Abstract
Study design
A thematic review.
Objectives
To evaluate if physical activity enhances muscle strength, improves balance, and reduces the fall frequency and the fracture incidence.
Background
One of the major medical problems of today is the increasing incidence of fragility fractures. Muscle strength and fall is one of the major determinants of a fracture. If physical activity could increase muscle strength, improve balance and reduce the fall frequency, then training could be recommended as prevention for fractures.
Methods
The review used Medline (Pub Med) and the search words exercise, physical activity, muscle strength, balance, falls, fractures. Randomized controlled trials (RCT) were predominantly included, although this not is a systematic review.
Results
The evidence that physical activity modifies the risk factors for fall is compelling, although RCT with fractures as end point are lacking. Physical activity is associated with improved muscle strength, co-ordination and balance. Physical training increases muscle strength also in octogenarians by up to 200%, i.e. a much more pronounced effect than the corresponding increase in muscle volume or bone mass. There is also evidence that physical activity decreases the actual number of falls. Observational cohort and case-control studies imply that physical activity is associated with reduced hip fracture risk. If exercise reduces the number of vertebral fractures and other fragility fractures are less evaluated.
Conclusions
Physical activity in older ages can be recommended to improve muscle strength and balance, to reduce the risk to fall and fractures, although the highest level of evidence – RCT with fracture as endpoint – is lacking.
Keywords: exercise, muscle strength, muscle mass, fall, fractures, physical activity
The increasing number of fragility fractures, usually defined as fractures of distal radius, proximal humerus, spine, pelvis, hip or tibial condyles due to a low energy trauma, has created an appreciable problem in the western world (1–3). Half of the female and one-third of the male population will, during their lifetime, suffer at least one fragility fracture, followed by increased morbidity and mortality (4, 5). Fragility fractures are associated with very high cost if sick-leave, visits to doctors, examination and pain medication are included (6). With this knowledge, the health care service and other sectors of society should unify their work and efforts, to try to decrease the number of fractures. Efforts during the last decades, both clinically and within research, have mainly been focused on how to increase bone mass (BM) or bone mineral content (BMC) or bone mineral density (BMD) (7–9). These treatment strategies are logical, as BMD has been shown to be one of the best predictors of bone strength. An increase in BMD by 10% (one standard deviation, SD) has been estimated to reduce the fracture incidence by 50% (10, 11).
Several double-blinded, prospective, randomized, controlled trials (RCT) clearly indicate that drug treatment could increase BMD by up to 10% and halve the fracture risk in both men and women with osteoporosis (7, 9), defined as BMD below 2.5 SD, corresponding to around 25% lower BMD value than in young individuals within the same gender (12). However, even if the relative risk to sustain a fracture is lower among individuals with osteopenia, i.e. a BMD reduced by 1–2.5 SD compared to young individuals of the same sex (12), the majority of fractures will occur in this larger group of individuals. At present, no data exist which imply that drug treatment in osteopenic individuals would reduce the number of fragility fractures (13), and lifelong treatment of everybody from e.g. 50 years of age is not demonstrated to be cost-effective (13).
A fracture seldom occurs without a trauma, usually a fall. Thus, strategies to reduce the number of falls would probably also reduce the number of fractures (14). Traits that are of importance for fall risk include muscle strength, muscle function and balance (15). This review evaluates existing data in the literature, to verify or oppose the hypothesis that physical exercise affects non-skeletal risk factors for fractures such as muscle mass, muscle strength, balance and fall frequency, and ultimately if physical activity is associated with reduced fracture incidence.
Method
Medline (Pub Med) was searched using the search words exercise, physical activity, muscle strength, balance, falls and fractures. Papers were scrutinized from 1966 and onwards and only papers in English were included. Randomized controlled trials (RCT) were used if available. From the relevant papers a continued search was undertaken by the link ‘related manuscripts’. If no RCT were found, the next level of evidence in the evidence-based hierarchy was scrutinized, i.e. non-randomized, controlled studies, then retrospective and prospective observation cohort studies and finally case-control studies. If a variety of papers were found within the same level of evidence, the authors tried to include the first published paper and the paper with the largest included cohorts and the longest follow-up.
Results and discussion
Physical activity and muscle function
Physical activity improves balance, co-ordination, muscle strength and reaction time. All these traits increase the possibility to break a tendency to fall or to protect from the full impact of an actual fall by the arms (16–20). There are reports that muscle strength increases by up to 200% even in octogenarians, a much larger increase than the 2–20% increases in muscle volume or the 1–2% increases in BM with a similar training program (16–18, 20, 21). Lord et al. reported that women above age 60 who exercised physically by aerobics twice per week improved balance, co-ordination and muscle strength (22). The muscle strength in the quadriceps muscle improved by 29% and the sway of the body was reduced by 6%, while the BM was unchanged during the intervention year. Heislein et al. reported that one hour of physical training twice a week increased the muscle strength in the quadriceps muscle by 21% and the grip strength by 14% with not more than eight weeks of physical training (23). Similarly, low to moderate physical activity during 16 weeks of physical training was associated with a 30–100% increase in muscle strength in both men and women while the BM at best increased by 3% (24). Adversely, a reduced level of physical training was followed by a fast decline in the muscle strength. Kontulainen et al. reported that muscle mass, expressed as a difference between dominant and non-dominant arm in tennis players, was reduced from 6 to 3% during 1–2 years of reduced level of physical activity (25) and Fiatarone et al. reported the muscle strength to decrease by 32% after only four weeks of reduced physical training (17).
Physical activity and the fall risk
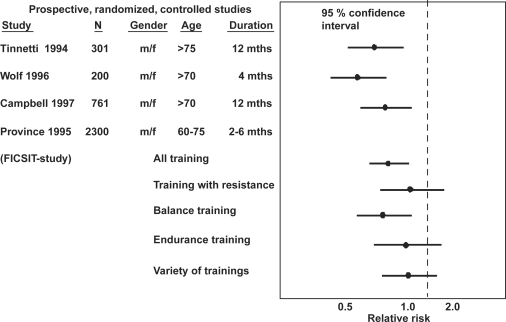
Several observational studies have reported that the risk to fall decreases with increased physical activity (15, 26). One of the first RCT that supported this notion was the ‘The Frail and Injuries: Cooperative Studies of Intervention Techniques (FICSIT)’ study (20) (Fig. 1). The FICSIT actually included several separate studies with different training modalities, but was pre-planned as a multi-center study. The study included 60–75-year old individuals subjected to an intervention program with 10–36 weeks training; endurance training, balance training and Tai Chi. The falls were reduced by 17% in the training group compared to the controls (20). The Tai Chi training showed the best results, with a 47% reduction in multiple falls during a four-month period (27, 28) (Fig. 1).
Fig. 1. .
The relative risk to sustain a fall. Published study, number of individuals (N), the gender (m = male, f = female), mean age of the cohort (years), duration of the prospective studies (months). The figure presents 95% confidence interval (95% CI) for odds ratio in active compared to inactive individuals.
One of the most cited prospective studies included a multiple-risk-factor intervention including a physical exercise program during one year, a strategy that actually reduced the fall frequency (29). A Swedish study also advocated a multiple-risk-factor intervention including a physical exercise program, a study that during the 34 weeks follow-up registered both a reduced fall frequency and a reduced hip fracture incidence (30). Another report infers that a home-based balance and strength training program reduced the fall frequency by 32% and reduced the number of fracture-induced falls by 38% (31). One of the more hopeful findings in this study was that the positive effect was retained one year after the physical training intervention (31). However, physical training was continued after the follow-up period in this study, probably influencing the inferences. There are, in contrast, other studies that oppose the notion of a fall-preventing effect, showing that any physical training-induced reduction in falls disappears within two years after cessation of the physical training (32). Furthermore, the prevention programs for fractures predominantly prevent falls in those with impairment or old age (20, 28, 29). Correspondingly, the effectiveness of physical exercise in fall prevention in middle-aged people is not good and therefore its benefit in fracture prevention may be as limited as that in bone preservation measures. There exists also a controversy if a physical exercise program in institutionalized, very frail individuals are of any benefit regarding fall reduction (33).
During the last years several RCT have been published that infer physical training to reduce the number of falls, sometimes with a significant outcome, sometimes only showing a trend (34–36). Gregg et al. reported in a meta-analysis including six studies that isolated physical training really reduced the number of individuals who fell (34). When thoroughly scrutinizing these intervention studies, it seems that fall reduction is most probable in individuals living by their own. In contrast, among elderly living in institutions, it seems questionable if an intervention program with physical activity can reduce the number of falls (30, 31, 35, 37). Maybe these individuals are too sick to be able to follow the physical training program or possibly, the physical training is too risky, leading to falls during the exercise sessions. In fact, there are studies supporting this hypothesis, reporting that individuals who sustain the most falls are those who are either most physically inactive or most physical active (36, 38, 39).
Physical activity and fracture risk in women
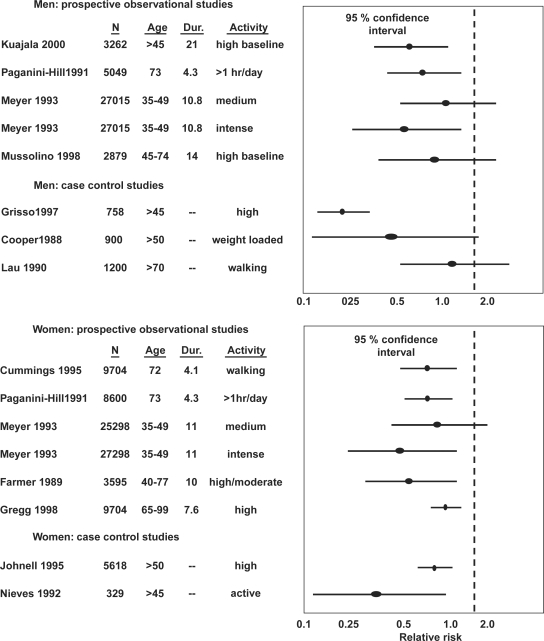
Several studies have reported that individuals with low physical activity, presently or previously, have an increased risk of sustaining a hip fracture (40) (Fig. 2). Other studies conclude that daily physical activity is associated with a reduced hip fracture risk (41). In one of the largest, prospective observational studies of osteoporotic fragility fractures, the Study of osteoporotic fractures (SOF) –study with a cohort of 9,704 women above age 65, the quintile with the highest physical activity had a 42% lower hip fracture risk compared to the women in the lowest quintile (40). Moderate physical activity was associated with a 30% reduction in hip fracture risk compared to physically inactive women in the Leisure World Study, a study including 8,600 middle aged and elderly women (42) (Fig. 2).
Fig. 2. .
Relative risk of sustaining a hip fracture with a higher level of exercise. Original study, numbers (N), age (years), duration of observation (years) and compared activity levels with 95% confidence interval (95% CI) for the odds ratio presented. A 95% CI below 1.0 indicate that exercise reduces the number of fractures.
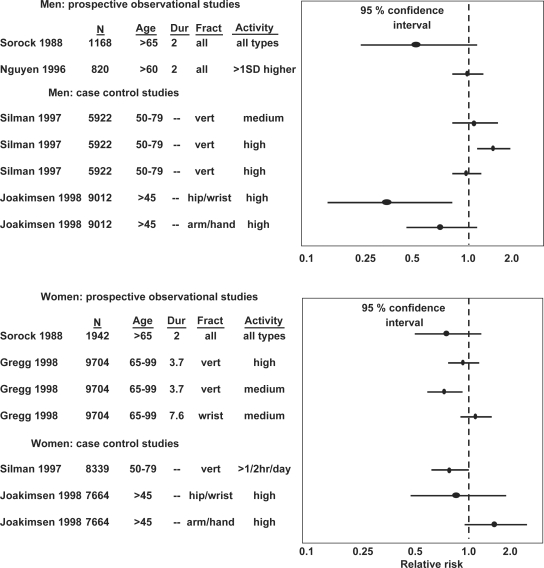
There are considerably less studies regarding physical activity and the risk to sustain a vertebral fracture. The previously mentioned SOF study shows that physical activity was associated with a 33% reduction in the number of vertebral fractures in active compared to inactive women (34, 40) (Fig. 3). This conclusion is supported by the European Vertebral Osteoporosis Study (EVOS), a study including 6,646 women, age 50–79 years. The study reported that physical activity 30 minutes or more every day was associated with a 30% reduction in the risk of sustaining a vertebral fracture, compared to inactive women (43) (Fig. 3).
Fig. 3. .
Relative risk of sustaining other fragility fractures with higher level of exercise. Original study, numbers (N), age (years), duration of observation (years) and compared activity levels with 95% confidence interval (95% CI) for the odds ratio presented. A 95% CI below 1.0 indicate that exercise reduce the number of fractures.
Physical activity and fracture risk in men
It is much more difficult to draw any conclusion as regards physical activity and the fracture incidence in men. Most studies in men are short-term studies with small cohorts, making a risk of type II error obvious (Fig. 2). One Finnish study including 3,262 Finnish men from age 50 onwards for two decades reported that a high physical activity at the time for the study start, reduced the risk to sustain a hip fracture by 38% (44). The Leisure World Study including 5,049 elderly men reported that increased training reduced the risk to sustain a hip fracture, as in women (42). There are now at least four large studies that follow well-defined groups, and all are in accordance when they report that regularly physical activity in men reduces the risk to sustain a hip fracture (42, 45, 46). Existing data thus support that physical activity reduces the risk of hip fractures also in men (Fig. 2).
The evidence regarding physical activity and vertebral fractures and other fragility fractures is even less clear in men than in women (Fig. 3). The previously described EVOS study including 5,922 men shows that physical activity did not reduce the number of vertebral fractures. Actually, men with the highest activity level had the highest prevalence of vertebral fractures (43) (Fig. 3). Other studies, on the other hand, show a trend of reduction in the number of vertebral fractures with increased physical activity (47, 48). In conclusion, there are too few studies and partly contradictory data to make conclusions regarding physical activity and the number of vertebral fractures or other fragility fractures in men.
Fracture risk with detraining
There are a few short-term prospective studies that evaluate changes in BMD with detraining, most indicating a higher age related loss in BMD associated with reduced training (49, 50). But fracture risk has not prospectively been evaluated in these studies. Studies that evaluate long-term effects of detraining are cross-sectional or observational studies but no RCT. These studies indicate that all benefits in BMD are lost with decades of detraining (51, 52), even if there possibly remains benefits in skeletal structure (52). These studies also infer that there is a lower fracture risk in individuals with a former (or current) high physical activity (49, 52). There is one study with a RCT-like design (53), a study that investigated 27 postmenopausal women aged 58–75 subjected to a two-year back strengthening program, compared with 23 controls. The intervention period resulted in increased back strength in the exercising women. After eight years, the intervention group still had a higher back strength but also a higher BMD and fewer spine fractures (14 fractures in 322 vertebral bodies examined, 4.3%, in the control group and six fractures in 378 vertebral bodies examined, 1.6%, in the intervention group). The relative risk for compression fracture was 2.7 times greater in the control group than in the intervention group. To our knowledge, this is the first and only prospective study reported in the literature demonstrating a possible long-term effect of strong back muscles on the reduction of vertebral fractures (53).
Conclusions
Existing data indicate that exercise, in both men and women and independently of age, improves muscle strength even when conducted during a short period. The muscle strength improves far more and much faster than the increase in muscle mass. Physical activity in elderly individuals living by their own seems to reduce the number of falls. It is more questionable if similar physical training programs could reduce the number of falls among institutionalized elderly. A physically activity lifestyle is associated with lower number of hip fractures in women and probably also in men. Effect on other fragility-related fractures is less evaluated.
Recommendations
The existing data would support to recommend physical activity to improve muscle strength, improve balance reduce the risk to fall and with probable effect also on the risk to sustain fractures.
Conflict of interest and funding
Financial support was obtained from the University Hospital Foundations, Centrum for Sports Medical Research (CIF), the Swedish Society of Medicine, and the Swedish Society of Medical Research.
References
- 1.Cooper C, Campion G, Melton LJ., 3rd Hip fractures in the elderly: a world-wide projection. Osteoporos Int. 1992;2:285–9. doi: 10.1007/BF01623184. [DOI] [PubMed] [Google Scholar]
- 2.Kannus P, Parkkari J, Sievanen H, Heinonen A, Vuori I, Jarvinen M. Epidemiology of hip fractures. Bone. 1996;18:57S–63S. doi: 10.1016/8756-3282(95)00381-9. [DOI] [PubMed] [Google Scholar]
- 3.Poor G, Atkinson EJ, O'Fallon WM, Melton LJ., 3rd Determinants of reduced survival following hip fractures in men. Clin Orthop. 1995;391:260–5. [PubMed] [Google Scholar]
- 4.Jarnlo GB, Thorngren KG. Background factors to hip fractures. Clin Orthop. 1993;287:41–9. [PubMed] [Google Scholar]
- 5.Cooper C, Atkinson EJ, Jacobsen SJ, O'Fallon WM, Melton LJ., 3rd Population-based study of survival after osteoporotic fractures. Am J Epidemiol. 1993;137:1001–5. doi: 10.1093/oxfordjournals.aje.a116756. [DOI] [PubMed] [Google Scholar]
- 6.Ray NF, Chan JK, Thamer M, Melton LJ., 3rd Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation. J Bone Miner Res. 1997;12:24–35. doi: 10.1359/jbmr.1997.12.1.24. [DOI] [PubMed] [Google Scholar]
- 7.Black DM, Cummings SR, Karpf DB, Caule JA, Thomson DE, Nevitt MC, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535–41. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 8.Jonsson B. Sweden: University of Lund; 1993. Life style and fracture risk. Thesis. [Google Scholar]
- 9.Orwoll E, Ettinger M, Weiss S, Miller P, Kendler DJG, Adami S, et al. Alendronate for the treatment of osteoporosis in men. N Engl J Med. 2000;343:604–10. doi: 10.1056/NEJM200008313430902. [DOI] [PubMed] [Google Scholar]
- 10.Cummings SR, Black DM, Nevitt MC, Browner W, Cauley J, Ensrud K, et al. Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet. 1993;341:72–5. doi: 10.1016/0140-6736(93)92555-8. [DOI] [PubMed] [Google Scholar]
- 11.Cummings SR, Nevitt MC, Browner WS, Stone K, Fox KM, Ensrud KE, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332:767–73. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 12.WHO. World Health Organ Tech Rep Ser. 1994. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis; pp. 1–129. Report of a WHO Study Group. [PubMed] [Google Scholar]
- 13.SBU95. Sweden: Stockholm; 1995. The Swedish council on technology assessment in health care: measurement of bone density. SBU report nr 127. [Google Scholar]
- 14.Jarvinen TL, Sievanen H, Khan KM, Heinonen A, Kannus P. Shifting the focus in fracture prevention from osteoporosis to falls. BMJ. 2008;336:124–6. doi: 10.1136/bmj.39428.470752.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319:1701–7. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- 16.Daley MJ, Spinks WL. Exercise, mobility and aging. Sports Med. 2000;29:1–12. doi: 10.2165/00007256-200029010-00001. [DOI] [PubMed] [Google Scholar]
- 17.Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA. 1990;263:3029–34. [PubMed] [Google Scholar]
- 18.Fiatarone MA, O'Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people [see comments] N Engl J Med. 1994;330:1769–75. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 19.Nelson ME, Fiatarone MA, Morganti CM, Trice I, Greenberg RA, Evans WJ. Effects of high-intensity strength training on multiple risk factors for osteoporotic fractures. A randomized controlled trial. JAMA. 1994;272:1909–14. doi: 10.1001/jama.1994.03520240037038. [DOI] [PubMed] [Google Scholar]
- 20.Province MA, Hadley EC, Hornbrook MC, Lipsitz LA, Miller JP, Mulrow CD, et al. The effects of exercise on falls in elderly patients. A preplanned meta-analysis of the FICSIT Trials. Frailty and Injuries: Cooperative Studies of Intervention Techniques [see comments] JAMA. 1995;273:1341–7. [PubMed] [Google Scholar]
- 21.Lexell J. Effects of strength and endurance training on skeletal muscle in the elderly; new muscles for old. J Swed Med Assoc (Läkartidningen) 1999;96:207–9. [PubMed] [Google Scholar]
- 22.Lord SR, Ward JA, Williams P, Strudwick M. The effect of a 12-month exercise trial on balance, strength, and falls in older women: a randomized controlled trial. J Am Geriatr Soc. 1995;43:1198–206. doi: 10.1111/j.1532-5415.1995.tb07394.x. [DOI] [PubMed] [Google Scholar]
- 23.Heislein DM, Harris BA, Jette AM. A strength training program for postmenopausal women: a pilot study. Arch Phys Med Rehabil. 1994;75:198–204. [PubMed] [Google Scholar]
- 24.Ryan AS, Treuth MS, Rubin MA, Miller JP, Nicklas BJ, Landis DM, et al. Effects of strength training on bone mineral density: hormonal and bone turnover relationships. J Appl Physiol. 1994;77:1678–84. doi: 10.1152/jappl.1994.77.4.1678. [DOI] [PubMed] [Google Scholar]
- 25.Kontulainen S, Kannus P, Haapasalo H, Heinonen A, Sievanen H, Oja P, et al. Changes in bone mineral content with decreased training in competitive young adult tennis players and controls: a prospective 4-yr follow-up. Med Sci Sports Exerc. 1999;31:646–52. doi: 10.1097/00005768-199905000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Greenspan SL, Myers ER, Maitland LA, Resnick NM, Hayes WC. Fall severity and bone mineral density as risk factors for hip fracture in ambulatory elderly. JAMA. 1994;271:128–33. [PubMed] [Google Scholar]
- 27.Wolf SL, Barnhart HX, Ellison GL, Coogler CE. The effect of Tai Chi Quan and computerized balance training on postural stability in older subjects. Phys Ther. 1997;77:371–81. doi: 10.1093/ptj/77.4.371. discussion 382–4. Atlanta FICSIT Group. Frailty and Injuries: Cooperative Studies on Intervention Techniques. [DOI] [PubMed] [Google Scholar]
- 28.Wolf SL, Barnhart HX, Kutner NG, McNeely E, Coogler C, Xu T. Reducing frailty and falls in older persons: an investigation of Tai Chi and computerized balance training. Atlanta FICSIT Group. Frailty and Injuries: Cooperative Studies of Intervention Techniques. J Am Geriatr Soc. 1996;44:489–97. doi: 10.1111/j.1532-5415.1996.tb01432.x. [DOI] [PubMed] [Google Scholar]
- 29.Tinetti ME, Baker DI, McAvay G, Claus EB, Garrett P, Gottschalk M, et al. A multifactorial intervention to reduce the risk of falling among elderly people living in the community [see comments] N Engl J Med. 1994;331:821–7. doi: 10.1056/NEJM199409293311301. [DOI] [PubMed] [Google Scholar]
- 30.Jensen J, Lundin-Olsson L, Nyberg L, Gustafson Y. Fall and injury prevention in older people living in residential care facilities. A cluster randomized trial. Ann Intern Med. 2002;136:733–41. doi: 10.7326/0003-4819-136-10-200205210-00008. [DOI] [PubMed] [Google Scholar]
- 31.Campbell AJ, Robertson MC, Gardner MM, Norton RN, Buchner DM. Falls prevention over 2 years: a randomized controlled trial in women 80 years and older [see comments] Age Ageing. 1999;28:513–8. doi: 10.1093/ageing/28.6.513. [DOI] [PubMed] [Google Scholar]
- 32.Wagner EH, LaCroix AZ, Grothaus L, Leveille SG, Hecht JA, Artz K, et al. Preventing disability and falls in older adults: a population-based randomized trial. Am J Public Health. 1994;84:1800–6. doi: 10.2105/ajph.84.11.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mulrow CD, Gerety MB, Kanten D, Cornell JE, DeNino LA, Chiodo L, et al. A randomized trial of physical rehabilitation for very frail nursing home residents [see comments] JAMA. 1994;271:519–24. [PubMed] [Google Scholar]
- 34.Gregg EW, Pereira MA, Caspersen CJ. Physical activity, falls, and fractures among older adults: a review of the epidemiologic evidence. J Am Geriatr Soc. 2000;48:883–93. doi: 10.1111/j.1532-5415.2000.tb06884.x. [DOI] [PubMed] [Google Scholar]
- 35.Lehtola S, Hanninen L, Paatalo M. The incidence of falls during a six month exercise trial and four month follow-up among home dwelling persons aged 70–75 years [Finnish] Liikunta Tiede. 2000;6:41–7. [Google Scholar]
- 36.O'Loughlin JL, Robitaille Y, Boivin JF, Suissa S. Incidence of and risk factors for falls and injurious falls among the community-dwelling elderly. Am J Epidemiol. 1993;137:342–54. doi: 10.1093/oxfordjournals.aje.a116681. [DOI] [PubMed] [Google Scholar]
- 37.Kallin K, Lundin-Olsson L, Jensen J, Nyberg L, Gustafson Y. Predisposing and precipitating factors for falls among older people in residential care. Public Health. 2002;116:263–71. doi: 10.1038/sj.ph.1900849. [DOI] [PubMed] [Google Scholar]
- 38.Tinetti ME, Doucette J, Claus E, Marottoli R. Risk factors for serious injury during falls by older persons in the community. J Am Geriatr Soc. 1995;43:1214–21. doi: 10.1111/j.1532-5415.1995.tb07396.x. [DOI] [PubMed] [Google Scholar]
- 39.Tinetti ME, Inouye SK, Gill TM, Doucette JT. Shared risk factors for falls, incontinence, and functional dependence. Unifying the approach to geriatric syndromes [see comments] JAMA. 1995;273:1348–53. [PubMed] [Google Scholar]
- 40.Gregg EW, Cauley JA, Seeley DG, Ensrud KE, Bauer DC. Physical activity and osteoporotic fracture risk in older women. Study of Osteoporotic Fractures Research Group [see comments] Ann Intern Med. 1998;129:81–8. doi: 10.7326/0003-4819-129-2-199807150-00002. [DOI] [PubMed] [Google Scholar]
- 41.Coupland C, Wood D, Cooper C. Physical inactivity is an independent risk factor for hip fracture in the elderly. J Epidemiol Community Health. 1993;47:441–3. doi: 10.1136/jech.47.6.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paganini-Hill A, Chao A, Ross RK, Henderson BE. Exercise and other factors in the prevention of hip fracture: the Leisure World study. Epidemiology. 1991;2:16–25. doi: 10.1097/00001648-199101000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Silman AJ, O'Neill TW, Cooper C, Kanis J, Felsenberg D. Influence of physical activity on vertebral deformity in men and women: results from the European Vertebral Osteoporosis Study. J Bone Miner Res. 1997;12:813–9. doi: 10.1359/jbmr.1997.12.5.813. [DOI] [PubMed] [Google Scholar]
- 44.Kujala UM, Kaprio J, Kannus P, Sarna S, Koskenvuo M. Physical activity and osteoporotic hip fracture risk in men. Arch Intern Med. 2000;160:705–8. doi: 10.1001/archinte.160.5.705. [DOI] [PubMed] [Google Scholar]
- 45.Farmer ME, Harris T, Madans JH, Wallace RB, Cornoni-Huntley J, White LR. Anthropometric indicators and hip fracture. The NHANES I epidemiologic follow-up study. J Am Geriatr Soc. 1989;37:9–16. doi: 10.1111/j.1532-5415.1989.tb01562.x. [DOI] [PubMed] [Google Scholar]
- 46.Meyer HE, Henriksen C, Falch JA, Pedersen JI, Tverdal A. Risk factors for hip fracture in a high incidence area: a case-control study from Oslo, Norway. Osteoporos Int. 1995;5:239–46. doi: 10.1007/BF01774013. [DOI] [PubMed] [Google Scholar]
- 47.Chan HH, Lau EM, Woo J, Lin F, Sham A, Leung PC. Dietary calcium intake, physical activity and the risk of vertebral fracture in Chinese. Osteoporos Int. 1996;6:228–32. doi: 10.1007/BF01622739. [DOI] [PubMed] [Google Scholar]
- 48.Greendale GA, Barrett-Connor E, Edelstein S, Ingles S, Haile R. Lifetime leisure exercise and osteoporosis. The Rancho Bernardo study. Am J Epidemiol. 1995;141:951–9. doi: 10.1093/oxfordjournals.aje.a117362. [DOI] [PubMed] [Google Scholar]
- 49.Nordstrom A, Karlsson C, Nyquist F, Olsson T, Nordstrom P, Karlsson M. Bone loss and fracture risk after reduced physical activity. J Bone Miner Res. 2005;20:202–7. doi: 10.1359/JBMR.041012. [DOI] [PubMed] [Google Scholar]
- 50.Valdimarsson O, Alborg HG, Duppe H, Nyquist F, Karlsson M. Reduced training is associated with increased loss of BMD. J Bone Miner Res. 2005;20:906–12. doi: 10.1359/JBMR.050107. [DOI] [PubMed] [Google Scholar]
- 51.Karlsson MK, Linden C, Karlsson C, Johnell O, Obrant K, Seeman E. Exercise during growth and bone mineral density and fractures in old age. Lancet. 2000;355:469–70. doi: 10.1016/s0140-6736(00)82020-6. [DOI] [PubMed] [Google Scholar]
- 52.Karlsson MK, Alborg HG, Obrant K, Nyquist F, Lindberg H, Karlsson C. Exercise during growth and young adulthood is associated with reduced fracture risk in old ages. J Bone Miner Res. 2002;17(Suppl 1):s297. [Google Scholar]
- 53.Sinaki M, Itoi E, Wahner HW, Wollan P, Gelzcer R, Mullan BP, et al. Stronger back muscles reduce the incidence of vertebral fractures: a prospective 10 year follow-up of postmenopausal women. Bone. 2002;30:836–41. doi: 10.1016/s8756-3282(02)00739-1. [DOI] [PubMed] [Google Scholar]