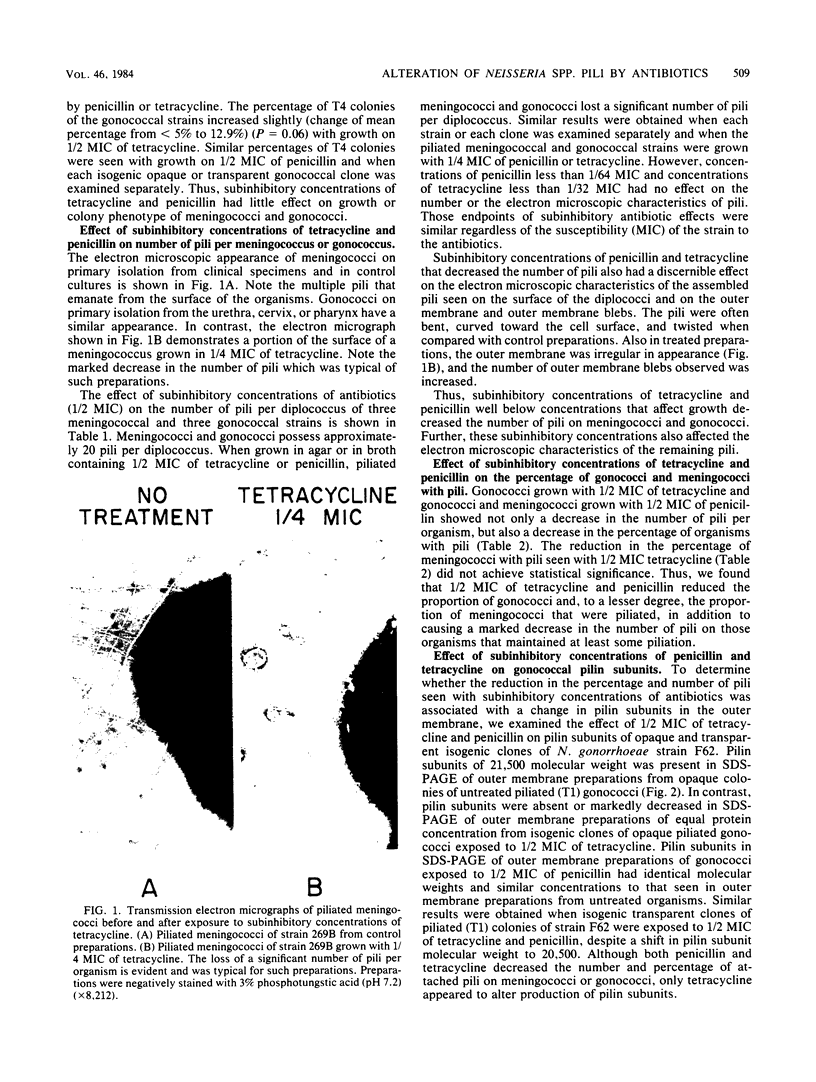
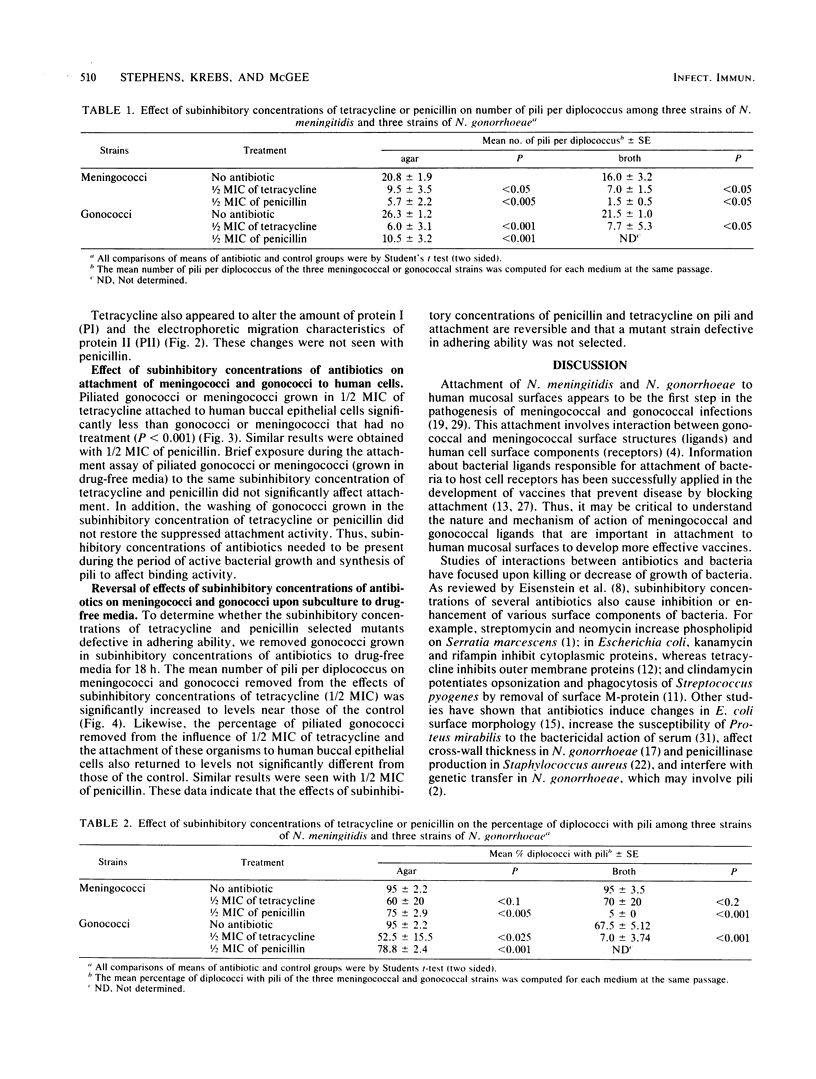
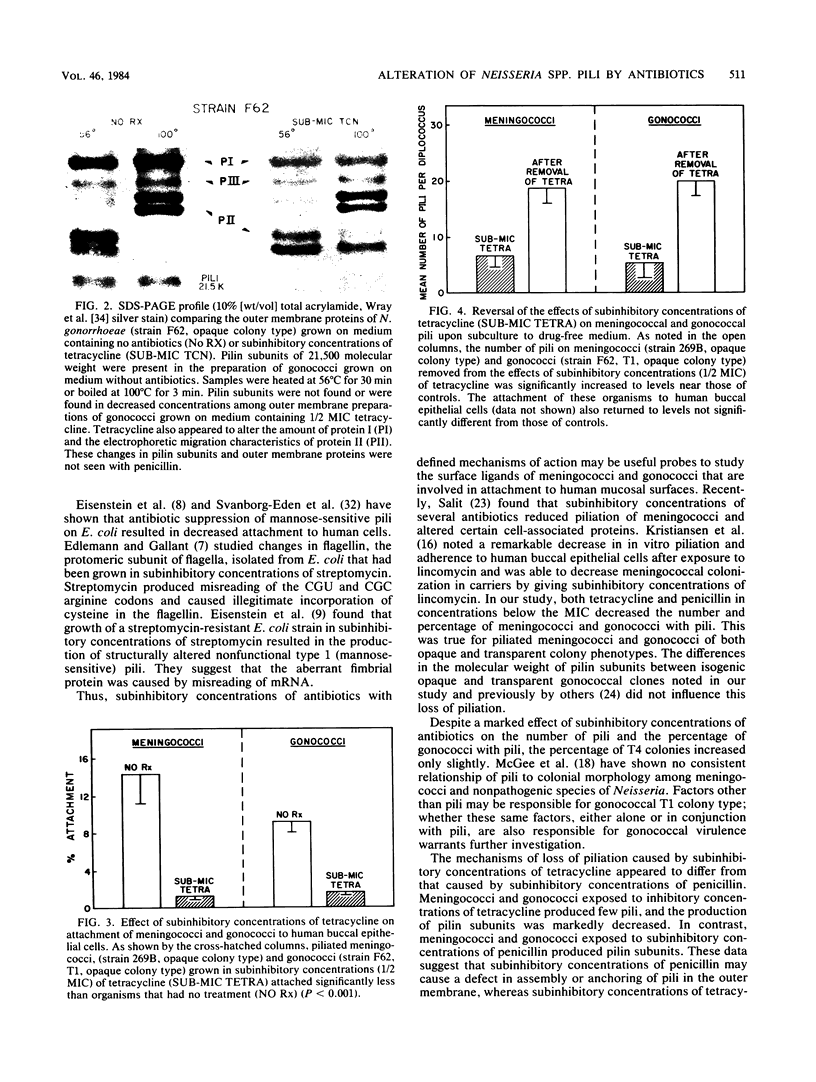
Abstract
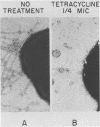
Recent evidence has suggested that surface structures of pathogenic bacteria, which are important in attachment to human mucosal surfaces, may be absent on bacteria grown in the presence of subinhibitory concentrations of antibiotics. We studied the effect of tetracycline and penicillin on meningococcal and gonococcal pili. Subinhibitory concentrations of tetracycline and penicillin were found to markedly reduce the number of pili per meningococcus or gonococcus and the percentage of meningococci or gonococci with pili, as determined by negative-staining electron microscopy. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of outer membrane preparations suggested that tetracycline decreased expression of pili by inhibiting synthesis of pilin subunits. In contrast, pilin subunit synthesis was unaltered by penicillin, suggesting a defect in assembly of pilin subunits or in anchoring of assembled pili. The decrease in the number of pili that occurred with subinhibitory concentrations of both tetracycline and penicillin was accompanied by a marked decrease in the ability of the organisms to attach to human cells. Gonococci or meningococci removed from the influence of subinhibitory concentrations of the antibiotics regained piliation, and attachment returned to levels near those of controls. The expression of meningococcal and gonococcal pili may be affected by factors that influence synthesis of pilin subunits or factors that interfere with the assembly and anchoring of pili in the outer membrane.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bermingham M. A., Deol B. S., Still J. L. Effect of streptomycin on lipid composition with particular reference to cyclic depsipeptide biosynthesis in Serratia marcescens and other micro-organisms. Biochem J. 1970 Oct;119(5):861–869. doi: 10.1042/bj1190861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas G. D., Sox T., Blackman E., Sparling P. F. Factors affecting genetic transformation of Neisseria gonorrhoeae. J Bacteriol. 1977 Feb;129(2):983–992. doi: 10.1128/jb.129.2.983-992.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- DeVoe I. W., Gilchrist J. E. Pili on meningococci from primary cultures of nasopharyngeal carriers and cerebrospinal fluid of patients with acute disease. J Exp Med. 1975 Feb 1;141(2):297–305. doi: 10.1084/jem.141.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmann P., Gallant J. Mistranslation in E. coli. Cell. 1977 Jan;10(1):131–137. doi: 10.1016/0092-8674(77)90147-7. [DOI] [PubMed] [Google Scholar]
- Eisenstein B. I., Beachey E. H., Ofek I. Influence of sublethal concentrations of antibiotics on the expression of the mannose-specific ligand of Escherichia coli. Infect Immun. 1980 Apr;28(1):154–159. doi: 10.1128/iai.28.1.154-159.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein B. I., Ofek I., Beachey E. H. Loss of lectin-like activity in aberrant type 1 fimbriae of Escherichia coli. Infect Immun. 1981 Feb;31(2):792–797. doi: 10.1128/iai.31.2.792-797.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemmell C. G., Peterson P. K., Schmeling D., Kim Y., Mathews J., Wannamaker L., Quie P. G. Potentiation of opsonization and phagocytosis of Streptococcus pyogenes following growth in the presence of clindamycin. J Clin Invest. 1981 May;67(5):1249–1256. doi: 10.1172/JCI110152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirashima A., Childs G., Inouye M. Differential inhibitory effects of antibiotics on the biosynthesis of envelope proteins of Escherichia coli. J Mol Biol. 1973 Sep 15;79(2):373–389. doi: 10.1016/0022-2836(73)90012-0. [DOI] [PubMed] [Google Scholar]
- Jit Sud I., Feingold D. S. Detection of agents that alter the bacterial cell surface. Antimicrob Agents Chemother. 1975 Jul;8(1):34–37. doi: 10.1128/aac.8.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Rutter J. M. Contribution of the K88 antigen of Escherichia coli to enteropathogenicity; protection against disease by neutralizing the adhesive properties of K88 antigen. Am J Clin Nutr. 1974 Dec;27(12):1441–1449. doi: 10.1093/ajcn/27.12.1441. [DOI] [PubMed] [Google Scholar]
- Kellogg D. S., Jr, Cohen I. R., Norins L. C., Schroeter A. L., Reising G. Neisseria gonorrhoeae. II. Colonial variation and pathogenicity during 35 months in vitro. J Bacteriol. 1968 Sep;96(3):596–605. doi: 10.1128/jb.96.3.596-605.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klainer A. S., Russell R. R. Effect of the inhibition of protein synthesis on the Escherichia coli cell envelope. Antimicrob Agents Chemother. 1974 Aug;6(2):216–224. doi: 10.1128/aac.6.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen B. E., Rustad L., Spanne O., Bjorvatn B. Effect of subminimal inhibitory concentrations of antimicrobial agents on the piliation and adherence of Neisseria meningitidis. Antimicrob Agents Chemother. 1983 Nov;24(5):731–734. doi: 10.1128/aac.24.5.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee Z. A., Dourmashkin R. R., Gross J. G., Clark J. B., Taylor-Robinson D. Relationship of pili to colonial morphology among pathogenic and nonpathogenic species of Neisseria. Infect Immun. 1977 Feb;15(2):594–600. doi: 10.1128/iai.15.2.594-600.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee Z. A., Johnson A. P., Taylor-Robinson D. Pathogenic mechanisms of Neisseria gonorrhoeae: observations on damage to human fallopian tubes in organ culture by gonococci of colony type 1 or type 4. J Infect Dis. 1981 Mar;143(3):413–422. doi: 10.1093/infdis/143.3.413. [DOI] [PubMed] [Google Scholar]
- McGee Z. A., Street C. H., Chappell C. L., Cousar E. S., Morris F., Horn R. G. Pili of Neisseria meningitidis: effect of media on maintenance of piliation, characteristics of Pili, and colonial morphology. Infect Immun. 1979 Apr;24(1):194–201. doi: 10.1128/iai.24.1.194-201.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T. F., Mlawer N., So M. Pilus expression in Neisseria gonorrhoeae involves chromosomal rearrangement. Cell. 1982 Aug;30(1):45–52. doi: 10.1016/0092-8674(82)90010-1. [DOI] [PubMed] [Google Scholar]
- Michel J., Stessman P., Stessman J. Effects of subminimal inhibitory concentrations of Erythromycin, Clindamycin, and Pristinamycin on the penicillinase production of Staphlyococcus aureus. Antimicrob Agents Chemother. 1980 Jan;17(1):13–15. doi: 10.1128/aac.17.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salit I. E., Blake M., Gotschlich E. C. Intra-strain heterogeneity of gonococcal pili is related to opacity colony variance. J Exp Med. 1980 Mar 1;151(3):716–725. doi: 10.1084/jem.151.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salit I. E. Effect of subinhibitory concentrations of antimicrobials on meningococcal adherence. Can J Microbiol. 1983 Mar;29(3):369–376. doi: 10.1139/m83-061. [DOI] [PubMed] [Google Scholar]
- Salit I. E., Morton G. Adherence of Neisseria meningitidis to human epithelial cells. Infect Immun. 1981 Jan;31(1):430–435. doi: 10.1128/iai.31.1.430-435.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoolnik G. K., Tai J. Y., Gotschlich E. C. A pilus peptide vaccine for the prevention of gonorrhea. Prog Allergy. 1983;33:314–331. [PubMed] [Google Scholar]
- Silverblatt F. J., Cohen L. S. Antipili antibody affords protection against experimental ascending pyelonephritis. J Clin Invest. 1979 Jul;64(1):333–336. doi: 10.1172/JCI109458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens D. S., Hoffman L. H., McGee Z. A. Interaction of Neisseria meningitidis with human nasopharyngeal mucosa: attachment and entry into columnar epithelial cells. J Infect Dis. 1983 Sep;148(3):369–376. doi: 10.1093/infdis/148.3.369. [DOI] [PubMed] [Google Scholar]
- Stephens D. S., McGee Z. A. Association of virulence of Neisseria meningitidis with transparent colony type and low-molecular-weight outer membrane proteins. J Infect Dis. 1983 Feb;147(2):282–292. doi: 10.1093/infdis/147.2.282. [DOI] [PubMed] [Google Scholar]
- Stephens D. S., McGee Z. A. Attachment of Neisseria meningitidis to human mucosal surfaces: influence of pili and type of receptor cell. J Infect Dis. 1981 Apr;143(4):525–532. doi: 10.1093/infdis/143.4.525. [DOI] [PubMed] [Google Scholar]
- Swanson J., Mayer L. W., Tam M. R. Antigenicity of Neisseria gonorrhoeae outer membrane protein(s) III detected by immunoprecipitation and Western blot transfer with a monoclonal antibody. Infect Immun. 1982 Nov;38(2):668–672. doi: 10.1128/iai.38.2.668-672.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]