lymph node metastasis: grading and mammaglobin staining
| Case number | ESBR grading(gland+pleomorphism+mitoses) | Signal intensity | Staining area (%) | Results |
|---|---|---|---|---|
| 1.Primary/Met | 3+3+1=7 | 3/0 | 10/0 | +/− |
| 2.Primary/Met | 3+3+3=9 | 3/3 | 90/90 | +/+ |
| 3.Primary/Met | 3+3+1=7 | 2/2 | 10/30 | +/+ |
| 4.Primary/Met | 2+3+1=6 | 0/0 | 0/0 | −/− |
| 5.Primary/Met | 3+3+1=7 | 3/3 | 75/75 | +/+ |
| 6.Primary/Met | 3+3+2=8 | 3/3 | 75/75 | +/+ |
| 7.Primary/Met | 3+3+2=8 | 3/3 | 70/10 | +/+ |
| 8.Primary/Met | 3+3+1=7 | 3/3 | 80/80 | +/+ |
| 9.Primary/Met | 3+3+1=7 | 0/2 | 0/1 | −/+ |
| 10.Primary/Met | 2+2+1=5 | 0/0 | 0/0 | −/− |
| 11.Primary/Met | 2+2+1=5/3+3+1=7 | 0/0 | 0/0 | −/− |
| 12.Primary/Met | 2+2+1=5/3+2+1=7 | 3/3 | 90/25 | +/+ |
| 13.Primary/Met | 3+2+2=7/3+3+3=9 | 0/2 | 0/20 | −/+ |
| 14.Primary/Met | 3+3+1=7 | 2/2 | 10/10 | +/+ |
| 15.Primary/Met | 3+3+1=7 | 3/0 | 50/0 | +/− |
| 16.Primary/Met | 2+3+1=6/3+3+1=7 | 3/3 | 80/20 | +/+ |
| 17.Primary/Met | 2+3+1=6/3+3+1=7 | 3/3 | 90/90 | +/+ |
| 18.Primary/Met | 2+2+1=5/3+2+1=6 | 3/3 | 90/90 | +/+ |
| 19.Primary/Met | 3+3+1=7/1+2+1=4 | 3/0 | 10/0 | +/− |
| 20.Primary/Met | 3+3+2=8/3+3+1=7 | 3/3 | 50/50 | +/+ |
| 21.Primary/Met | 1+2+1=4/3+2+1=6 | 0/0 | 0/0 | −/− |
| 22.Primary/Met | 3+3+1=7/2+2+1=5 | 3/3 | 80/80 | +/+ |
| 23.Primary/Met | 2+2+1=5 | 3/2 | 80/10 | +/+ |
| 24.Primary/Met | 3+3+2=8 | 0/0 | 0/0 | −/− |
| 25.Primary/Met | 3+3+1=7 | 3/3 | 15/20 | +/+ |
| 26.Primary/Met | 3+3+1=7 | 3/3 | 10/15 | +/+ |
| 27.Primary/Met | 3+3+1=7 | 3/3 | 75/80 | +/+ |
| 28.Primary/Met | 3+3+1=7 | 3/3 | 10/10 | +/+ |
| 29.Primary/Met | 3+1+1=7 | 3/3 | 90/90 | +/+ |
| 30.Primary/Met | 3+3+1=7 | 3/3 | 10/10 | +/+ |
| 31.Primary/Met | 3+1+1=7 | 3/3 | 80/90 | +/+ |
| 32.Primary/Met | 2+3+1=6/3+3+1/7 | 3/3 | 30/70 | +/+ |
| 33.Primary/Met | 2+3+1=6/3+3+1=7 | 0/0 | 0/0 | −/− |
| 34.Primary/Met | 2+3+1=6/3+3+1=7 | 2/2 | 10/20 | +/+ |
| 35.Primary/Met | 2+3+1=6 | 2/3 | 20/80 | +/+ |
| 36.Primary/Met | 3+3+1=7 | 2/2 | 10/20 | +/+ |
| 37.Primary/Met | 3+2+1=7 | 3/3 | 90/90 | +/+ |
| 38.Primary/Met | 3+3+1=7 | 0/0 | 0/0 | −/− |
| 39.Primary/Met | 2+3+1=6/3+3+1=7 | 0/2 | 0/10 | −/+ |
| 40.Primary/Met | 2+3+1=6/3+3+1=7 | 3/3 | 20/30 | +/+ |
| 41.Primary/Met | 3+3+1=7 | 3/3 | 30/15 | +/+ |
All the positive mammaglobin immunostains showed cytoplasmic pattern. There are four profiles in the breast carcinoma cases: profile1, positive staining for both primary breast and their paired metastatic carcinomas (31 of 41 cases, 76%); profile 2, positive staining for primary breast carcinomas (34 of 41 cases, 83%); profile 3, positive staining for metastatic breast carcinomas (34 of 41 cases, 83%); profile 4 negative staining for both primary and their paired metastatic carcinoma (7 of 41 cases, 17%) (Table 1 and Figure 1).
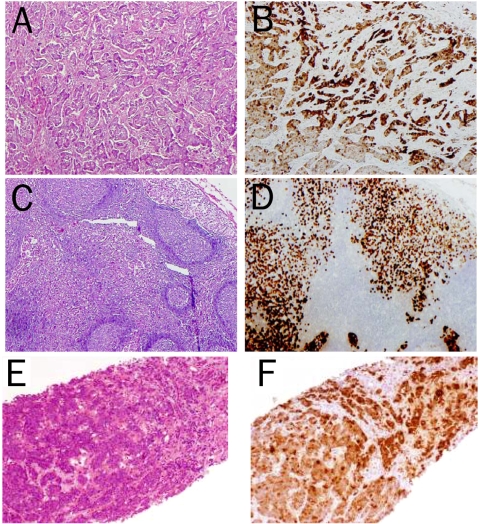
Figure 1.
Immunohistochemical staining of mammaglobin in breast cancers. Representative H&E-stained sections of primary (A) and metastatic breast cancer to lymph node (C) and liver (E), and their corresponding immunohistochemical staining of mammaglobin in the cancer cells (B,D and F).
In addition, both breast carcinoma with distant metastases (to liver and kidney, respectively) showed strong mammaglobin staining (Figure 1). Patchy positive stains of benign breast ductal epithelium were also evident at the periphery of 12 (29%) primary breast carcinomas (Figure 2).
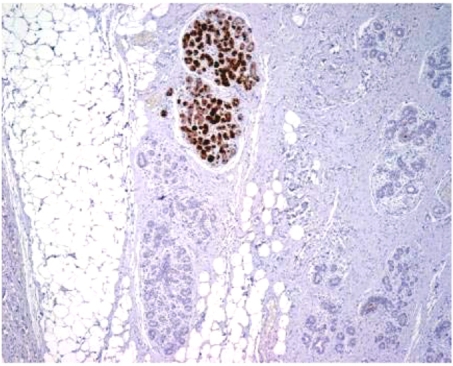
Figure 2.
Immunohistochemical staining of mammaglobin in normal breast glands in the periphery of primary breast cancer. The staining is random and only a small number of normal breast glands are stained.
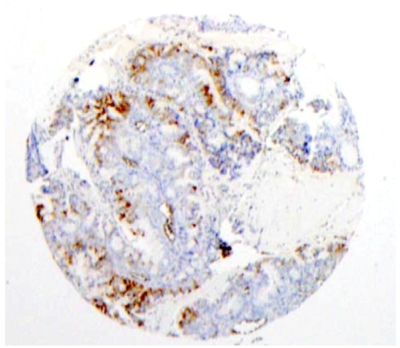
Eleven of the 63 cases of endometrial carcinoma on the tissue microarray were positive (17%) (Figure 3). One carcinoid tumor was positive. Among the normal tissues on the microarray, 6 of 10 endometrium specimens were positive. One uterus and one spleen were also positive (Table 2).
Figure 3.
Immunohistochemical staining of mammaglobin in a microarrayed endometrial carcinoma. Among the 59 cases of endometrial carcinoma on the tissue microarray, positive cytoplasmic staining for mammaglobin was detected in 7 cases.
Table 2.
Immunoreactivity profile of mammaglobin in 161 non-breast neoplasms and 39 normal tissues
| Neoplastic tissue type | Positive/Total | |
|---|---|---|
| Non-breast neoplasms | ||
| Endometrial Ca | 11/63 | |
| Colon Ca | 0/31 | |
| Lung Ca | 0/21 | |
| Prostate Ca | 0/14 | |
| Renal cell Ca | 0/4 | |
| Gastric Ca | 0/1 | |
| Hepatocellular Ca | 0/2 | |
| Ovarian Ca | 0/2 | |
| Pancreatic Ca | 0/2 | |
| Thyroid Ca | 0/1 | |
| Undifferentiated | 0/2 | |
| Ca | ||
| Astrocytoma | 0/2 | |
| Carcinoid Tumor | 1/2 | |
| Ewing’s sarcoma | 0/2 | |
| Hodgkin | 0/2 | |
| lymphoma | ||
| Leiomyoma | 0/2 | |
| MFH | 0/2 | |
| Melanoma | 0/2 | |
| Mesothelioma | 0/2 | |
| Rhabdosarcoma | 0/2 | |
| Normal tissues | ||
| Endometrium | 6/10 | |
| Thyroid | 0/2 | |
| Spleen | 1/2 | |
| Uterus | 1/1 | |
| Prostate gland | 0/2 | |
| Ovary | 0/1 | |
| Testes | 0/2 | |
| Pancreas | 0/2 | |
| Lung | 0/2 | |
| Liver | 0/2 | |
| Kidney | 0/2 | |
| Heart | 0/2 | |
| Adrenal gland | 0/2 | |
| Brain | 0/2 | |
| Colon | 0/2 | |
| Small intestine | 0/2 | |
| Stomach | 0/1 |
Overall, the number of true positive staining was 64 (31 primary breast cancer + 31 lymph node metastasis + 2 metastasis to liver or kidney); false positive was 20 (11 endometrial carcinoma, 1 carcinoid tumor, 6 normal endometrium, 1 normal spleen and 1 normal uterus); false negative staining was 20 (10 primary breast cancer and 10 ipsilateral lymph node metastasis); true negative staining was 180 (149 non-breast neoplasm + 31 normal tissues). Therefore, sensitivity is 76% with a specificity of 90%.
Discussion
The majority of mammaglobin expression studies conducted to date have been designed to detect gene expression levels. There have been studies that examined protein expression in primary and metastatic breast cancer using anti-mammaglobin antibodies [6, 13]. Han et al [13] reported improved sensitivity for the detection of breast cancer with an anti-mammaglobin antibody (84.3% positivity) when compared with BRST-1 (75.7%) and GCDFP-15 (44.3%) in a series of 70 breast cancer cases. Additionally Bhargava et al [3] conducted a survey comparing the sensitivity and specificity of a mammaglobin antibody cocktail to anti-GCDFP-15 on a series of 29 breast carcinomas with matched lymph node metastases (whole tissue sections) and 63 breast carcinomas. The authors reported mammaglobin positivity in 67 of 121 (55.4%) breast carcinomas and GCDFP-15 immunoreactivity in 28 of 121 cases (23.1%). The authors further reported that in the majority of cases, anti-mammaglobin produced a higher staining intensity and percentage of positively staining cells than anti-GCDFP-15.
Due to the focal expression of some biomarkers, the use of microarrays to evaluate the diagnostic utility of new markers can lead to false negative results [14]. In this study, we chose to use whole tissue sections from paraffin embedded blocks of both primary and lymph node metastasis of breast cancer to more accurately determine the sensitivity and specificity of mammaglobin as a breast carcinoma tumor marker. To our knowledge, this is the largest cohort of metastatic carcinoma which is matched with the breast primaries to assess the value of mammaglobin.
Breast cancer prognosis correlates with axillary lymph node status. Identifying metastasis in the axillary lymph nodes is essential in the tumor staging. The use of a cohort in which each breast cancer had a matched axillary lymph node metastasis enhances the significance of this study. In the majority of cases, the primary cancer and the metastases received the same histological grades. However, in a subset of cases the histological grades for the primary tumor and lymph node metastasis were discordant (Table 1). Among these cases, the difference in the grades was subtle for the majority of cases, and could represent the heterogeneous nature in differentiation of the breast cancer. In some cases there were also differences between the primary and the metastatic tumor which reflected the overall staining patterns for mammaglobin. Most strikingly, certain cases only exhibited positive staining in the metastasis. We postulate that this may be due to either sampling bias or a possible de-differentiation in a separate milieu.
The clinical utility of mammaglobin as a diagnostic marker is exemplified by the final diagnosis of two breast cancer cases with distant metastasis to the liver and kidney, respectively. Although the mammaglobin staining was focal, strong positive immunostaining intensity enabled us to determine that the tumor was of breast origin without difficulty.
We also recognized mammaglobin expression in certain endometrial carcinomas. Although this is not a surprising finding because both breast and endometrium are subject to estrogen regulation, we do recommend caution in interpreting a positive mammaglobin staining in case of a metastatic carcinoma. Inclusion of additional markers and clinical information is necessary to establish the final diagnosis in such scenarios.
In summary, the expression profile of monoclonal mouse anti-human mammaglobin, as demonstrated by this study, supports the clinical utility of this marker in the diagnosis of metastatic breast carcinoma, and adding anti-mammaglobin to the current antibody panel will improve the sensitivity and specificity in the diagnosis of metastatic breast carcinoma and help elucidate metastatic carcinomas whose primary origin cannot be identified. In addition, endometrial origin should be considered as a major differential diagnosis when tumor cells are positive for mammaglobin.
References
- 1.Nadji M, Gomez-Fernandez C, Ganjei-Azar P, Morales AR. Immunohistochemistry of estrogen and progesterone receptors reconsidered: experience with 5,993 breast cancers. Am J Clin Pathol. 2005;123:21–27. doi: 10.1309/4wv79n2ghj3x1841. [DOI] [PubMed] [Google Scholar]
- 2.Tornos C, Soslow R, Chen S, Akram M, Hummer AJ, Abu-Rustum N, Norton L, Tan LK. Expression of WT1, CA 125, and GCDFP-15 as useful markers in the differential diagnosis of primary ovarian carcinomas versus metastatic breast cancer to the ovary. Am J Surg Pathol. 2005;29:1482–1489. doi: 10.1097/01.pas.0000176429.88702.36. [DOI] [PubMed] [Google Scholar]
- 3.Bhargava R, Beriwal S, Dabbs DJ. Mammaglobin vs GCDFP-15: An Immunohistologic Validation Survey for Sensitivity and Specificity. Am J Clin Pathol. 2007;127:1–11. doi: 10.1309/TDP92PQLDE2HLEET. [DOI] [PubMed] [Google Scholar]
- 4.Watson MA, Fleming TP. Mammaglobin, a mammary-specific member of the uteroglobin gene family, is overexpressed in human breast cancer. Cancer Res. 1996;56:860–865. [PubMed] [Google Scholar]
- 5.Lehrer RI, Xu G, Abduragimov A, Dinh NN, Qu XD, Martin D, Glasgow BJ. Lipophilin, a novel heterodimeric protein of human tears. FEBS Lett. 1998;432:163–167. doi: 10.1016/s0014-5793(98)00852-7. [DOI] [PubMed] [Google Scholar]
- 6.Watson MA, Dintzis S, Darrow CM, Voss LE, DiPersio J, Jensen R, Fleming TP. Mammaglobin expression in primary, metastatic, and occult breast cancer. Cancer Res. 1999;59:3028–3031. [PubMed] [Google Scholar]
- 7.Carter D, Douglass JF, Cornellison CD, Retter MW, Johnson JC, Bennington AA, Fleming TP, Reed SG, Houghton RL, Diamond DL, Vedvick TS. Purification and characterization of the mammaglobin/lipophilin B complex, a promising diagnostic marker for breast cancer. Biochemistry. 2002;41:6714–6722. doi: 10.1021/bi0159884. [DOI] [PubMed] [Google Scholar]
- 8.O’Brien N, Maguire TM, O’Donovan N, Lynch N, Hill AD, McDermott E, O'Higgins N, Duffy MJ. Mammaglobin a: A promising marker for breast cancer. Clin Chem. 2002;48:1362–1364. [PubMed] [Google Scholar]
- 9.Zehentner BK, Dillon DC, Jiang Y, Xu J, Bennington A, Molesh DA, Zhang X, Reed SG, Persing D, Houghton RL. Application of a multigene reverse transcription-PCR assay for detection of mammaglobin and complementary transcribed genes in breast cancer lymph nodes. Clin Chem. 2002;48:1225–1231. [PMC free article] [PubMed] [Google Scholar]
- 10.Sjödin A, Guo D, Hofer PA, Henriksson R, Hedman H. Mammaglobin in Normal Human Sweat Glands and Human Sweat Gland Tumors. J Invest Dermatol. 2003;121:428–429. doi: 10.1046/j.1523-1747.2003.12374.x. [DOI] [PubMed] [Google Scholar]
- 11.Fanger GR, Houghton RL, Retter MW, Hendrickson RC, Babcook J, Dillon DC, Durham MD, Reynolds LD, Johnson JC, Carter D, Fleming TP, Roche PC, Persing DH, Reed SG. Detection of mammaglobin in the sera of patients with breast cancer. Tumour Biol. 2002;23:212–221. doi: 10.1159/000067251. [DOI] [PubMed] [Google Scholar]
- 12.Bernstein JL, Godbold JH, Raptis G, Watson MA, Levinson B, Aaronson SA, Fleming TP. Identification of mammaglobin as a novel serum marker for breast cancer. Clin Cancer Res. 2005;11:6528–6535. doi: 10.1158/1078-0432.CCR-05-0415. [DOI] [PubMed] [Google Scholar]
- 13.Han JH, Kang Y, Shin HC, Kim HS, Kang YM, Kim YB, Oh SY. Mammaglobin expression in lymph nodes is an important marker of metastatic breast carcinoma. Arch Pathol Lab Med. 2003;127:1330–1334. doi: 10.5858/2003-127-1330-MEILNI. [DOI] [PubMed] [Google Scholar]
- 14.Wan WH, Fortuna MB, Furmanski P. A rapid and efficient method for testing immunohistochemical reactivity of monoclonal antibodies against multiple tissue samples simultaneously. J Immunol Methods. 1987;103:121–129. doi: 10.1016/0022-1759(87)90249-3. [DOI] [PubMed] [Google Scholar]