Abstract
Alzheimer disease (AD) is characterized by numerous senile plaques (SP) in addition to widespread neocortical neurofibrillary tangles (NFT). Some elderly have pathologic aging (PA), which is characterized by numerous SP composed of diffuse amyloid deposits with few or no NFT confined to the limbic lobe. Both AD and PA represent a range of Alzheimer type pathology (ATP). Some cases of progressive supranuclear palsy (PSP) have concurrent ATP, but the relationship between ATP and PSP has not been addressed. In this study, a consecutive series of PSP cases were divided into three groups according to the degree of concurrent ATP – pure PSP, PSP/PA and PSP/AD. Braak NFT stage was significantly greater in PSP/AD compared with both PSP/PA and PSP. Among the pathologic variables studied in middle frontal, superior temporal and motor cortices, there were no differences between PSP and PSP/PA except for SP. In PSP/AD, there was greater neuronal tau pathology (pretangles, NFT and neuropil threads) in middle frontal and superior temporal cortices, probably a reflection of ATP since there was no comparable increase in PSP-related glial tau pathology in these regions. The APOEɛ4 allele frequency was significantly higher in PSP/PA and PSP/AD than in PSP. These results strongly argue that ATP in PSP represents independent disease processes even when present in the same brain.
Keywords: Alzheimer's disease, progressive supranuclear palsy, senile plaque, tau, apolipoprotein E
Introduction
Alzheimer disease (AD) is the most common age-related neurodegenerative disease and the most common cause of dementia among the elderly. AD pathology is characterized by senile plaques (SP) in addition to widespread neocortical neurofibrillary tangles (NFT) and neuropil threads composed of tau protein [1]. The clinical symptoms of AD include memory loss and eventually global cognitive dysfunction. Advanced age, female sex and apolipoprotein E (APOE) ɛ4 are risk factors for sporadic AD [2]. Some neurologically normal individuals have many SP, but few or no NFT, a process referred to as pathological aging (PA) [3]. AD and PA represent a range of Alzheimer's type pathology (ATP), with the major difference being the distribution of NFT.
ATP is detected in some cases of progressive supranuclear palsy (PSP), an uncommon parkinsonian disorder associated with tau pathology in both neurons and glia. The relationship of ATP to tau pathology in PSP has not been addressed. To explore this question, we studied cases of pure PSP, as well as cases of PSP with PA (PSP/PA) and AD (PSP/AD).
Material and Methods
Selection of Cases
All cases met NINDS neuropathological criteria for PSP [4, 5]. AD was diagnosed if there were numerous SP (sufficient to meet Khachaturian criteria for AD) [6], including at least some neuritic plaques in multiple cortical sections and a Braak stage of IV and greater [1]. These criteria are in accord with National Institute on Aging and the Reagan Institute Working Group criteria for “high likelihood” of AD [7]. PA is a term we use to describe cases with numerous cortical SP, composed mostly of diffuse, non-neuritic amyloid deposits, and with a Braak NFT stage of III or less [3]. Cases with few or no SP and Braak stage III or less were considered to have “pure” PSP.
PSP cases were obtained from the Society for PSP Brain Bank at Mayo Clinic in Jacksonville, Florida. At the time of this study, the PSP Brain Bank had 586 cases of PSP (306 men and 280 women; average age at death: 74.9 ± 8.0 years). Of these 586 cases, 137 cases (23%) had ATP consistent with either PA (n=79) or AD (n=58). Other common pathologic processes are argyrophilic grain disease (AGD; n=154, 26%), vascular disease (VaD; n=77, 13%) and Lewy bodies (LBs; n=45, 8%). Cases of PSP lacking PA, AD, AGD, VaD and LBs (i.e. “pure” PSP) account for less than half the cases (n=274, 47%). Comparing pure PSP to PSP/PA and PSP/AD revealed some expected demographic differences. Both PSP/AD and PSP/PA were older than pure PSP (78 ± 8 and 76 ± 7 vs 72 ± 8 years). In addition, the male-to-female ratio was different between PSP/AD, PSP/PA and pure PSP (31% men and 34% men vs. 58% men). Therefore, for the quantitative comparison of effects of ATP on tau-related pathology, we matched 20 cases each of PSP, PSP/PA and PSP/AD for age and sex (Table 1).
Table 1.
Demographics of PSP cases with respect to Alzheimer type pathology
| PSP | PSP/PA | PSP/AD | |
|---|---|---|---|
| Number | 20 | 20 | 20 |
| Age at death, y (mean ± sd) | 78.2 ± 6.0 | 76.6 ± 5.9 | 79.3 ± 6.3 |
| Sex (F:M) | 11:9 | 11:9 | 11:9 |
| Brain weight, g (mean ± sd) | 1109 ± 122 | 1113± 128 | 1131± 106 |
| Braak NFT stage (median, 25th, 75th %-tile) | II, I, III | II, II, III | V, IV, V# |
| Cortical SP score (median, 25th, 75th %-tile) | 0, 0, 1* | 3, 2, 3 | 3, 2, 3 |
| APOEɛ4 (percent; number studied) | 0% (17)* | 60% (15) | 53% (15) |
| MAPT H1H1 (percent, number studied) | 71% (14) | 71% (7) | 83% (6) |
Y = year; F = female; M=male; sd = standard deviation
P<0.05 compared to PSP/PA and PSP/AD
P<0.05 compared to PSP and PSP/PA
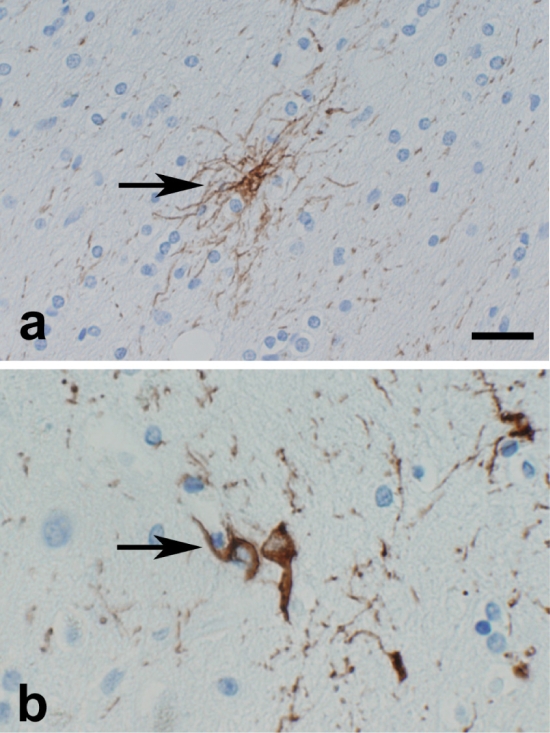
The types of tau pathology assessed in multiple brain regions included pretangles, tangles, neuropil threads, as well as glial pathology that is specific to PSP and not found in AD, namely, tufted astrocytes and coiled bodies. Immunohistochemistry with a monoclonal antibody to phospho-tau, CP13, is a sensitive marker for both neuronal and gliallesions [8]. Pretangles were defined as neuronal tau immunoreactivity with diffuse or granular, rather than fibrillar, cytoplasmic staining [9, 10]. Tufted astrocytes were defined as star-like tufts of tau-positive abnormal fibers (Figure 1a) [11-13]. Coiled bodies were defined as tau-positive cells in white matter, and have been shown to be within oligodendroglia (Figure 1b) [14, 15]. The density of pretangles, NFT, tufted astrocytes, neuropil threads and coiled bodies were scored in middle frontal, superior temporal and motor cortices with tau immunohistochemistry. The density of SP was scored by thioflavin-S fluorescent microscopy.
Figure 1.
Typical appearance of tufted astrocyte (a, arrow) and coiled bodies (b, arrow) by tau immunohistochemistry. Bar=25µm (a), 37.5µm (b)
The density of pretangles or NFT, tufted astrocytes and coiled bodies was semiquantitatively graded as 0 (absent or rare), 1 (low density: 1-3 per × 200 field), 2 (high density: 4-6 per × 200 field) or 3 (very high density: 7 or more per × 200 field). The density of neuropil threads was graded as 0 (absent or rare), 1 (low density), 2 (high density) or 3 (very high density). The density of SP was graded as 0 (absent or rare), 1 (low density: 1-10 per × 100 field), 2 (high density: 10-30 per × 100 field) or 3 (very high density: 30 or more per × 100 field).
Immunohistochemistry
Blocks of tissue from formalin-fixed brain were embedded in paraffin and sections cut at 5 µm thickness. Deparaffinized and rehydrated sections were steamed for 30 min for antigen retrieval, followed by blocking of endogenous peroxidase with 3% H2O2. Sections were treated with 5% normal goat serum for 20 min and then incubated with the phospho-tau monoclonal antibody, CP13, at room temperature for 45 min. After incubation with the primary antibody, the sections were treated with biotinylated primary antibody anti-mouse IgG secondary antibody Envision-Plus, Peroxidase, Mouse (DAKO, Santa Barbara, CA) for 30 min. Peroxidase labeling was visualized with 3, 3′-diaminobenzidine.
APOE and MAPT Genotype Analysis
Genomic DNA was extracted from frozen brains using the QIAamp DNA mini kit (Qiagen, Bothell, WA). Amplification was carried out using the Single-day APOE genotyping method [16] or MAPT [17] with minor modifications [18].
Statistical Analysis
The differences among three groups were examined with Kruskal-Wallis One Way Analysis of Variance on Ranks. If there was a significant difference, Dunn's Method was performed for pair-wise comparisons. The difference in the frequencies of APOE alleles between diagnostic categories were tested using Chi-Square test. Statistical analyses were performed with SigmaStat 3 (Systat Software, Inc., Point Richmond, CA) and a probability value of p<0.05 was regarded as significant for all analyses.
Results
We screened 586 cases of pathologically confirmed PSP for this study. Cases included in the semiquantitative analysis of the effects of ATP on tau pathology had to be free of AGD, VaD and LBs. 20 cases of PSP/AD were matched to 20 cases of pure PSP and 20 cases of PSP/PA (Table 1).
As expected tufted astrocytes, coiled bodies, pretangles, NFT and neuropil threads were detected in all cases. Also as expected based upon selection criteria, the Braak NFT stage was significantly greater in PSP/AD compared to both PSP/PA and PSP and the densities of SP were significantly greater in PSP/PA and PSP/AD than in PSP (Table 1). PSP did not differ from PSP/PA with respect to Braak NFT stage and PSP/PA did not differ from PSP/AD with respect to SP density.
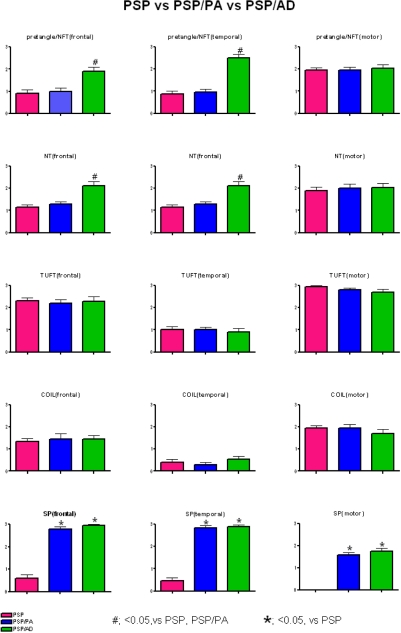
Among the tau pathologic variables studied (Figure 2), there were no differences between PSP and PSP/PA, but there were differences for PSP/AD. In particular, PSP/AD had greater neuronal tau pathology (pretangles and NFT) as well as neuropil threads in middle frontal and superior temporal cortices. A similar tendency was noted in motor cortex, but it did not reach statistical significance. The explanation for increased cortical neuronal tau pathology likely reflects the effects of concurrent AD.
Figure 2.
The density of pretangles and NFT, neuropil threads (NT), tufted astrocytes (TUFT), coiled bodies (COIL), and senile plaques (SP) in each region.
Discussion
Given the age at death of most patients with PSP (>75 years of age in the PSP Brain Bank series), it is not surprising that mixed pathology is common. In the PSP Brain Bank, fewer than 50% of PSP cases were “pure” when one excludes cases with concomitant ATP, AGD, VaD or LBs. Previous studies have documented concurrent pathologic processes in PSP, including AD [19], LBs [19-22], AGD [23] and VaD [24]. Rarely, mixed cases in PSP have features of Pick disease [25] or multiple system atrophy (MSA) [26, 27]. The coexistence of PSP and AD is uncommon (10% of the cases in the PSP Brain Bank), but it is not rare. The clinical significance of concomitant pathology in PSP has not been systematically assessed, but may contribute to diagnostic difficulties [28]. For example, focal neurologic deficits due to VaD may lead to erroneous diagnosis of corticobasal degeneration (CBD).
PSP is a sporadic neurodegenerative disease characterized by neuronal loss, globose NFT, and glial pathology in cortex, basal ganglia and brainstem [5]. Clinically, it presents as an atypical parkinsonism with frequent early falls, failure to respond to dopaminergic therapy and progressive impairment of eye motility, especially vertical eye movements [29]. The major risk factor is a haplotype of the tau gene (MAPT) [17], which is of interest since the neuronal and glial lesions that are histopathologic hallmarks of PSP are composed of tau protein.
In human brain, six tau isoforms are produced by alternative splicing of mRNA [30, 31]. Three isoforms contain three microtubule-binding repeats in the C-terminal region (3-repeat tau: 3R tau). The other three isoforms contain four microtubule-binding repeats by the addition of exon 10 derived 31 amino acid sequences (4 repeat tau: 4R tau). Biochemical analyses have revealed that insoluble tau in PSP, CBD and AGD are composed predominantly of 4R tau [18, 32, 33], while it is a mixture of both 3R and 4R tau in AD [34]. Because of selective incorporation of 4R tau in the lesions in PSP, CBD and AGD, they are sometimes referred to as “4R tauopathies.” Although AGD is common in both PSP (20% of cases) or CBD (40% of cases) [18], only a few cases have been reported that share pathological features of both PSP and CBD [35, 36]. Komori and co-workers suggested that tufted astrocytes and astrocytic plaques, the histopathologic lesion of CBD [14], are highly characteristic for each disease, so they are mutually exclusive [37]. The result of the present study suggests that the presence of 3R tau due to concomitant AD does not influence the 4R tauopathy of PSP.
In addition to NFT, it is recognized that non-filamentous precursors to NFT, so-called pretangles, occur not only in aging and AD [9, 10], but also in PSP and other 4R tauopathies, where they may be more common than NFT. Tau-immunoreactive astrocytes in PSP are referred to as tufted astrocytes, while lesions in oligodendrocytes are referred to as coiled bodies. Tufted astrocytes are frequent in motor cortex and striatum in PSP, but also detected in multimodal association cortices of the frontal, temporal and parietal lobes, usually at a much lower density than in the primary motor cortex. Among subcortical structures that are vulnerable to tufted astrocytes is the midbrain tectum. They have diagnostic significance for PSP, particularly as mentioned in the differential diagnosis of CBD [37]. Coiled bodies are most numerous in white matter and are also found in other tauopathies, such as AGD [18].
Glial tau pathology is minimal in AD, although a subset of elderly individuals have tau immunoreactive astrocytes in the basal forebrain and medial temporal lobe affecting fibrous astrocytes in subpial and perivascular locations [38]. These so-called “thorn-shaped” astrocytes [39], lack diagnostic significance and can be detected in clinically normal individuals, as well as AD.
Tau pathology in the limbic lobe is minimal in PSP unless there is concurrent ATP, AGD or thorn-shaped astrocytes. Since ATP was the frame of reference of this study, we had to choose brain regions where tau pathology is less consistent and severe in PA and AD [1, 3], therefore, we studied the impact of ATP on PSP-related tau pathology (specifically tufted astrocytes and coiled bodies) in three cortical regions - middle frontal gyrus, superior temporal gyrus and primary motor cortex. The latter is minimally affected in AD, but consistently affected in PSP. If ATP pathology facilitated PSP-related tauopathy, we predicted that glial pathology would be greater in cortical areas vulnerable to ATP. We did not find this to be the case.
In humans, APOE is a single gene located on chromosome 19q13.2 with three major allelic variants (ɛ2, ɛ3 and ɛ4) encoding three protein isoforms [40]. The ɛ4 allele, which is the major risk factor for AD, affects the rate of Aβ aggregation with less consistent influence on neurofibrillary degeneration, when compared to individual who inherit the more common ɛ3 allele [2]. The ɛ2 allele is associated with reduced risk of AD and thus may constitute a protective factor [41]. There is little evidence to suggest that APOE is a risk factor for or that it influences the pathology in PSP [42-44]. On the other hand, there is evidence to suggest that APOEɛ4 carrier status predisposes to ATP in PSP [45], along with other well recognized risk factors for ATP, namely, advanced age and female sex. These observations were confirmed in the present study. In addition, the present study showed that ɛ2 allele was not significantly associated with reduced risk of ATP in PSP.
In summary, the major finding of this study is that presence of Aβ deposits and 3R tau due to ATP does not influence PSP-related 4R tauopathy, specifically glial tau lesions in cortical areas, adding further support to the notion that PSP and ATP are independent disease processes, even when they occur in the same brain.
Acknowledgments
The authors thank Virginia Philips, Linda Rousseau and Monica Castanedes-Casey for their expert technical assistance. We appreciate the gift of CP13 from Peter Davies, Albert Einstein College of Medicine. The cases used in this study were donated to the Society of Progressive Supranuclear Palsy brain bank and generous donations of family members in this endeavor are greatly appreciated. This study was supported by NIH grants P50-NS40256, P50-AG25711, P50-AG16574 and P01-AG17216, as well as the Mayo foundation.
References
- 1.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 2.Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dickson DW, Crystal HA, Mattiace LA, Masur DM, Blau AD, Davies P, Yen SH, Aronson MK. Identification of normal and pathological aging in prospectively studied nondemented elderly humans. Neurobiol Aging. 1992;13:179–189. doi: 10.1016/0197-4580(92)90027-u. [DOI] [PubMed] [Google Scholar]
- 4.Litvan I, Hauw JJ, Bartko JJ, Lantos PL, Daniel SE, Horoupian DS, McKee A, Dickson D, Bancher C, Tabaton M, Jellinger K, Anderson DW. Validity and reliability of the preliminary NINDS neuropathologic criteria for progressive supranuclear palsy and related disorders. J Neuropathol Exp Neurol. 1996;55:97–105. doi: 10.1097/00005072-199601000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Hauw JJ, Daniel SE, Dickson D, Horoupian DS, Jellinger K, Lantos PL, McKee A, Tabaton M, Litvan I. Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy) Neurology. 1994;44:2015–2019. doi: 10.1212/wnl.44.11.2015. [DOI] [PubMed] [Google Scholar]
- 6.Khachaturian ZS. Diagnosis of Alzheimer's disease. Arch Neurol. 1985;42:1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- 7.Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:1095–1097. doi: 10.1097/00005072-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Ishizawa T, Mattila P, Davies P, Wang D, Dickson DW. Colocalization of tau and alpha-synuclein epitopes in Lewy bodies. J Neuropathol Exp Neurol. 2003;62:389–397. doi: 10.1093/jnen/62.4.389. [DOI] [PubMed] [Google Scholar]
- 9.Bancher C, Brunner C, Lassmann H, Budka H, Jellinger K, Wiche G, Seitelberger F, Grundke-Iqbal I, Iqbal K, Wisniewski HM. Accumulation of abnormally phosphorylated tau precedes the formation of neurofibrillary tangles in Alzheimer's disease. Brain Res. 1989;477:90–99. doi: 10.1016/0006-8993(89)91396-6. [DOI] [PubMed] [Google Scholar]
- 10.Braak E, Braak H, Mandelkow EM. A sequence of cytoskeleton changes related to the formation of neurofibrillary tangles and neuropil threads. Acta Neuropathol (Berl) 1994;87:554–567. doi: 10.1007/BF00293315. [DOI] [PubMed] [Google Scholar]
- 11.Hauw JJ, Verny M, Delaere P, Cervera P, He Y, Duyckaerts C. Constant neurofibrillary changes in the neocortex in progressive supranuclear palsy. Basic differences with Alzheimer's disease and aging. Neurosci Lett. 1990;119:182–186. doi: 10.1016/0304-3940(90)90829-x. [DOI] [PubMed] [Google Scholar]
- 12.Yamada T, McGeer PL, McGeer EG. Appearance of paired nucleated, Tau-positive glia in patients with progressive supranuclear palsy brain tissue. Neurosci Lett. 1992;135:99–102. doi: 10.1016/0304-3940(92)90145-w. [DOI] [PubMed] [Google Scholar]
- 13.Yamada T, Calne DB, Akiyama H, McGeer EG, McGeer PL. Further observations on Tau-positive glia in the brains with progressive supranuclear palsy. Acta Neuropathol (Berl) 1993;85:308–315. doi: 10.1007/BF00227727. [DOI] [PubMed] [Google Scholar]
- 14.Feany MB, Dickson DW. Widespread cytoskeletal pathology characterizes corticobasal degeneration. Am J Pathol. 1995;146:1388–1396. [PMC free article] [PubMed] [Google Scholar]
- 15.Iwatsubo T, Hasegawa M, Ihara Y. Neuronal and glial tau-positive inclusions in diverse neurologic diseases share common phosphorylation characteristics. Acta Neuropathol (Berl) 1994;88:129–136. doi: 10.1007/BF00294505. [DOI] [PubMed] [Google Scholar]
- 16.Crook R, Hardy J, Duff K. Single-day apolipoprotein E genotyping. J Neurosci Methods. 1994;53:125–127. doi: 10.1016/0165-0270(94)90168-6. [DOI] [PubMed] [Google Scholar]
- 17.Baker M, Litvan I, Houlden H, Adamson J, Dickson D, Perez-Tur J, Hardy J, Lynch T, Bigio E, Hutton M. Association of an extended haplotype in the tau gene with progressive supranuclear palsy. Hum Mol Genet. 1999;8:711–715. doi: 10.1093/hmg/8.4.711. [DOI] [PubMed] [Google Scholar]
- 18.Togo T, Cookson N, Dickson DW. Argyrophilic grain disease: neuropathology, frequency in a dementia brain bank and lack of relationship with apolipoprotein E. Brain Pathol. 2002;12:45–52. doi: 10.1111/j.1750-3639.2002.tb00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gearing M, Olson DA, Watts RL, Mirra SS. Progressive supranuclear palsy: neuropathologic and clinical heterogeneity. Neurology. 1994;44:1015–1024. doi: 10.1212/wnl.44.6.1015. [DOI] [PubMed] [Google Scholar]
- 20.Uchikado H, DelleDonne A, Ahmed Z, Dickson DW. Lewy bodies in progressive supranuclear palsy represent an independent disease process. J Neuropathol Exp Neurol. 2006;65:387–395. doi: 10.1097/01.jnen.0000218449.17073.43. [DOI] [PubMed] [Google Scholar]
- 21.Tsuboi Y, Wszolek ZK, Graff-Radford NR, Cookson N, Dickson DW. Tau pathology in the olfactory bulb correlates with Braak stage, Lewy body pathology and apolipoprotein epsilon4. Neuropathol Appl Neurobiol. 2003;29:503–510. doi: 10.1046/j.1365-2990.2003.00453.x. [DOI] [PubMed] [Google Scholar]
- 22.Mori H, Oda M, Komori T, Arai N, Takanashi M, Mizutani T, Hirai S, Mizuno Y. Lewy bodies in progressive supranuclear palsy. Acta Neuropathol (Berl) 2002;104:273–278. doi: 10.1007/s00401-002-0555-3. [DOI] [PubMed] [Google Scholar]
- 23.Togo T, Dickson DW. Ballooned neurons in progressive supranuclear palsy are usually due to concurrent argyrophilic grain disease. Acta Neuropathol (Berl) 2002;104:53–56. doi: 10.1007/s00401-002-0520-1. [DOI] [PubMed] [Google Scholar]
- 24.Togo T, Dickson DW. Tau accumulation in astrocytes in progressive supranuclear palsy is a degenerative rather than a reactive process. Acta Neuropathol (Berl) 2002;104:398–402. doi: 10.1007/s00401-002-0569-x. [DOI] [PubMed] [Google Scholar]
- 25.Arima K, Murayama S, Oyanagi S, Akashi T, Inose T. Presenile dementia with progressive supranuclear palsy tangles and Pick bodies: an unusual degenerative disorder involving the cerebral cortex, cerebral nuclei, and brain stem nuclei. Acta Neuropathol (Berl) 1992;84:128–134. doi: 10.1007/BF00311384. [DOI] [PubMed] [Google Scholar]
- 26.Takanashi M, Ohta S, Matsuoka S, Mori H, Mizuno Y. Mixed multiple system atrophy and progressive supranuclear palsy: a clinical and pathological report of one case. Acta Neuropathol (Berl) 2002;103:82–87. doi: 10.1007/s004010100433. [DOI] [PubMed] [Google Scholar]
- 27.Uchikado H, Delledonne A, Uitti R, Dickson DW. Coexistence of PSP and MSA: a case report and review of the literature. Acta Neuropathol (Berl) 2006;111:186–192. doi: 10.1007/s00401-005-0022-z. [DOI] [PubMed] [Google Scholar]
- 28.Josephs KA, Dickson DW. Diagnostic accuracy of progressive supranuclear palsy in the Society for Progressive Supranuclear Palsy brain bank. Mov Disord. 2003;18:1018–1026. doi: 10.1002/mds.10488. [DOI] [PubMed] [Google Scholar]
- 29.Dickson DW, Rademakers R, Hutton ML. Progressive supranuclear palsy: pathology and genetics. Brain Pathol. 2007;17:74–82. doi: 10.1111/j.1750-3639.2007.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron. 1989;3:519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- 31.Andreadis A, Brown WM, Kosik KS. Structure and novel exons of the human tau gene. Biochemistry. 1992;31:10626–10633. doi: 10.1021/bi00158a027. [DOI] [PubMed] [Google Scholar]
- 32.Sergeant N, Wattez A, Delacourte A. Neurofibrillary degeneration in progressive supranuclear palsy and corticobasal degeneration: tau pathologies with exclusively “exon 10” isoforms. J Neurochem. 1999;72:1243–1249. doi: 10.1046/j.1471-4159.1999.0721243.x. [DOI] [PubMed] [Google Scholar]
- 33.Arai T, Ikeda K, Akiyama H, Shikamoto Y, Tsuchiya K, Yagishita S, Beach T, Rogers J, Schwab C, McGeer PL. Distinct isoforms of tau aggregated in neurons and glial cells in brains of patients with Pick's disease, corticobasal degeneration and progressive supranuclear palsy. Acta Neuropathol (Berl) 2001;101:167–173. doi: 10.1007/s004010000283. [DOI] [PubMed] [Google Scholar]
- 34.Sergeant N, David JP, Goedert M, Jakes R, Vermersch P, Buee L, Lefranc D, Wattez A, Delacourte A. Two-dimensional characterization of paired helical filament-tau from Alzheimer's disease: demonstration of an additional 74-kDa component and age-related biochemical modifications. J Neurochem. 1997;69:834–844. doi: 10.1046/j.1471-4159.1997.69020834.x. [DOI] [PubMed] [Google Scholar]
- 35.Katsuse O, Iseki E, Arai T, Akiyama H, Togo T, Uchikado H, Kato M, de Silva R, Lees A, Kosaka K. 4-repeat tauopathy sharing pathological and biochemical features of corticobasal degeneration and progressive supranuclear palsy. Acta Neuropathol (Berl) 2003;106:251–260. doi: 10.1007/s00401-003-0728-8. [DOI] [PubMed] [Google Scholar]
- 36.Tan CF, Piao YS, Kakita A, Yamada M, Takano H, Tanaka M, Mano A, Makino K, Nishizawa M, Wakabayashi K, Takahashi H. Frontotemporal dementia with co-occurrence of astrocytic plaques and tufted astrocytes, and severe degeneration of the cerebral white matter: a variant of corticobasal degeneration? Acta Neuropathol (Berl) 2005;109:329–338. doi: 10.1007/s00401-004-0933-0. [DOI] [PubMed] [Google Scholar]
- 37.Komori T, Arai N, Oda M, Nakayama H, Mori H, Yagishita S, Takahashi T, Amano N, Murayama S, Murakami S, Shibata N, Kobayashi M, Sasaki S, Iwata M. Astrocytic plaques and tufts of abnormal fibers do not coexist in corticobasal degeneration and progressive supranuclear palsy. Acta Neuropathol (Berl) 1998;96:401–408. doi: 10.1007/s004010050911. [DOI] [PubMed] [Google Scholar]
- 38.Schultz C, Ghebremedhin E, Del Tredici K, Rub U, Braak H. High prevalence of thorn-shaped astrocytes in the aged human medial temporal lobe. Neurobiol Aging. 2004;25:397–405. doi: 10.1016/S0197-4580(03)00113-1. [DOI] [PubMed] [Google Scholar]
- 39.Ikeda K, Akiyama H, Kondo H, Haga C, Tanno E, Tokuda T, Ikeda S. Thorn-shaped astrocytes: possibly secondarily induced tau-positive glial fibrillary tangles. Acta Neuropathol. 1995;90:620–625. doi: 10.1007/BF00318575. [DOI] [PubMed] [Google Scholar]
- 40.Weisgraber KH, Mahley RW. Human apolipoprotein E: the Alzheimer's disease connection. Faseb J. 1996;10:1485–1494. doi: 10.1096/fasebj.10.13.8940294. [DOI] [PubMed] [Google Scholar]
- 41.Corder EH, Saunders AM, Risch NJ, Strittmatter WJ, Schmechel DE, Gaskell PC, Jr, Rimmler JB, Locke PA, Conneally PM, Schmader KE, et al. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet. 1994;7:180–184. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- 42.Morris HR, Schrag A, Nath U, Burn D, Quinn NP, Daniel S, Wood NW, Lees AJ. Effect of ApoE and tau on age of onset of progressive supranuclear palsy and multiple system atrophy. Neurosci Lett. 2001;312:118–120. doi: 10.1016/s0304-3940(01)02190-5. [DOI] [PubMed] [Google Scholar]
- 43.Anouti A, Schmidt K, Lyons KE, Hubble JP, Schellenberg G, Golbe LI, Lang AE, Galvez-Jimenez N, Hershey L, Koller WC. Normal distribution of apolipoprotein E alleles in progressive supranuclear palsy. Neurology. 1996;46:1156–1157. doi: 10.1212/wnl.46.4.1156. [DOI] [PubMed] [Google Scholar]
- 44.Tabaton M, Rolleri M, Masturzo P, Cammarata S, Angelini G, Hansen LA, Saitoh T, Petersen RB, Perry G, Richey P, et al. Apolipoprotein E epsilon 4 allele frequency is not increased in progressive supranuclear palsy. Neurology. 1995;45:1764–1765. doi: 10.1212/wnl.45.9.1764. [DOI] [PubMed] [Google Scholar]
- 45.Tsuboi Y, Josephs KA, Cookson N, Dickson DW. APOE E4 is a determinant for Alzheimer type pathology in progressive supranuclear palsy. Neurology. 2003;60:240–245. doi: 10.1212/01.wnl.0000044340.37138.a9. [DOI] [PubMed] [Google Scholar]